Abstract
Background
The purpose of this study is to examine patterns of rotator cuff tear size progression in degenerative rotator cuff tears and to compare tear progression risks for tears with and without anterior supraspinatus tendon disruption.
Methodology
Asymptomatic full-thickness rotator cuff tears with minimum 2-year follow-up were examined with annual shoulder ultrasounds. Integrity of the anterior 3mm of the supraspinatus tendon determined classification of cable-intact versus disrupted tears. Tear enlargement was defined as an increase of 5mm or greater in width. Tear propagation direction was calculated from measured changes in tear width in reference to the biceps tendon on serial ultrasounds.
Results
The cohort included 139 full-thickness tears with a mean subject age of 63.3 years and follow-up duration of 6.0 years. Ninety-six (69.1%) of the tears were considered cable-intact. Cable-disrupted tears were larger at baseline (median 19.0mm vs. 10.0mm, p<0.0001) than cable-intact tears. There was no difference in the risk of enlargement (52.1% vs. 67.4%, p=0.09) or time to enlargement (3.2 vs. 2.2 years, p=0.37) for cable-intact compared to cable-disrupted tears.
There was no difference in the magnitude of enlargement for cable-intact and cable-disrupted tears (median 7.0mm vs.9.0mm, p=0.18). Cable-intact tears propagated a median of 5mm anteriorly and 4mm posteriorly, whereas cable-disrupted tears propagated posteriorly.
Conclusions
The majority of degenerative rotator cuff tears spare the anterior supraspinatus tendon. Although tears classified as cable-disrupted are larger at baseline than cable-intact tears, tear enlargement risks are similar for each tear type.
Keywords: Rotator cuff tear, progression, rotator cable, ultrasound
Introduction
Identification of “at-risk” rotator cuff tears will better refine surgical indications for rotator cuff disease. Recent research has better illustrated the general risks of tear enlargement of degenerative rotator cuff tears through the monitoring of asymptomatic tears9,15,17. Although the time-dependent risks of tear progression have been better defined, longitudinal analysis of the common patterns of tear enlargement has not been performed to our knowledge. Descriptive analysis of tear propagation patterns is fundamental for further defining the natural history of rotator cuff disease and may also help refine surgical indications. This is particularly clinically relevant given the high prevalence of degenerative rotator cuff tears in association with age23,25 and the lack of consensus regarding appropriate surgical indications for symptomatic tears3.
Traditionally, most degenerative rotator cuff tears have been felt to begin as partial-thickness tendon defects occurring at the undersurface of the anterior supraspinatus tendon2,6,10,14. Others have suggested that these tears are most likely to initiate within the rotator crescent with the majority of tears sparing the anterior cable insertion of the tendon12,18. Recent data has suggested that these degenerative tears may enlarge within the crescent propagating in both the anterior and posterior directions12; however, there is little data defining these patterns of tear propagation in a prospective, longitudinal fashion. Furthermore, little is known regarding the risks of tear progression for tears isolated to the rotator crescent compared to those involving the anterior aspect of the tendon. Loss of the anterior supraspinatus origin of the rotator cable is associated with an increased risk of fatty muscle degeneration13 but, to date, little information exists about associated progression risks. A better understanding of the patterns of tear progression is fundamental in defining the natural history of rotator cuff disease and the clinical relevance is magnified given the influence of tear location on rotator cuff muscle degeneration.
The purpose of this study is to examine the temporal-based patterns of rotator cuff tear size progression in full-thickness, degenerative rotator cuff tears. Additionally, we sought to compare the risks and patterns of tear progression in shoulders with and without disruption of the anterior rotator cable origin.
Methodology
Institutional Review Board approval was obtained prior to study initiation (IRB # 201103230). Subjects for the present study belong to a cohort of individuals with asymptomatic rotator cuff tears that have been followed longitudinally for the purpose of defining the risks of tear enlargement and pain development over time. Subjects presented to the physician with shoulder pain secondary to rotator cuff disease and were found to have an asymptomatic rotator cuff tear in the contralateral shoulder with shoulder ultrasonography. After tear identification subjects were confirmed to be asymptomatic at baseline on the study side and were followed annually with a repeat clinical examination, shoulder ultrasonography and shoulder radiographs according to a previously published protocol9,16. Exclusion criteria included: the presence of shoulder pain as previously defined, a history of shoulder trauma or injury, isolated subscapularis tears, preexisting glenohumeral arthritis, a history of inflammatory arthritis and prior surgery on the study shoulder.
Shoulder Ultrasonography
Shoulder ultrasonography was performed according to a previously described protocol21,22 in real time with a Siemens Elegra or Antares (Siemens Medical Solutions, Mountain View, CA, USA) or GE E8 or E9 (General Electric, Madison, WI, USA) scanner and a variable high-frequency linear array transducer (7.5 to 13 MHz) by one of three radiologists with extensive experience in musculoskeletal ultrasonography. The accuracy of this modality in our institution has been well documented20–22,24. The maximum anteroposterior dimension of the tear was measured in transverse views (perpendicular to the long axis of the cuff) and designated as the width of the tear. This is analogous to the sagittal plane size of the tear. The maximum degree of retraction was measured in longitudinal views (parallel to the long axis of the cuff) and designated as the length of the tear. This is analogous to the amount of tear retraction in the coronal plane. The distance of the anterior aspect of the supraspinatus tear to the biceps tendon or the lateral aspect of the biceps groove if the tendon was absent was measured to determine the integrity of the anterior aspect of the supraspinatus tendon.
Tear Propagation Analysis
For this analysis we studied only full-thickness tears, either classified as full-thickness at baseline or later converted to a full-thickness tear during follow-up. We chose to analyze full-thickness tears as shoulder ultrasonography is more accurate in defining the tear size of full-thickness compared to partial-thickness tears. All full-thickness tears with a minimum of 2 years follow-up without tear enlargement and any full-thickness tear with width enlargement on consecutive ultrasounds regardless of the length of follow-up were included. A tear was considered enlarged only if the width was increased by 5 mm or greater compared to baseline ultrasound dimensions. Serial ultrasounds reports were analyzed referencing the dimensions of the tear and the distance of the anterior aspect of the tear in reference to the long head biceps tendon. We categorized tears as cable-intact if the anterior supraspinatus footprint (immediately posterior to the biceps tendon/groove) was intact for more than 3 mm posterior to the biceps. Cable-disrupted tears were defined as tears that involved the anterior 3 mm of the supraspinatus tendon footprint. Based on the width of the tear and the location of the anterior edge of the tear to the biceps, the location of each tear within the supraspinatus and infraspinatus footprint can be mapped. By comparing the change in width of the tear and the change in location of anterior aspect of the tear in reference to the biceps after an enlargement event, the direction (either anterior, posterior or both) and the magnitude of tear propagation within the tendon insertion footprint can be calculated (Figure 1). For tears with multiple width enlargement events, the final tear dimensions were compared against baseline values.
Figure 1.
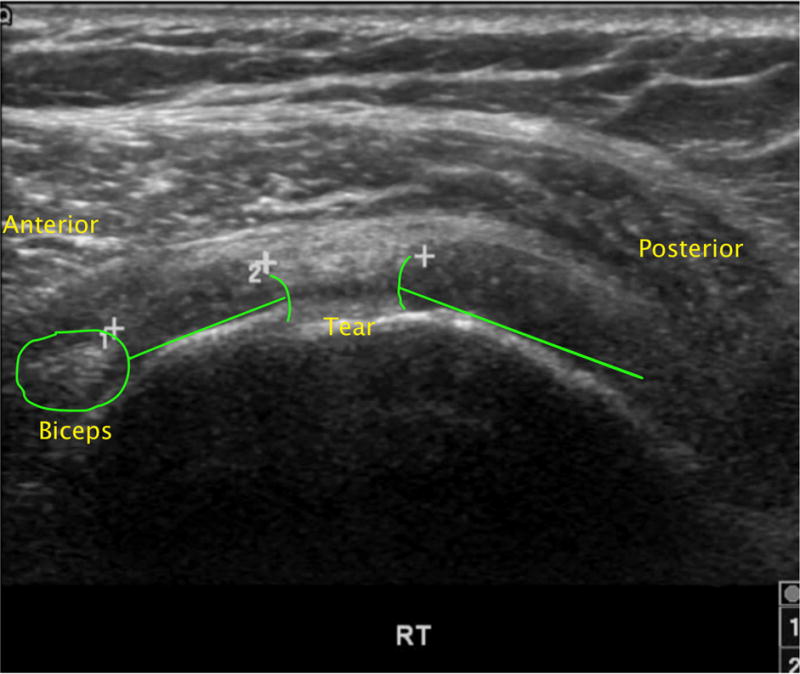
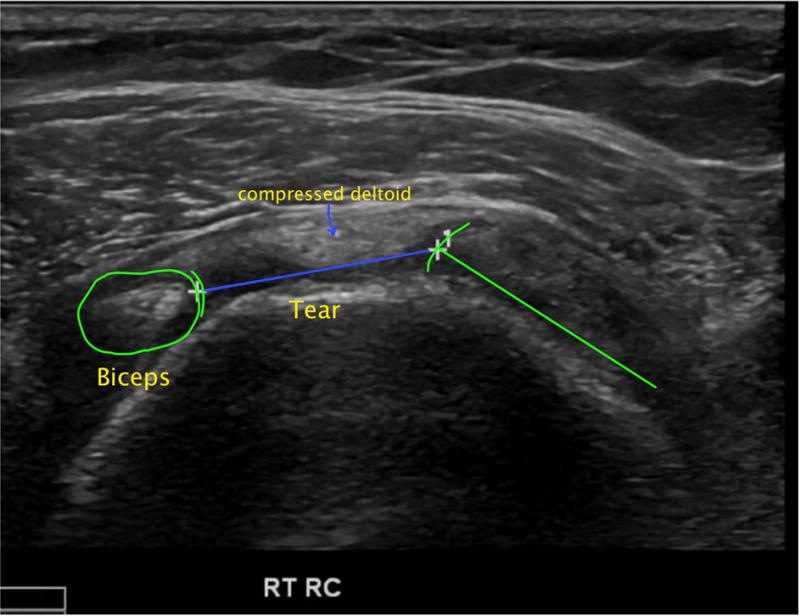
Transverse sonographic image of full-thickness rotator cuff tear.
A) Cable-intact full thickness rotator cuff tear. Intact cuff demarcated by green line. Intact cuff is noted between the anterior tear edge and the biceps tendon.
B) Tear has progressed 2 years later to involve the entire supraspinatus tendon, therefore is now classified as cable-disrupted. The transverse tear dimension (tear width) is shown with a blue line.
Statistical Analysis
Between-group comparisons of continuous variables were performed using analysis of variance (ANOVA). When more than two groups were compared and the overall ANOVA was significant (p<0.05), Tukey-Kramer p-values adjusted for multiple comparisons were used to determine which groups were significantly different. Continuous variables that were not normally distributed were rank-transformed prior to the ANOVA. Chi-square tests were used for between-group comparisons of categorical variables.
Results
Tear Propagation Analysis
A total of 181 shoulders from the prospective cohort were identified to have a full-thickness cuff tear and a minimum of 2-year follow-up. Forty-two shoulders were excluded due to missing ultrasound data regarding the distance of the tear from the biceps tendon/groove. Therefore, 139 full-thickness tears possessed adequate data and follow-up for tear enlargement analysis. Thirty-three of these shoulders were originally enrolled as partial-thickness tears and 5 were originally designated as controls (no tear). Once these tears progressed to full-thickness defects, longitudinal surveillance examining further tear enlargement was performed for this analysis. The mean age of the subjects at enrollment was 63.3 years and the median length of study follow-up was 6.0 years (Table 1). Six of the shoulders had a decrease in tear width during follow-up; however, 5 of these had a tear enlargement event as well (4 before and 1 after the recognized decrease in tear size). These 5 shoulders were considered enlarged in the analysis to determine the direction of propagation.
Table 1.
Subject and tear characteristics for the entire full-thickness tear cohort and by cable integrity (N=139).
| Variable | Entire Cohort (N=139) |
By Cable Disruption | ||
|---|---|---|---|---|
| Disrupted (n=43) |
Intact (n=96) |
p-value | ||
| Age at enrollment (years), mean (SD) | 63.3 (8.7) range 33.9 – 84.5 |
64.3 (7.9) | 62.8 (9.1) | 0.35* |
| Study period duration (years), median [IQR] | 6.0 [5.3] range 0.3 – 11.4 |
3.9 [5.0] | 6.1 [5.5] | 0.09† |
| Baseline tear width (mm), median [IQR] | 11.0 [10.0] range 3.0 – 42.0 |
19.0 [10.0] | 10.0 [6.0] | <0.0001† |
| Width enlargement, n (% of group): no yes |
60 (43%) 79 (57%) |
14 (33%) 29 (67%) |
46 (48%) 50 (52%) |
0.09‡ |
| Width Enlargers (n=79) | ||||
| Duration of follow-up until width enlargement (years), median [IQR] | 2.8 [4.6] range 0.0 – 10.3 |
2.2 [3.4] | 3.2 [5.0] | 0.37† |
SD = standard deviation; IQR = interquartile range; n = number of patients; mm = millimeters
P-value compares disrupted vs. intact cable by analysis of variance
P-value compares disrupted vs. intact cable by analysis of variance using rank-transformed data
P-value compares the proportion of tears that have width enlargement for tears with a disrupted vs. intact cable by chi-square test.
Of the 139 subjects with full-thickness tears, 96 (69.1%) were classified as cable-intact and 43 (30.9%) tears had disruption of the anterior cable. The median width of the cable-intact tears prior to enlargement (10.0 mm) was less than the cable-disrupted tears (19.0 mm, p<0.0001, Table 1). Fifty (52.1%) of the cable-intact tears showed progression of tear width at a median of 3.2 years from enrollment, whereas, 29 (67.4%) of the cable-disrupted tears enlarged in width at a median of 2.2 years from enrollment. There was no statistically significant difference in the risk of tear progression (p=0.09) or the time from study enrollment to progression (p=0.37) between cable-intact and disrupted tears. With the current sample sizes, this study had a 40% power to detect this observed effect regarding risk of tear enlargement at an alpha level of 0.05. With these sample sizes and an alpha of 0.05 for a two-tailed chi-square test, the study had 80% power to detect a between-group difference in the incidence of width enlargement of at least 25.2%.
Of the 79 shoulders with full-thickness tears that enlarged in width, 68 had adequate measurements of tear location in reference to the biceps tendon/groove both before and after width enlargement. These shoulders were analyzed for the direction of tear enlargement in the transverse plane within the insertional footprint of the tendon. Forty-three (63.2%) of the 68 tears were classified as cable-intact and 25 (36.8%) as cable-disrupted (Table 2). There were no significant differences in age and length of follow-up between groups. The cable-disrupted tears were larger at baseline (19.0 vs. 8.0 mm, p<0.0001). There was no difference in the magnitude of width enlargement in the cable-intact (7.0 mm) compared to the cable-disrupted (9.0 mm, p=0.18) shoulders during follow-up.
Table 2.
Tear characteristics and propagation data separately by cable integrity (N=68).
| Variable | Cable Integrity at Baseline
|
p-value¶ | |
|---|---|---|---|
| Intact (n=43) | Disrupted (n=25) | ||
|
| |||
| Patient Characteristics | |||
|
| |||
| Baseline age (years), mean (SD) | 65.5 (8.7) | 67.3 (8.6) | 0.42 |
|
| |||
| Age at final enlargement (years), mean (SD) | 67.2 (8.8) | 68.8 (8.6) | 0.46 |
|
| |||
| Duration (years) from study enrollment to final enlargement, mean (SD) | 4.8 (3.0) | 3.6 (2.2) | 0.09 |
|
| |||
| Tear Characteristics | |||
|
| |||
| Tear width (mm), median [IQR]: | |||
| Baseline | 8.0 [6.0] | 19.0 [11.0] | <0.0001** |
| Final enlargement | 18.0 [11.0] | 30.0 [17.0] | n/a |
| Change† | 7.0 [8.0] | 9.0 [5.0] | 0.18** |
|
| |||
| Tear distance from biceps (mm), median [IQR]: | |||
| Baseline | 10.0 [5.0] | 0.0 [0.0] | n/a |
| Final enlargement | 6.0 [9.0] | 0.0 [0.0] | n/a |
| Change‡(same as anterior enlargement) | 5.0 [9.0] | 0.0 [0.0] | n/a |
|
| |||
| Enlargement Data | |||
|
| |||
| Anterior enlargement (mm)‡, median [IQR] | 5.0 [9.0] | 0.0 [0.0] | n/a |
|
| |||
| Posterior enlargement (mm)§, median [IQR] | 4.0 [7.0] | 9.0 [5.0] | n/a |
|
| |||
| Direction of enlargement of ≥ 3mm, n (%): | |||
| Anterior only | 16 (37%) | 0 (0%) | n/a |
| Posterior only | 16 (37%) | 24 (96%) | n/a |
| Anterior and posterior | 11 (26%) | 1 (4%) | n/a |
SD = standard deviation; IQR = interquartile range; n = number of patients; n/a = analysis not performed; mm = millimeters
Calculated as: (widthfinal - widthbaseline).
Anterior enlargement is defined as the change in tear distance from biceps between Baseline and the final enlargement event (distancebaseline - distancefinal).
Posterior enlargement is defined as the change in tear width minus the anterior enlargement between Baseline and the final enlargement event [(widthfinal - widthbaseline) - (distancebaseline - distancefinal)].
P-value compares tears with the cable-intact versus disrupted by analysis of variance.
Statistical analysis performed using rank-transformed data
The median distance of the cable-intact tears from the biceps tendon was 10 mm at baseline and 6 mm at most recent follow-up compared to 0 mm at baseline for the cable-disrupted tears. The cable intact-tears increased width a median of 5 mm in the anterior and 4 mm in the posterior directions (Table 2). Using a change in width of 3mm as a threshold, 37% of the cable intact tears enlarged anteriorly, 37% posteriorly and 26% in both directions. There was no significant relationship between the baseline tear width and the direction of tear propagation (p=0.22, Table 3). The magnitude of width enlargement was greater for tears that enlarged in both directions (15.0mm, compared to either anterior (6.5mm) or posterior (5.5mm, p<0.0001)).
Table 3.
Tear characteristics and enlargement data separately by the direction of the enlargement for cable-intact tears at Baseline and that enlarged ≥ 3mm in the specified direction (n=43).
| Variable | Direction Of Enlargement Of ≥ 3mm: | p-value¶ | ||
|---|---|---|---|---|
| Anterior only (n=16) | Posterior only (n=16) | Anterior and Posterior (n=11) | ||
| Tear Characteristics | ||||
| Tear width (mm), median [IQR]: | ||||
| Baseline | 8.0 [5.0] | 7.0 [6.5] | 11.0 [7.0] | 0.22 |
| Final enlargement | 18.5 [9.5] | 15.0 [5.5] | 28.0 [9.0] | n/a |
| Change† | 6.5 [6.0] | 5.5 [2.5] | 15.0 [6.0]** | <0.0001 |
| Tear distance from biceps (mm), median [IQR]: | ||||
| Baseline | 12.5 [4.5] | 10.0 [5.5] | 9.0 [3.0] | n/a |
| Final enlargement | 2.5 [6.5] | 9.5 [6.0] | 0.0 [0.0] | n/a |
| Change‡ (same as anterior enlargement) | 7.5 [8.5] | 0.0 [2.0] | 8.0 [4.0] | n/a |
| Enlargement Data | ||||
| Anterior enlargement‡ (mm), median [IQR] | 7.5 [8.5] | 0.0 [2.0] | 8.0 [4.0] | n/a |
| Posterior enlargement§ (mm), median [IQR] | −0.5 [1.5] | 6.0 [3.0] | 7.0 [5.0] | n/a |
IQR = interquartile range; n/a = analysis not performed.
Calculated as: (widthfinal - widthbaseline).
Anterior enlargement is defined as the change in tear distance from biceps between Baseline and the final enlargement event (distancebaseline - distancefinal).
Posterior enlargement is defined as the change in tear width minus the anterior enlargement between Baseline and the final enlargement event [(widthfinal - widthbaseline) - (distancebaseline - distancefinal)].
P-value compares tears with anterior only versus posterior only versus anterior and posterior enlargement by analysis of variance (ANOVA) using rank-transformed data. When significant, all pairwise between-group comparisons were performed and comparisons with a significant Tukey-Kramer adjusted p-value are footnoted.
P-value < 0.05 compared to anterior only (p = 0.0003) and compared to posterior only (p < 0.0001) by Tukey-Kramer adjusted pairwise comparisons within the ANOVA.
Discussion
Understanding common patterns of tear enlargement is fundamental for both surgical indications as well as surgical repair strategies. To properly illustrate directions of tear enlargement or propagation patterns of tears, a longitudinal analysis is ideal as direct comparisons of changing tear dimensions can be established in a prospective fashion. The asymptomatic cuff tear may also be ideal for this analysis as treatments that may influence disease progression are not rendered given the tear is painless on presentation. To this point, the risks of enlargement for tears that are isolated to the rotator crescent compared to tears with propagation into the rotator cable have not been studied.
The results of this study demonstrate that the majority of atraumatic degenerative rotator cuff tears are isolated to the rotator crescent. In this cohort, approximately 30% of full-thickness tears involved the most anterior aspect of the supraspinatus tendon. An earlier report of this cohort demonstrated that less than 5% of small full-thickness (<10mm) tears had complete disruption of the anterior supraspinatus footprint12. In the previous report, the most common locations for full-thickness tears were within the rotator crescent, approximately 13–17 mm posterior to the biceps tendon with diminishing frequency seen anterior and posterior to this region. These findings contradict previous theories that degenerative rotator cuff tears begin at the articular aspect of the supraspinatus tendon adjacent to the biceps tendon2,6,10,11,14.
The rotator cable consists of a thickening cuff tissue which arcs from the inferior infraspinatus tendon to the anterior supraspinatus tendon. The anterior cable is continuous with the coracohumeral ligament and blends with the upper subscapularis tendon4,8. Burkhart proposed the purpose of the rotator cable was to stress-shield or buffer abnormal forces created by tears that develop within the rotator crescent1. The importance of the supraspinatus tendon and rotator cable in maintaining normal glenohumeral abduction torque has been suggested biomechanically5,19. Additionally, the anterior aspect of the supraspinatus tendon has been shown to have the highest tensile strength compared to the middle and posterior regions of the tendon7. Mesiha et al demonstrated the importance of the anterior supraspinatus insertion noting increased gapping, stiffness and regional tendon strains in experimental tears of the anterior supraspinatus tendon compared to tears isolated to the rotator crescent16. Little is known regarding the influence of tear extension into the rotator cable on the risks of tear progression over time. In the current study, we found a nonsignificant trend towards a greater risk of tear enlargement for cable-disrupted compared to cable-intact tears (67 vs. 52%). Our data suggests that there are significant risks of tear propagation for both cable-intact (isolated) and cable-disrupted tears. The theoretical benefit of stress shielding of cable intact tears does not appear to significantly mitigate the risks of tear progression into the anterior cable over time. Additionally, we found no difference in the magnitude of enlargement or the time to enlargement between tear types; however, given the potentially greater risks of tear enlargement coupled with established risks of muscle degeneration when the anterior supraspinatus is torn13, we feel that tears with anterior cable involvement to be high risk and should be closely monitored.
A fundamental question regarding cable-intact and cable-disrupted tears is whether these tears have a common or variable location of initiation. Because the width of cable-disrupted tears is considerably larger (19 vs. 10 mm) compared to isolated crescent tears, we hypothesize that these tears may simply reflect a more chronic or later disease stage and that they have a similar location of tear initiation. This theory is supported by a previous study demonstrating a much lower incidence of anterior supraspinatus involvement with full-thickness tears less than 10 mm in width12. We recognize that this theory can only be inferred given the unknown chronicity of the tears and lack of data regarding the tear location at the time of tear initiation for the majority of this cohort.
This longitudinal analysis is the first to describe direction of tear enlargement in a prospective fashion. The tear propagation analysis suggests that cable-intact tears commonly enlarge in both an anterior and posterior direction with no significant predilection for one direction over another. Our previous research suggested similar findings; however, given the single point in time analysis, the direction of propagation was theoretical12. Over time, many tears appear to grow within the rotator crescent in both directions and can eventually compromise the anterior edge of the supraspinatus tendon. For cable-intact tears the baseline tear size had no apparent influence on the direction of tear enlargement. This suggests that larger tears, which are already closer to the anterior cable, are not protected from further anterior enlargement. The cable-disrupted tears were found to propagate in the posterior direction into the thinner crescent tissue, as expected. We did not analyze the incidence of biceps tendon pathology or extension into the upper subscapularis tendon between cable-intact and disrupted tears. Further investigation is warranted to define these associated pathologies.
This study is unique in that tear enlargement was described in a yearly longitudinal manner rather than a single point in time analysis. We used a validated method of assessing cuff tear size in the form of shoulder ultrasonography performed by experienced radiologists. Furthermore, to minimize errors in size measurements, only included full-thickness tear for this analysis as both ultrasound and magnetic resonance imaging are more accurate in measuring tear dimension for full-thickness rather than partial thickness tears. There are also several important limitations that warrant discussion. We defined tear enlargement as a five-millimeter increase in size to maintain consistency with previous research; however, we recognize that a clinically relevant tear size increase threshold has not been established. We emphasized the width or sagittal plane tear size rather than the length or degree of retraction for this analysis. We felt this to be clinically relevant given the emphasis of the study was to examine tear enlargement compared to the integrity of the anterior supraspinatus cable attachment and to describe directions of propagation within the rotator crescent. Also, the chronicity of the majority of tears in this study is unknown. This potentially confounds definitions of cable-intact and disrupted tears as these may simply represent tears in differing stages of disease; however, we do feel the descriptive analysis of tear propagation remains valid. In addition, we recognize that disruption of the anterior aspect of the supraspinatus tendon (defined as cable-disrupted tears) likely does not completely compromise the function of the anterior cable given the expansion of this tissue into the adjacent biceps sling and upper subscapularis. With the available number of subjects, there was not a statistically significant difference in tear enlargement risk between cable-intact and disrupted tears; however, our data does suggest a trend towards a greater risk of enlargement in cable-disrupted tears. The clinical relevance of a 15% greater risk of tear progression in the cable-disrupted tears is of uncertain importance.
Conclusions
The majority of asymptomatic degenerative rotator cuff tears do not involve the anterior aspect of the supraspinatus tendon. Tears defined as cable-disrupted are larger than cable-intact tears. Cable-intact tears appear to propagate in both the anterior and posterior direction while cable-disrupted tears propagate posteriorly within the rotator crescent. The risks, magnitude and timeline of tear progression for cable-intact and cable-disrupted full-thickness tears appear similar and, thus, both have a similar high risk for progression that should be considered in surgical indications.
Acknowledgments
Study funded by the National Institute of Health: NIH R01-AR051026
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Level of evidence: Level I, Prognosis Study
Please publish Figures in color
IRB Approval: Washington University - #201103230
J.D.K., A.C.M. and K.Y. have potential conflicts that are not related to the content of this study
References
- 1.Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge”. Arthroscopy. 1993;9:611–6. doi: 10.1016/s0749-8063(05)80496-7. [DOI] [PubMed] [Google Scholar]
- 2.Codman EA, Akerson IB. The pathology associated with rupture of the supraspinatus tendon. Ann Surg. 1931;93:348–59. doi: 10.1097/00000658-193101000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn WR, Schackman BR, Walsh C, Lyman S, Jones EC, Warren RF, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg. 2005;87(9):1978–84. doi: 10.2106/JBJS.D.02944. [DOI] [PubMed] [Google Scholar]
- 4.Fallon J, Blevins FT, Vogel K, Trotter J. Functional morphology of the supraspinatus tendon. J Orthop Res. 2002;20(5):920–6. doi: 10.1016/s0736-0266(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 5.Halder AM, O’Driscoll SW, Heers G, Mura N, Zobitz ME, An KN, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg. 2002;84-A(5):780–5. doi: 10.2106/00004623-200205000-00013. no doi number available. [DOI] [PubMed] [Google Scholar]
- 6.Hijioka A, Suzuki K, Nakamura T, Hojo T. Degenerative change and rotator cuff tears. An anatomical study in 160 shoulders of 80 cadavers. Arch Orthop Trauma Surg. 1993;112(2):61–4. doi: 10.1007/BF00420255. [DOI] [PubMed] [Google Scholar]
- 7.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578–84. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 8.Kask K, Kolts I, Lubienski A, Russlies M, Leibecke T, Busch LC. Magnetic resonance imaging and correlative gross anatomy of the ligamentum semicirculare humeri (rotator cable) Clin Anat. 2008;21(5):420–6. doi: 10.1002/ca.20639. [DOI] [PubMed] [Google Scholar]
- 9.Keener JD, Galatz LM, Teefey SA, Middleton WD, Steger-May K, Stobbs-Cucchi G, et al. A prospective evaluation of survivorship of asymptomatic degenerative rotator cuff tears. J Bone Joint Surgery. 2015;97(2):89–98. doi: 10.2106/JBJS.N.00099. doi.10.2106/JBJS.N.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keyes EL. Observations on rupture of supraspinatus tendon. Based upon a study of seventy-three cadavers. Ann Surg. 1933;97:849–56. doi: 10.1097/00000658-193306000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyes EL. Anatomical observations on senile changes in the shoulder. J Bone Joint Surg. 1935;17:953–60. [Google Scholar]
- 12.Kim HM, Dahiya N, Teefey SA, Middleton WD, Stobbs G, Steger-May K, et al. Location and initiation of degenerative rotator cuff tears: an analysis of three hundred and sixty shoulders. J Bone Joint Surg. 2010;92(5):1088–96. doi: 10.2106/JBJS.I.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. The J Bone Joint Surg. 2010;92(4):829–39. doi: 10.2106/JBJS.H.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54(1):30–1. [PubMed] [Google Scholar]
- 15.Mall NA, Kim HM, Keener JD, Steger-May K, Teefey SA, Middleton WD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623–33. doi: 10.2106/JBJS.I.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesiha MM, Derwin KA, Sibole SC, Erdemir A, McCarron JA. The biomechanical relevance of anterior rotator cuff cable tears in a cadaveric shoulder model. J Bone Joint Surg Am. 2013;95(20):1817. doi: 10.2106/JBJS.L.00784. [DOI] [PubMed] [Google Scholar]
- 17.Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249–55. doi: 10.2106/JBJS.L.00185. [DOI] [PubMed] [Google Scholar]
- 18.Namdari S, Donegan RP, Dahiya N, Galatz LM, Yamaguchi K, Keener JD. Characteristics of small to medium-sized rotator cuff tears with and without disruption of the anterior supraspinatus tendon. J Shoulder Elbow Surg. 2014;23(1):20–7. doi: 10.1016/j.jse.2013.05.015. doi.10.1016/j.jse.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Oh JH, Jun BJ, McGarry MH, Lee TQ. Does a critical rotator cuff tear stage exist?: a biomechanical study of rotator cuff tear progression in human cadaver shoulders. J Bone Joint Surg Am. 2011;93(22):2100–9. doi: 10.2106/JBJS.J.00032. [DOI] [PubMed] [Google Scholar]
- 20.Prickett WD, Teefey SA, Galatz LM, Calfee RP, Middleton WD, Yamaguchi K. Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surg Am. 2003;85-A(6):1084–9. doi: 10.2106/00004623-200306000-00016. no doi available. [DOI] [PubMed] [Google Scholar]
- 21.Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg. 2000;82(4):498–504. [PubMed] [Google Scholar]
- 22.Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708–16. no doi available. [PubMed] [Google Scholar]
- 23.Teunis T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913–21. doi: 10.1016/j.jse.2014.08.001. doi.10.1016/j.jse.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Wall LB, Teefey SA, Middleton WD, Dahiya N, Steger-May K, Kim HM, et al. Diagnostic performance and reliability of ultrasonography for fatty degeneration of the rotator cuff muscles. J Bone Joint Surg Am. 2012;94(12):e83. doi: 10.2106/JBJS.J.01899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88(8):1699–704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]


