Abstract
Chimeric antigen receptor (CAR) T-cell therapy utilizes genetic engineering to redirect a patient’s own T cells to target cancer cells. The remarkable results in hematological malignancies prompted investigating this approach in solid tumors such as pancreatic cancer. The complex tumor microenvironment, stromal hindrance in limiting immune response, and expression of checkpoint blockade on T cells poses hurdles. Herein, we summarize the opportunities, challenges, and state of knowledge in targeting pancreatic cancer with CAR T-cell therapy.
Keywords: Chimeric antigen receptor, CAR T cells, immunotherapy, adoptive cell therapy, checkpoint blockade
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is projected to become the second leading cause of cancer death in the United States by 2030 [1]. Despite recent advances in systemic chemotherapy for metastatic PDAC with approval of several chemotherapy regimens in the last decade, median overall survival remains poor for this disease (<1 year) and novel treatment strategies are needed [2–4]. Although initial studies of single agent immunotherapy agents with checkpoint inhibitors in PDAC have been disappointing, much promise and interest in novel targeted and immunotherapy strategies for PDAC remains given our emerging understanding of the role the immune system in pancreatic cancer [5].
In pancreatic cancer, published studies have shown that the presence of tumor-infiltrating lymphocytes (TILs) in surgical resection specimens is associated with improved prognosis among patients who undergo surgical resection of their early stage cancers [6,7]. Infusion of T cells as treatment of solid tumors has been shown to be effective, as demonstrated by TIL therapy for melanoma, where complete remission (CR) was seen in up to 22% of patients with metastatic disease [8]. TIL therapy relies on the ability to isolate and expand tumor-reactive T cells from within the patient tumor, which is challenging to implement. Progress in genetic engineering and a greater understanding of T-cell biology have allowed us now to translate the complex cytotoxic T-cell response into a synthetic molecule that can be introduced into T cells. Adoptive cell therapy using genetically engineered T cells is a promising approach that specifically targets cancer cells to eradicate tumor burden. The two main approaches currently used in the clinic to engineer T cells are to target a tumor antigen either via a T-cell receptor (TCR) or a chimeric antigen receptor (CAR). While TCRs are restricted to human leukocyte antigen (HLA), CARs directly bind to cancer cell-surface proteins, carbohydrates, or glycolipids, which allows them to be limited by HLA down regulation commonly seen in solid tumors [9]. First generation CARs were developed in 1989 and consisted of a TCR stimulatory domain attached to a single chain variable antibody fragment [10]; the subsequent addition of a costimulatory domain (second generation CAR) has greatly enhanced its success by improving T-cell survival and proliferation upon encounter with antigen-expressing target cells [11]. Two costimulatory domains, CD28 and 4-1BB, were utilized by multiple investigators to link to CD3z domain, an intracellular signaling domain that imparts cytolytic ability to the T-cell. The addition of both costimulatory domains (CD28 and 4-1BB) to the CD3z domain, which is designated a third generation CAR, does not consistently perform better than the second generation CAR [12–14]; consequently, most clinical trials today use a second generation CAR (Figure 1). The use of CAR-modified T cells that target CD19, a B-cell receptor, has been shown to induce durable remissions in patients with chemorefractory B-cell malignancies.[15–17]. Patients with B-cell acute lymphocytic leukemia (ALL) treated with second generation CARs after lymphodepletion exhibit CR of 80–90% in relapsed and refractory patients [18].
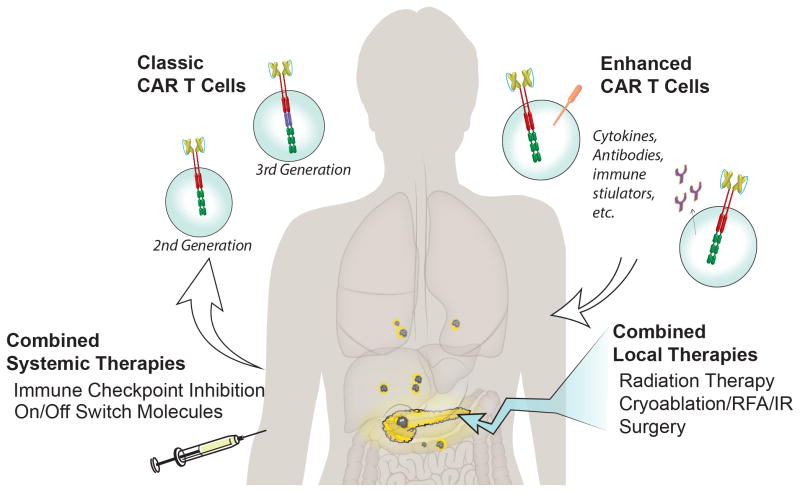
Figure 1. CAR T-cell therapy for pancreatic cancer.
Second and third generation CAR T cells, containing the CD3z domain with 1 or 2 cosignaling domains, respectively, are being used in active clinical trials for pancreatic cancer. “Enhanced CAR T cells,” coexpressing or secreting additional molecules with the CAR, such as dominant negative PD-1, checkpoint inhibitor antibodies, costimulatory molecules, cytokines, or other proteins, are being tested in solid tumors. Combining CAR T-cell therapy with additional local and systemic therapies may further enhance efficacy.
The excitement generated by unprecedented responses and survival rates in otherwise incurable relapsed B-cell ALL patients in early phase CAR T-cell trials sets a very optimistic stage for solid tumors, which has yet to yield the same level of success [19]. In a first generation CAR T-cell clinical trial for neuroblastoma, CRs were observed in 3 of 19 patients treated [20,21]. All 3 patients who achieved CR exhibited persistence of circulating CAR T cells for 46 weeks. Pancreatic cancer, which is still in early phase CAR T-cell testing, exhibits a number of tumor-specific antigens and, conceptually, is a promising candidate tumor for investigating CAR T-cell therapy. Herein, we summarize the opportunities, challenges, and current state of knowledge in using CAR T-cell therapy to target pancreatic cancer.
CLINICAL TRIALS
Multiple early phase CAR T-cell clinical trials for solid tumors, which include pancreatic cancer patients, are currently active across the United States and internationally. Target antigens in these trials include mesothelin (MSLN), prostate stem cell antigen (PSCA), carcinoembryonic antigen (CEA), HER2, MUC1, and CD133. These trials differ in preconditioning regimens, concurrent drug delivery, route of administration, type of CAR, and dose (Table 1). All of the current trials utilize second or third generation CARs, as T cells transduced with either of these CARs persist longer than first generation CAR transduced T cells. In addition to delivering the CAR-transduced T cells, some trials incorporate co-delivery of additional drugs or molecules, such as IL-2 and rimiducid, to induce costimulatory domain expression or SIR Y-90 microspheres in patients with liver metastases (Table 1). Many of the available CARs utilize murine scFv elements, which have been shown to be effective in patients with ALL [18], but are potentially immunogenic and can lead to induction of neutralizing antibodies [22]. Additionally, cardiac arrest from an anaphylactic reaction to the murine-based CAR has been reported [23]. Aiming to increase persistence and reduce the potential for side effects from the mouse scFv-based CAR, investigators have been co-administering the CD19-targeting CAR to concomitantly eliminate the B cells that express CD19 and produce anti-mouse antibodies that are responsible for CAR T-cell elimination and side effects observed in prior patients. At our institution, we currently have ongoing human MSLN-targeted CAR T-cell clinical trials for patients with metastatic breast cancer, lung cancer, and pleural mesothelioma; we are expanding the trial to include pancreatic cancer patients.
Table 1.
CAR T-cell Clinical Trials for Pancreatic Cancer
| Target | Institution | Preconditioning | Costim domain | Human/Mouse scFv | Population | Estimated completion | Route | Outcomes | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Mesothelin | NCI | Cy/Flu | CD28 | Murine | PDAC, ovarian, and mesothelioma | 2019 | IV | N/A | NCT01583686 |
| Mesothelin | MSKCC | Cy | CD28 | Human | PDAC, breast, mesothelioma | 2018 | IV | N/A | NCT02792114 Pending |
| Mesothelin | UPenn | None | 4-1BB | Murine | Chemo-refractory metastatic PDAC | 2015 | IV | 1 PDAC patients reported; no off target toxicity; transient PET response and temporary reduction in ascites tumor burden. | NCT01897415 |
| Mesothelin | UPenn | With/without Cy | 4-1BB | Murine | PDAC failed at least one treatment | 2017 | IV | N/A | NCT02159716 |
| Mesothelin, CD19 | UPenn, UCSF | Cy | 4-1BB | Murine Mesothelin with Humanized CD19 | PDAC | 2018 | IV | N/A | NCT02465983 |
| Mesothelin | Renji Hospital, China | Cy | 4-1BB | N/A | PDAC, non-surgical candidate failed at least one treatment | 2018 | Trans-cathether arterially infused | N/A | NCT02706782 |
| Mesothelin | Shanghai Cancer Hospital, China | N/A | N/A | N/A | PDAC spread to liver, non-surgical candidate, failed at least one chemo | 2018 | Intratumoral injection or vascular IR delivery | N/A | NCT01897415 |
| Mesothelin | Chinese PLA General Hospita, China | N/A | N/A | N/A | Relapsed or refractory mesothelin positive cancer | 2018 | IV | MTD | NCT02580747 |
| PSCA | Baylor Sammons Cancer Center | N/A | AP1903 inducible MyD88/CD40 | N/A | Non-resectable PDAC | 2020 | IV | N/A | NCT02744287 |
| CEA | Roger Williams Medical Center, RI | N/A | CD28 | N/A | CEA expressing carcinomas with liver mets | 2017 | Hepatic artery | N/A | NCT02850536, NCT02416466 |
| CEA | Southwest Hospital, China | N/A | N/A | N/A | Relapsed or refractory CEA positive PDAC | 2019 | IV | N/A | NCT02349724 |
| Muc1 | Hefei Binhu Hospital, China | Cy/Flu | CD28/4-1BB | Murine | PDAC without curative options; non-R1 resected PDAC | 2018 | N/A | N/A | NCT02587689 |
| CD133 | Chinese PLA General Hospita, China | Nab-Paclitaxel and Cy | 4-1BB | Murine | Refractory or relapsed CD133 positive cancer | 2018 | N/A | MTD | NCT02541370 |
| HER2 | Southwest Hospital, China | N/A | N/A | N/A | Relapsed or refractory HER2 positive cancer | 2019 | IV | MTD | NCT02713984 |
Definitions: CEA, carcinoembryonic antigen; PSCA, prostate stem cell antigen; Muc1, mucin 1; HER2, human epidermal growth factor receptor 2; MTD, maximum tolerated dose; Cy, cyclophosphamide; Flu, fludarabine
In a MSLN-targeted CAR T-cell clinical trial at University of Pennsylvania, a patient with pancreatic cancer had stable disease after completing 3 weeks of intravenous CAR T-cell therapy. By FDG PET/CT imaging, a decrease in the maximum standardized uptake value (SUVmax) was seen transiently in all sites of disease after therapy, but the effect was not sustained on repeat imaging. Analysis of ascitic fluid on days 3 and 15 after start of therapy revealed a 40% decrease in the concentration of tumor cells that coexpressed MSLN and c-Met [23]. Given that the CAR in this trial was expressed transiently by mRNA electroporation of T cells, as a cautionary measure, using a mouse hybridoma-derived scFv that could have been eliminated by an endogenous immune reaction [23], the transient tumor response exhibited here was exciting and encouraging.
PRECONDITIONING
The role of preconditioning prior to CAR T-cell treatment for solid tumors has not been well established, but experience from CAR-treated leukemia patients shows lymphodepletion prior to CAR T-cell therapy increases the expansion, persistence, inflammatory cytokine release, and efficacy of CD19-targeted CARs [24] [25]. TIL-treated melanoma patients experience similar benefits from lymphodepletion [26].
The rationale for lymphodepletion is multifold: 1) it allows infused CAR T cells, which are more differentiated than most of the endogenous circulating lymphocytes, to compete for cytokines and nutrients enough to engraft, expand, and reach the tumor in adequate numbers over adequate time; 2) it helps eliminate some of the protumor immune cells in the microenvironment such as Tregs and myeloid suppressor cells; 3) it may enhance endogenous immune functions such as antigen-presenting cell activation; and 4) it may have meaningful and direct antitumor or cytoreductive effects.
The most common regimen includes cyclophosphamide or cyclophosphamide with fludarabine (Cy/Flu) [27]. The addition of fludarabine to cyclophosphamide improved CAR T-cell expansion and disease-free survival in B-cell ALL [28]. In prostate cancer, a Phase I study using Cy/Flu preconditioning (with IL-2 support) showed good CAR T-cell engraftment (defined as ≥20%) in 3 out of 5 patients [29] and a similar regimen has been used in many pancreatic cancer CAR T-cell clinical trials (Table 1).
While Cy and Flu are the most common preconditioning agents used in CAR T-cell trials, these agents have not been used to treat PDAC. Common PDAC chemotherapeutic agents include 5-FU, gemcitabine (GEM), and combination therapies such as FOLFIRINOX (5-FU, leukcovorin, irinotecan, oxaliplatin) [30]. These chemotherapeutic agents, as well as commonly used radiation regimens, can modify the tumor microenvironment and host immune responses via several underlying mechanisms, such as immunogenic cell death, local T-cell infiltration, and eradication of immunosuppressing Tregs and myeloid derived suppressor cells (MDSCs); this provides a rationale for their use neoadjuvantly in CAR T-cell therapy [31]. The number of CD4+ and CD8+ cells increased significantly after neoadjuvant chemoradiation using gemcitabine and TS-1 in mice and higher CD4 count was shown to be a good prognostic marker [32]. Multimodal chemotherapy using GEM, cyclophosphamide, and taxane decreased Tregs in the PDAC microenvironment [33]. In PDAC patients, neoadjuvant treatment with chemoradiotherapy was associated with statistically lower Treg accumulation in the tumor, while the total number of CD4 and CD8 T cells did not change [34]. The effect of chemotherapeutics on MDSCs is controversial—Zheng et al. [33] showed that GEM and 5-FU have a direct killing effect on MDSCs, however, Takeuchi et al. [35] reported that GEM can increase MDSC numbers through increases in GM-CSF levels. Whether these specific chemotherapies or other treatments, such as radiation therapy, provide additional benefit to CAR T-cell therapy for patients with PDAC remains both intriguing and presently untested.
TARGET ANTIGENS
An ideal target antigen for solid tumor CAR T-cell therapy is overexpressed on tumor cells with limited or no expression on normal cells and is expressed in the majority of patients. Association with aggressiveness and evidence of spontaneous beneficial immune responses against a candidate antigen adds strength in selecting a target antigen for CAR T-cell therapy. Pancreatic cancer preclinical models thus far have focused on various cancer cell surface antigens—MSLN, CEA, MUC1, PSCA, CD24, HER2, and natural killer (NK) receptors.
Mesothelin
Mesothelin is a cell surface antigen [36] that is highly expressed in mesothelioma, ovarian, and pancreatic cancers [37]. In normal tissue, it is expressed at low levels only in the pleura, pericardium, and peritoneum [38]. In cancer cells, MSLN is involved in cell proliferation [39], adhesion [40,41], cell signaling [40], and metastases [42,43]. Mesothelin consists of two different proteins: membrane-bound MSLN and soluble MSLN-related peptide (SMRP) [44]. Soluble MSLN-related peptide cleaved from MSLN-expressing tumors can be measured in serum [45,46]. Production of SMRP could be related to abnormal splicing events leading to synthesis of a secreted protein or to an enzymatic cleavage from membrane-bound MSLN; evidence exists for both mechanisms [47]. For the purpose of CAR targeting, the membrane-bound version of MSLN is most relevant, although serum levels may be useful as a screening and therapy response tumor marker indicative of the presence of MSLN-expressing tumor cells [48]. Mesothelin-targeting immunotoxin SS1P has shown in vivo specificity and antitumor activity [38,49], thus validating MSLN as a target. In a pancreatic cancer vaccine trial [50], patients with survival advantage had strong and consistent CD8+ T-cell response to MSLN epitopes [51]. Specific T-cell epitopes derived from MSLN were shown to activate human T cells to efficiently lyse human tumors expressing MSLN [52]. Therefore, there is strong evidence that adoptive immunotherapy using a MSLN receptor will be tumor-specific and beneficial for pancreatic cancer patients.
Mesothelin is expressed in 80% of pancreatic cancers [53–55] and, among positive tumors, 25–100% of cells express the antigen [56]. Among normal tissue in the abdomen, MSLN is expressed on the common bile duct and mesothelium [56]. While several clinical trials utilize MSLN CARs to target pancreatic cancer, preclinical justification extends primarily from research on other MSLN-expressing solid tumors [57–59]. We have demonstrated that MSLN CAR T cells administered regionally were more effective compared with systemically administered MSLN CAR T cells, even at a reduced dose. This therapeutic benefit has been shown to be CD4 CAR T-cell-mediated [60]. Serum SMRP neither interfered with MSLN CAR T-cell targeting to the tumor nor activated CAR T cells. In our ongoing clinical trial for patients with pleural malignancies, MSLN-targeted CAR T cells are delivered intrapleurally (NCT02414269).
In a preclinical model where T cells engineered to express an affinity-enhanced TCR against MSLN in a genetically engineered model of autochthonous PDAC, engineered T cells preferentially accumulate in PDAC and induce tumor cell death and stromal remodeling. However, TILs become progressively dysfunctional, a limitation successfully overcome by serial T-cell infusions that resulted in a near one-fold increase in survival without overt toxicities [61].
Carcinoembryonic antigen
Carcinoembryonic antigen is a glycoprotein expressed in nearly 75% of pancreatic cancers [62,63]. Expression is also observed in chronic pancreatitis and at lower levels on epithelial cells in the colon and other gastrointestinal organs, often in a luminal distribution [64]. In an immunocompetent transgenic mouse expressing CEA in the intestinal and pulmonary tracts, a single, second generation CAR T-cell injection of 10 million cells produced long-term tumor eradication in 67% of mice bearing established orthotopic pancreatic cancer [65]. Lymphodepletion was neither required for achieving therapeutic efficacy nor did it result in autoimmunity. T-cell targeting to the tumor site occurred even in the presence of soluble CEA at clinically relevant concentrations. The CAR T cells had a central memory phenotype and became activated at the targeted tumor site, however, they converted to an exhausted phenotype after prolonged persistence [65,66]. While pancreatic cancer-specific CAR T-cell trials targeting CEA have not been reported, preliminary results from a Phase I dose escalation trial targeting a range of CEA-positive cancers (breakdown by disease not listed) using CAR T cells with high-dose IL-2 found that 7 out of 13 patients had stable disease 6 weeks after treatment, while 6 patients progressed [67]. On a cautionary note, this trial was stopped at the second dose escalation level of 4 planned patients due to pulmonary toxicity [67,68].
CD24
The finding that not all cancer cells are equally capable of repopulating or growing a tumor and a small population of “cancer stem cells” may be responsible for the vast majority of tumor growth provided a rationale to target these progenitor cells with CARs specifically. CD24 is one cancer stem cell marker, along with CD44 and CD133 [69], that is believed to play a role in pancreatic carcinogenesis [70]. Using a second generation CAR targeting CD24 in an orthotopic human pancreatic cancer xenograft model, Maliar et al. successfully eliminated tumors where CD24+ cells were the minority [66]. In this study, 10 million CAR T cells were injected intratumorally or intravenously on 3 or 4 alternating days, and mice were given intraperitoneal IL-2 injections twice daily for 10 days with 2 Gy of radiation used for preconditioning. Interestingly, although more tumor cells expressed HER2 than CD24, the CD24 CAR was more effective at curing mice than the HER2 CAR and the majority of mice treated with HER2 CAR T-cell therapy that progressed still responded to CD24 CAR therapy [66].
HER2
While expression of the HER2/neu receptor tyrosine kinase on pancreatic cancer is somewhat controversial [71], some studies have found it on 20–60% of PDACs [72,73]. In the same CD24 study described above, a HER2-targeting second generation CAR using the scFv from the trastuzumab antibody was tested against human orthotopic pancreatic cancer in immunodeficient mice. The HER2 CAR dramatically reduced the size of the tumors within one week, even in cases of high tumor burden and multiple metastases [66]. The most effective treatment strategy was sequential treatment with the HER2 CAR, followed by the CD24 CAR 2 months later, leading to 90% survival at 14 weeks [66].
MUC1
MUC1 is a mucin overexpressed in roughly 90% of pancreatic cancers [74] and is glycosylated in a predictable way (O-linked glycosylation) in pancreatic and other cancers [75]. A second generation CAR targeting glycosylated MUC1 on pancreatic cancer was tested in an orthotopic human pancreatic cancer xenograft model. In this test, administration of 500 000 CAR T cells led to 100% survival (6/6 mice) at 16 weeks compared with 40% survival (2/5 mice) among mice treated with untransduced T cells [76], thereby establishing MUC1 as a promising target for pancreatic cancer.
Prostate stem cell antigen
Prostate stem cell antigen is a glycoprotein of unknown function [77] expressed by 60–80% of PDAC tumors [78,79] with low basal expression in prostate epithelium, urinary bladder, kidney, esophagus, stomach, and placenta [80]. A theoretical benefit of targeting PSCA is that it appears to be upregulated early in pancreatic cancer development, even in the premalignant PanIN stage [81]. Prostate stem cell antigen-targeted CARs have been created and tested against pancreatic cancer by 2 groups, both showing efficacy [14,82]. Interestingly, a second-generation CAR containing CD28 induced a more potent antitumor effect than a third generation CAR containing CD28 and 41BB in this human pancreatic cancer mouse model [14].
Natural killer receptors
Natural killer cells interact with their targets via a complex array of inhibitory and activating receptors, and, similar to T cells, are capable of directly killing tumor cells. NKp46 is thought to be the main activating receptor for human NK cells [83], which recognizes heparan sulfate proteoglycans that are aberrantly expressed on transformed cell types, including pancreatic cancer [84]. The NKp46 extracellular signaling domain was used to create a second generation CAR that was effective in vitro against several tumor types, including pancreatic cancer cells [85].
STROMAL TARGETING
Pancreatic cancer is characterized by an intense stromal reaction that is believed to promote tumor growth and act as a barrier to the delivery of effective agents. Therefore, stromal targeting in PDAC has been an important goal for therapy. The stroma includes myofibroblasts, endothelial cells, immune cells, and cytokines within a complex extracellular matrix [86]. Pancreatic stellate cells are located in the periductal and periacinar regions and are critical in stromal biosynthesis [87]. When activated by cytokines secreted by PDAC, stellate cells transform into myofibroblasts that generate ECM proteins [88], which in turn act on the PDAC to promote epithelial–mesenchymal transformation (EMT) and a stem cell phenotype in the cancer cells. This promotes their dissemination, recurrence and resistance to chemotherapy and radiation [89].
Fibroblast activation protein (FAP) is a marker of a major subset of cancer associated stromal cells and depletion of these cells using CAR T cells targeting FAP reduced extracellular matrix proteins, decreased tumor vascular density, and restrained growth of syngeneic murine pancreatic cancers [90]. On a cautionary note, FAP-targeted CARs have been tested in the context of other tumor models where they were also found to recognize multipotent bone marrow stromal cells, thus causing lethal bone toxicity and cachexia [91]. However, novel mechanisms for targeting the stroma in pancreatic cancer remain an active area of investigation. A method for overcoming the physical limitations of the tumor microenvironment is to engineer CAR T cells to overexpress heparanase [92]. This approach was tested in melanoma and neuroblastoma solid tumors, and resulted in increased extracellular matrix degradation, improved T-cell infiltration, and increased antitumor activity [92]. An additional strategy to further enrich CAR T-cell accumulation in solid tumors is to engineer CAR T cells to coexpress chemokine receptors that attract the T cells to tumor sites, as has been demonstrated in neuroblastoma [93], Hodgkin lymphoma [94], and mesothelioma [95].
TOXICITY
The major CAR T-cell toxicities seen in solid tumors differ somewhat from those seen in liquid tumors. The major side effects observed thus far in solid tumor studies have been on-target, off-tumor effects [96,97], or an immune reaction to a murine-based CAR design [98]. This exemplifies the care required when choosing a target antigen. In ALL patients, cytokine release syndrome (CRS) and neurotoxicity are the most common severe side effects. Cytokine release syndrome has been associated with disease burden, CAR T-cell dose, and response to therapy in ALL patients [15,17,99], and is sometimes seen in CAR-treated solid tumor patients. The symptoms of CRS include hypotension, fevers, coagulopathy, respiratory/renal insufficiency, myalgias, and neurological complications. Physiologically, CRS is potentially caused by the release of inflammatory cytokines produced by large numbers of activated CAR T cells [15] and endogenous myeloid cells [100], and C reactive protein levels correlate with the severity of CRS in leukemia [101]. Treatment includes high-dose steroids, vasopressors, ventilatory support, supportive care [15], and potentially anti-IL-6R antibody (tocilizumab), which has been beneficial in some [15,17], but not all CRS patients [16]. The generally reversible, but occasionally severe, neurological toxicity sometimes seen in leukemia-treated patients [15,102], the pathophysiology of which is currently not known, has fortunately not been observed in pancreatic cancer or other CAR T-cell treated solid tumor patients. However, neurotoxicity has been observed in TIL-treated melanoma patients [103], characterized pathologically by multifocal necrotizing leukoencephalopathy and thought to be due to cross-reactivity against intracerebral MAGE-A12 antigen, once again exemplifying the importance of careful target selection.
Concern for toxicity from genetically modified T cells inspired the development of multiple sophisticated suicide gene systems, such as herpes simplex virus thymidine kinase, Escherichia coli nitroreductase, inducible Caspase9, CD20, OmomycER, and EGFRt [104]. The price of including a suicide system is the genetic space it occupies, which is limited in current transduction strategies, and the fact that any non-human gene introduced may be immunogenic and lead to premature elimination of the CAR T cells and/or toxicity [105]. Additional safety strategies include dual targeting where binding of two separate tumor antigen targets is required for full activation of the CAR T-cell [106–108] or where binding to a normal tissue antigen inhibits CAR activation [109]. It is also possible to sterically block the tumor-binding scFv domain of the CAR with a substrate that is cleaved in the presence of matrix metalloproteinases that are enriched within the tumor microenvironment as a way to limit functionality outside of the tumor microenvironment [110]. Additionally, the opposite of a suicide switch has been created and tested in mice where the antigen-binding and intracellular-signaling domains of a receptor are separated into two components that assemble and function only in the presence of a small-molecule dimerizer (i.e., an “on switch”) [111]. While suicide genes are often incorporated as an additional safety measure in current clinical trials, their deployment in clinical practice has not yet been required.
Pancreatic cancer-specific considerations
Many pancreatic cancer-specific characteristics have been cited as possible contributors to the poor response exhibited thus far in immune checkpoint blockade for PDAC patients, which may similarly affect CAR T-cell therapy. PDAC is well known for having a dense fibrous stroma that raises the question of whether this physical barrier excludes T cells [112]. Immunohistochemical analysis of resected normal pancreas and PDAC reveals the number and percentage of T cells infiltrating tumors actually increased relative to normal pancreatic tissue controls [113], thereby suggesting that infiltration is possible and can occur naturally. The observation that patients with higher levels of CD4 and CD8 T-cell infiltration are associated independently with increased survival is further evidence that the infiltrating T cells are functionally active and important [6].
Despite this, greater immune cell infiltration may not equate universally with better response. T cells can become inactive due to intrinsic mechanisms such as by activation without costimulation (leading to anergy) or through expression of inhibitory receptors. Fortunately, second generation CAR T cells are endowed with simultaneous stimulation and costimulation, which overcoming the natural tendency toward anergy. However, expression of inhibitor receptors, such as PD-1 or CTLA-4, is an expected outcome of activation and may limit the ability of CAR T cells in the tumor [114]. PDAC is known to directly express both PD-L1 [115] and CTLA-4 receptors [116]. CTLA-4 is a CD28 receptor family member that increases on conventional T cells after activation. CTLA-4 competes with CD28 to bind with B7, which, in turn, reduces costimulation of the T-cell and suppresses response. Given this mechanism, it is interesting to speculate whether second generation BBz CAR T cells have exhaustive properties that differ from 28z CAR T cells. PD-1 binds to inhibitory ligands, PD-L1 or PD-L2, and is expressed on APCs and cancer cells. PD-1 is also an activation marker and, while its presence often indicates exhaustion, it is also a reassuring indication that the T-cell has been reacting to a target and further immune response may be possible.
T cells may also become deactivated by extrinsic influences, mainly through cytokines produced by Tregs or MDSCs. Increased Treg populations, both within the tumor and peripherally, are associated with decreased tumor grade and survival in PDAC [117]. Both Tregs and myeloid cells are enriched in pancreatic tumors relative to normal tissue [118]. Tregs can arise from a subset of thymocytes that bypass negative selection in the thymus despite self-recognition or from naïve CD4 T cells in the periphery that differentiate in the presence of cytokines such as TGF-β. Tregs suppress CAR T cells, endogenous T cells, and a multitude of other immune cells primarily by secreting inhibitory cytokines such as TGF-β and IL-10. One mechanism by which PDAC accumulates Tregs is by upregulating chemokine receptor type 5 (CCR5), which is involved in Treg homing and activation [119]. Additionally, PDAC can directly secrete TGF-β and, thereby, inhibit T-cell activation while promoting Treg differentiation [120,121].
M2 macrophages, one subset of MDSCs, are associated with increased tumor growth and poorer prognosis [122]. While the mechanism by which M2 macrophages exert strong immunosuppressive influences remains only partially understood, infiltration of MDSCs into the tumor correlates with a lack of CD8+ CTLs that are capable of killing tumor [123]. The presence of M2 macrophages at the tumor periphery is associated with increased metastasis, increased recurrence rate, and decreased survival [124,125], and the level of circulating MDSCs correlates with PDAC disease stage [126]. The strong presence of these immunosuppressive cells and cytokines are challenges to CAR T-cell therapy for PDAC that will need to be overcome.
FUTURE PERSPECTIVES
Despite the aforementioned promising advances in treatment, pancreatic cancer is still a disease that responds poorly to conventional therapy and has had no major improvements in survival over the last several decades. CAR T-cell therapy has thus far been effective against pancreatic cancer in cell culture and mice. In humans, impressive CR rates of 80–100% in relapsed B-cell ALL have generated optimism that CAR T-cell therapy may provide new hope for metastatic pancreatic cancer patients. However, it is clear that solid tumors have additional challenges compared with liquid tumors and the success of CAR T-cell therapy in pancreatic cancer and other solid tumors may require more sophisticated strategies. For example, co-administration of checkpoint blocking antibodies [127] or cell intrinsic PD-1 resistance mechanisms, such as expression of a dominant negative receptor [64,128], may further improve CAR T-cell efficacy in pancreatic cancer (Figure 1).
In leukemia patients, higher tumor bulk appears to be associated with worse response to CAR T-cell therapy; therefore, it is conceivable that ablative approaches, such as radiation, cryoablation, radiofrequency ablation (RFA), and even debulking surgery, may improve CAR T-cell outcomes. Radiation therapy has additional theoretical benefits such as potentiating an immune response to tumors, forming an in situ vaccine, and overcoming tumor microenvironment immunosuppression [129,130]. Similar theoretical benefits have been posited for RFA [131] and it will be interesting to see whether these modalities improve CAR T-cell efficacy against PDAC.
While a higher ratio of TH2 to TH1 subtypes in PDAC has been associated with worse survival [132], the optimal ratio of infused CAR TH2 to TH1 T cells for pancreatic cancer patients is currently unknown. Additionally, factors, such as IL-12 and IL-27, promote TH1 differentiation [133] and CAR T cells that express these cytokines may improve efficacy [134].
Depleting Tregs may become an important aspect of CAR T-cell therapy for PDAC. This can be achieved via preconditioning with traditional agents, such as cyclophosphamide and/or fludarabine, or through novel approaches. CTLA-4 inhibition reduced Treg accumulation in mice with PDAC [135] and it is a rational combinatorial approach with CAR T cells. CD25-targeting has also been used for Treg depletion and similar to how combining cyclophosphamide with an anti-CD25 monoclonal antibody enhanced vaccine efficacy in a PDAC mouse model [136], a comparable benefit may be afforded to CAR T-cell therapy.
Small molecule drugs used traditionally in benign diseases may have new uses with CAR T-cell therapy for cancer. For example, rosiglitazone (a treatment for diabetes) significantly reduced PDAC progression and metastases in mice, and limited early MDSC and intratumoral Treg accumulation [137]; this provided the rationale for combining rosiglitazone with CAR T-cell therapy to treat PDAC. Another intriguing combination with CAR T-cell therapy is metformin (a common diabetes medication), which increases the number of CD8(+) TILs and protects them from apoptosis and exhaustion in mice [138]. Metformin has also been associated with improved survival in PDAC patients [139].
The ability to generate armored CARs that secrete cytokines opens the door to a plethora of opportunities. Given the numerous immunosuppressive mechanisms of TGF-β, it is not surprising that inhibiting TGF-β with a stable siRNA injected IV promotes systemic immune activation in mice and prolongs their survival [140]. Efficacy was dependent on CD8 T cells in this model and provides a strong rationale for combining this therapy with CAR T cells [140]. TNF-a agonism may be another beneficial approach as intrapleural administration of an agonistic anti-glucocorticoid-induced TNF receptor monoclonal antibody suppressed Treg function and inhibited tumor growth in a mouse PDAC model [141].
Activated macrophages present antigens and can promote tumor elimination. Blocking CSF1 can increase macrophage activation in mice [142] and, since macrophages constitute a large portion of PDAC tumors, CAR T cells co-delivered with, or directly expressing, a CSF1 inhibitor may be an effective treatment strategy for PDAC. Similarly, since CCR2 is important in recruiting M2 macrophages to the tumor microenvironment and they are currently being tested in PDAC patients who are not receiving CAR T-cell therapy [143], co-administration or expression of a CCR2 inhibitor with CAR T cells may be even more potent.
Successfully targeting the PDAC stroma may be accomplished in multiple novel ways other than targeting FAP or expressing heparinase, as described above. If additional enzymes capable of ECM degradation, such as hyaluronidase, are expressed by T cells, they may prove efficacious as systemic administration of this enzyme normalizes the high interstitial fluid pressure of tumors in mice, re-expands the microvasculature, and permanently remodels the tumor microenvironment, which then leads to a doubling of overall survival that was achieved with gemcitabine treatment [144]. Secreted protein acidic and rich in cysteine (SPARC) is produced by tumor-associated fibroblasts in PDAC and its overexpression is associated with an adverse prognosis [145]. SPARC has a strong affinity for albumin, which provides a rationale for attaching albumin to agents in order to enhance accumulation within the tumor. Justifying this approach, a Phase III study using an albumin-bound formulation of paclitaxel (nab-paclitaxel), in combination with GEM, in PDAC patients with advanced disease had improved survival compared with GEM alone [3], while the addition of non-albumin-bound paclitaxel was not associated with any benefits. It stands to reason that a similar strategy, such as co-expressing albumin (or its derivatives) on the CAR T-cell surface, may enhance CAR T-cell efficacy in PDAC patients.
Tyrosine kinase inhibitors (TKI) enjoyed a wave of anti-cancer success before immunotherapies emerged and, although both strategies can be very effective, the combination of TKIs with CAR T cells has not been tested. Bruton tyrosine kinase (BTK) is a non-receptor enzyme of the Tec kinase family that is expressed on B cells, myeloid cells, and mast cells. BTK inhibitors promote M1 antitumor macrophage differentiation over M2 macrophages and block the release of IL-8, MPC-1, and TNFα from mast cells, all of which play a role in fibrosis. The BTK inhibitor ibrutinib showed efficacy against pancreatic insulinoma in mice through its immune modulating mechanism [146], thus providing a rationale for combining a BTK inhibitor with PDAC CAR T cells.
Although CAR T-cell therapy has advanced within the last decade, its application as a treatment for PDAC remains in its infancy, albeit with promising potential. Strategies that overcome local immunosuppressive myeloid cells, inhibitory cytokines secreted by the tumor or surrounding host cells, regulatory T cells, hypoxia, nutrient deprivation, stimulatory cytokine depletion, immunosuppressive upregulation, cytotoxic receptor upregulation, and physical barriers of the dense stroma are all currently being implemented by various groups. We are optimistic that these approaches, alone or in combination, will allow CAR T cells to provide the same level of benefit to pancreatic cancer patients as they do for liquid tumor patients.
Synopsis.
Adoptive T-cell therapy, which redirects a patient’s own T cells against a tumor antigen using chimeric antigen receptor (CAR) gene transfer technology, has had remarkable success in hematological tumors within the past several years. Herein, we review the current progress and future perspectives, both clinical and preclinical, of CAR T-cell therapy for pancreatic cancer.
Acknowledgments
Funding Statement: The author’s laboratory is supported by funding from Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. This research was also supported by the National Institutes of Health (P30 CA008748), U.S. Department of Defense (PR101053, LC110202, and BC132124), Memorial Sloan Kettering’s Office of Technology Development, and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center.
We thank Alex Torres of the Memorial Sloan Kettering Thoracic Surgery Service for his editorial assistance.
Footnotes
Disclosure Statement: None of the authors have disclosures.
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 5.Chang JH, Jiang Y, Pillarisetty VG. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine. 2016;95:e5541. doi: 10.1097/MD.0000000000005541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. British journal of cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer discovery. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 12.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abate-Daga D, Lagisetty KH, Tran E, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25:1003–1012. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125:3392–3400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CE, Adusumilli PS. Next frontiers in CAR T-cell therapy. Molecular therapy oncolytics. 2016;3:16028. doi: 10.1038/mto.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 23.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer immunology research. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geyer MB, Brentjens RJ. Review: Current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy. 2016;18:1393–1409. doi: 10.1016/j.jcyt.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junghans RP, Ma Q, Rathore R, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate. 2016;76:1257–1270. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- 30.Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Therapeutic advances in medical oncology. 2015;7:68–84. doi: 10.1177/1758834014564775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchikawa T, Takeuchi S, Nakamura T, et al. Clinical impact of chemotherapy to improve tumor microenvironment of pancreatic cancer. World journal of gastrointestinal oncology. 2016;8:786–792. doi: 10.4251/wjgo.v8.i11.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homma Y, Taniguchi K, Murakami T, et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Annals of surgical oncology. 2014;21:670–676. doi: 10.1245/s10434-013-3390-y. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Dou Y, Duan L, et al. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cellular immunology. 2015;294:54–59. doi: 10.1016/j.cellimm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchikawa T, Hirano S, Tanaka E, et al. Novel aspects of preoperative chemoradiation therapy improving anti-tumor immunity in pancreatic cancer. Cancer science. 2013;104:531–535. doi: 10.1111/cas.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi S, Baghdadi M, Tsuchikawa T, et al. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res. 2015;75:2629–2640. doi: 10.1158/0008-5472.CAN-14-2921. [DOI] [PubMed] [Google Scholar]
- 36.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–2912. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors. Cancer discovery. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bharadwaj U, Li M, Chen C, Yao Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol Cancer Res. 2008;6:1755–1765. doi: 10.1158/1541-7786.MCR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res. 2008;6:186–193. doi: 10.1158/1541-7786.MCR-07-0254. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen SH, Hung WC, Wang P, et al. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Scientific reports. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 44.Robinson BW, Creaney J, Lake R, et al. Soluble mesothelin-related protein--a blood test for mesothelioma. Lung Cancer. 2005;49(Suppl 1):S109–111. doi: 10.1016/j.lungcan.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Robinson BW, Creaney J, Lake R, et al. Soluble mesothelin-related protein--a blood test for mesothelioma. Lung Cancer. 2005;49(Suppl 1):S109–S111. doi: 10.1016/j.lungcan.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Park EK, Sandrini A, Yates DH, et al. Soluble mesothelin-related protein in an asbestos-exposed population: the dust diseases board cohort study. Am J Respir Crit Care Med. 2008;178:832–837. doi: 10.1164/rccm.200802-258OC. [DOI] [PubMed] [Google Scholar]
- 47.Sapede C, Gauvrit A, Barbieux I, et al. Aberrant splicing and protease involvement in mesothelin release from epithelioid mesothelioma cells. Cancer science. 2008;99:590–594. doi: 10.1111/j.1349-7006.2007.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolkova P, Nakladalova M, Zapletalova J, et al. Validity of mesothelin in occupational medicine practice. International journal of occupational medicine and environmental health. 2016;29:395–404. doi: 10.13075/ijomeh.1896.00637. [DOI] [PubMed] [Google Scholar]
- 49.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 50.Lutz E, Yeo CJ, Lillemoe KD, et al. A Lethally Irradiated Allogeneic Granulocyte-Macrophage Colony Stimulating Factor-Secreting Tumor Vaccine for Pancreatic Adenocarcinoma: A Phase II Trial of Safety, Efficacy, and Immune Activation. Ann Surg. 2011 doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. JExpMed. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Einama T, Kamachi H, Nishihara H, et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1276–1282. doi: 10.1097/MPA.0b013e318221bed8. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu A, Hirono S, Tani M, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer science. 2012;103:739–746. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter JM, Tang LH, Klimstra DS, et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PloS one. 2012;7:e40157. doi: 10.1371/journal.pone.0040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 57.Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18:2478–2489. doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kachala SS, Bograd AJ, Villena-Vargas J, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20:1020–1028. doi: 10.1158/1078-0432.CCR-13-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tozbikian G, Brogi E, Kadota K, et al. Mesothelin expression in triple negative breast carcinomas correlates significantly with basal-like phenotype, distant metastases and decreased survival. PloS one. 2014;9:e114900. doi: 10.1371/journal.pone.0114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science translational medicine. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stromnes IM, Schmitt TM, Hulbert A, et al. T Cells Engineered against a Native Antigen Can Surmount Immunologic and Physical Barriers to Treat Pancreatic Ductal Adenocarcinoma. Cancer cell. 2015;28:638–652. doi: 10.1016/j.ccell.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albers GH, Fleuren G, Escribano MJ, Nap M. Immunohistochemistry of CEA in the human pancreas during development, in the adult, chronic pancreatitis, and pancreatic adenocarcinoma. Am J Clin Pathol. 1988;90:17–22. doi: 10.1093/ajcp/90.1.17. [DOI] [PubMed] [Google Scholar]
- 63.Allum WH, Stokes HJ, Macdonald F, Fielding JW. Demonstration of carcinoembryonic antigen (CEA) expression in normal, chronically inflamed, and malignant pancreatic tissue by immunohistochemistry. J Clin Pathol. 1986;39:610–614. doi: 10.1136/jcp.39.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nap M, Mollgard K, Burtin P, Fleuren GJ. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 1988;9:145–153. doi: 10.1159/000217555. [DOI] [PubMed] [Google Scholar]
- 65.Chmielewski M, Hahn O, Rappl G, et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology. 2012;143:1095–1107. e1092. doi: 10.1053/j.gastro.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 66.Maliar A, Servais C, Waks T, et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology. 2012;143:1375–1384. e1371–1375. doi: 10.1053/j.gastro.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Thistlethwaite F. A CRUK phase I trial of adoptive transfer of autologous tumour antigen-specific T cells with pre-conditioning chemotherapy and intravenous IL2 in patients with advanced CEA positive tumours. NCRI Poster Session. 2010 [Google Scholar]
- 68.Hawkins R. A trial looking at MFEz T cells, chemotherapy and IL-2 (interleukin 2) for cancers that test positive for carcinoembryonic antigen (CEA) (PH1/105) Cancer Research UK Clinical Trial Listings. 2010 [Google Scholar]
- 69.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 70.Sagiv E, Kazanov D, Arber N. CD24 plays an important role in the carcinogenesis process of the pancreas. Biomed Pharmacother. 2006;60:280–284. doi: 10.1016/j.biopha.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 71.te Velde EA, Franke AC, van Hillegersberg R, et al. HER-family gene amplification and expression in resected pancreatic cancer. Eur J Surg Oncol. 2009;35:1098–1104. doi: 10.1016/j.ejso.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Safran H, Steinhoff M, Mangray S, et al. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol. 2001;24:496–499. doi: 10.1097/00000421-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Komoto M, Nakata B, Amano R, et al. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer science. 2009;100:1243–1247. doi: 10.1111/j.1349-7006.2009.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qu CF, Li Y, Song YJ, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. British journal of cancer. 2004;91:2086–2093. doi: 10.1038/sj.bjc.6602232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham RA, Burchell JM, Taylor-Papadimitriou J. The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother. 1996;42:71–80. doi: 10.1007/s002620050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Posey AD, Jr, Schwab RD, Boesteanu AC, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16:3533–3538. doi: 10.1158/1078-0432.CCR-09-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 79.Wente MN, Jain A, Kono E, et al. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119–125. doi: 10.1097/01.mpa.0000173459.81193.4d. [DOI] [PubMed] [Google Scholar]
- 80.Gu Z, Thomas G, Yamashiro J, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 81.Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224–232. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katari UL, Keirnan JM, Worth AC, et al. Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 2011;13:643–650. doi: 10.1111/j.1477-2574.2011.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 84.Bloushtain N, Qimron U, Bar-Ilan A, et al. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173:2392–2401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 85.Tal Y, Yaakobi S, Horovitz-Fried M, et al. An NCR1-based chimeric receptor endows T-cells with multiple anti-tumor specificities. Oncotarget. 2014;5:10949–10958. doi: 10.18632/oncotarget.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Javle M, Golan T, Maitra A. Changing the course of pancreatic cancer--Focus on recent translational advances. Cancer treatment reviews. 2016;44:17–25. doi: 10.1016/j.ctrv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Current opinion in gastroenterology. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krantz SB, Shields MA, Dangi-Garimella S, et al. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-beta signaling. Mol Cancer Res. 2011;9:1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochemical and biophysical research communications. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 90.Lo A, Wang LC, Scholler J, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tran E, Chinnasamy D, Yu Z, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Craddock JA, Lu A, Bear A, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer immunology research. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Stegen SJ, Davies DM, Wilkie S, et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: identifying a window of therapeutic opportunity? J Immunol. 2013;191:4589–4598. doi: 10.4049/jimmunol.1301523. [DOI] [PubMed] [Google Scholar]
- 101.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Molecular therapy oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minagawa K, Zhou X, Mineishi S, Di Stasi A. Seatbelts in CAR therapy: How Safe Are CARS? Pharmaceuticals (Basel) 2015;8:230–249. doi: 10.3390/ph8020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riddell SR, Elliott M, Lewinsohn DA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 106.Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lanitis E, Poussin M, Klattenhoff AW, et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer immunology research. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilkie S, van Schalkwyk MC, Hobbs S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 109.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Science translational medicine. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desnoyers LR, Vasiljeva O, Richardson JH, et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Science translational medicine. 2013;5:207ra144. doi: 10.1126/scitranslmed.3006682. [DOI] [PubMed] [Google Scholar]
- 111.Wu CY, Roybal KT, Puchner EM, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seo YD, Pillarisetty VG. T-cell programming in pancreatic adenocarcinoma: a review. Cancer gene therapy. 2016 doi: 10.1038/cgt.2016.66. [DOI] [PubMed] [Google Scholar]
- 113.Emmrich J, Weber I, Nausch M, et al. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion. 1998;59:192–198. doi: 10.1159/000007488. [DOI] [PubMed] [Google Scholar]
- 114.Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 116.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 118.Shibuya KC, Goel VK, Xiong W, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PloS one. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trapani JA. The dual adverse effects of TGF-beta secretion on tumor progression. Cancer cell. 2005;8:349–350. doi: 10.1016/j.ccr.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 121.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 122.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 123.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 124.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. The Journal of surgical research. 2011;167:e211–219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 125.Yoshikawa K, Mitsunaga S, Kinoshita T, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer science. 2012;103:2012–2020. doi: 10.1111/j.1349-7006.2012.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 128.Chen N, Morello A, Tano Z, et al. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2017 doi: 10.1080/2162402X.2016.1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vatner RE, Cooper BT, Vanpouille-Box C, et al. Combinations of immunotherapy and radiation in cancer therapy. Frontiers in oncology. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gameiro SR, Higgins JP, Dreher MR, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PloS one. 2013;8:e70417. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tassi E, Braga M, Longhi R, et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PloS one. 2009;4:e7234. doi: 10.1371/journal.pone.0007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochemical Society transactions. 2016;44:412–418. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sandin LC, Eriksson F, Ellmark P, et al. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology. 2014;3:e27614. doi: 10.4161/onci.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clinical and translational science. 2008;1:228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bunt SK, Mohr AM, Bailey JM, et al. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62:225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amin S, Mhango G, Lin J, et al. Metformin Improves Survival in Patients with Pancreatic Ductal Adenocarcinoma and Pre-Existing Diabetes: A Propensity Score Analysis. The American journal of gastroenterology. 2016;111:1350–1357. doi: 10.1038/ajg.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ellermeier J, Wei J, Duewell P, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 2013;73:1709–1720. doi: 10.1158/0008-5472.CAN-11-3850. [DOI] [PubMed] [Google Scholar]
- 141.Aida K, Miyakawa R, Suzuki K, et al. Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-alpha gene therapy for pancreatic cancer. Cancer science. 2014;105:159–167. doi: 10.1111/cas.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. The Lancet Oncology. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Neuzillet C, Tijeras-Raballand A, Cros J, et al. Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer metastasis reviews. 2013;32:585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- 146.Soucek L, Buggy JJ, Kortlever R, et al. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia. 2011;13:1093–1100. doi: 10.1593/neo.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]