Abstract
Background
Aspirin desensitization followed by daily aspirin provides therapeutic benefits to patients with aspirin exacerbated respiratory disease (AERD). It is not well understood how eicosanoid levels change during aspirin treatment.
Objective
To investigate associations between clinical outcomes of aspirin treatment and plasma eicosanoid levels in AERD patients.
Methods
Thirty-nine AERD patients were offered aspirin treatment (650 mg twice daily) for four weeks. Respiratory parameters and plasma levels of multiple eicosanoids were recorded at baseline and after four weeks of aspirin therapy using the Asthma Control Test (ACT) and Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). Respiratory function was evaluated using the forced expiratory volume in 1 second (FEV1) and nasal peak flow (NPF).
Results
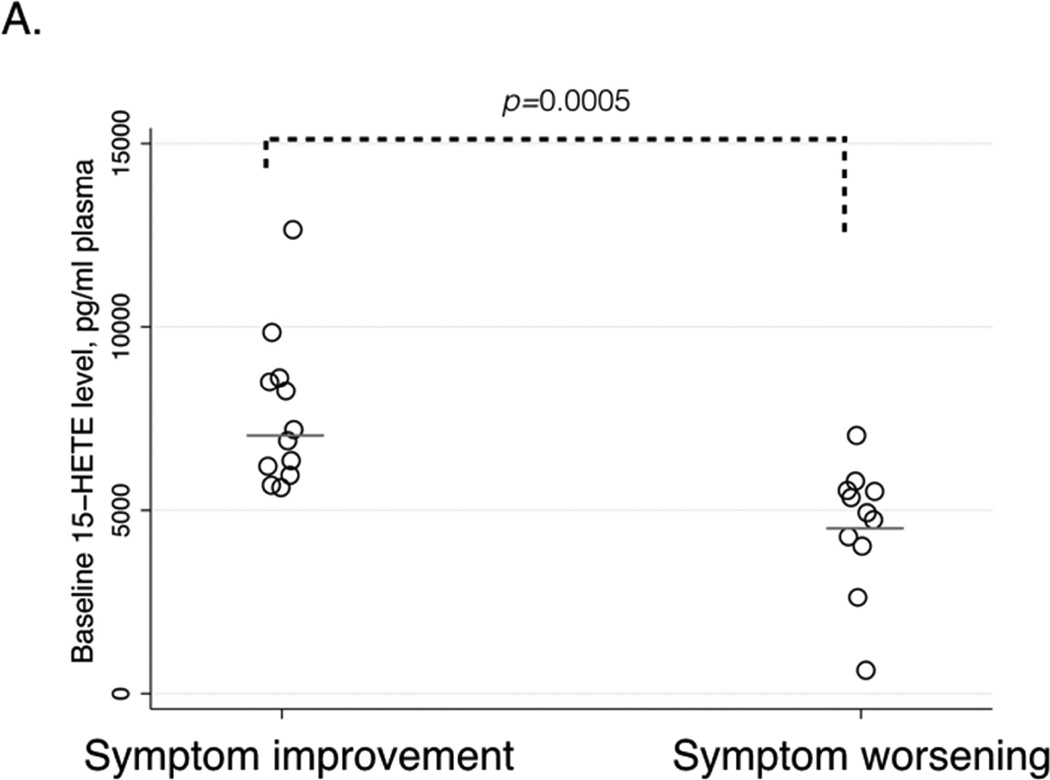
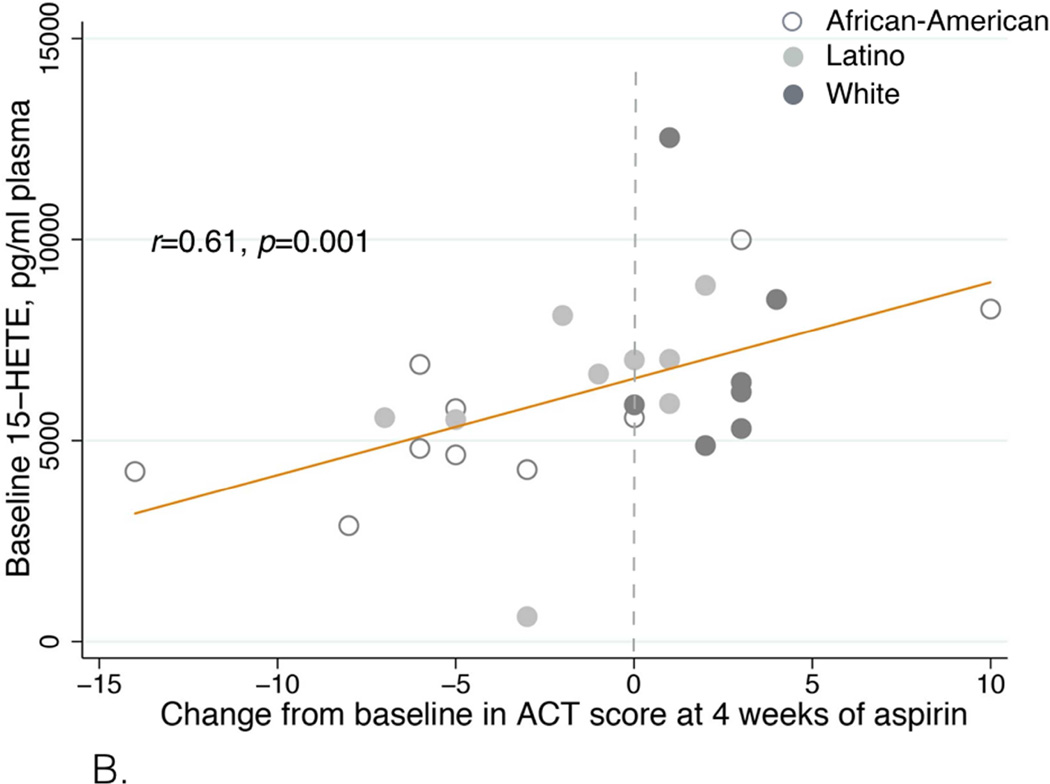
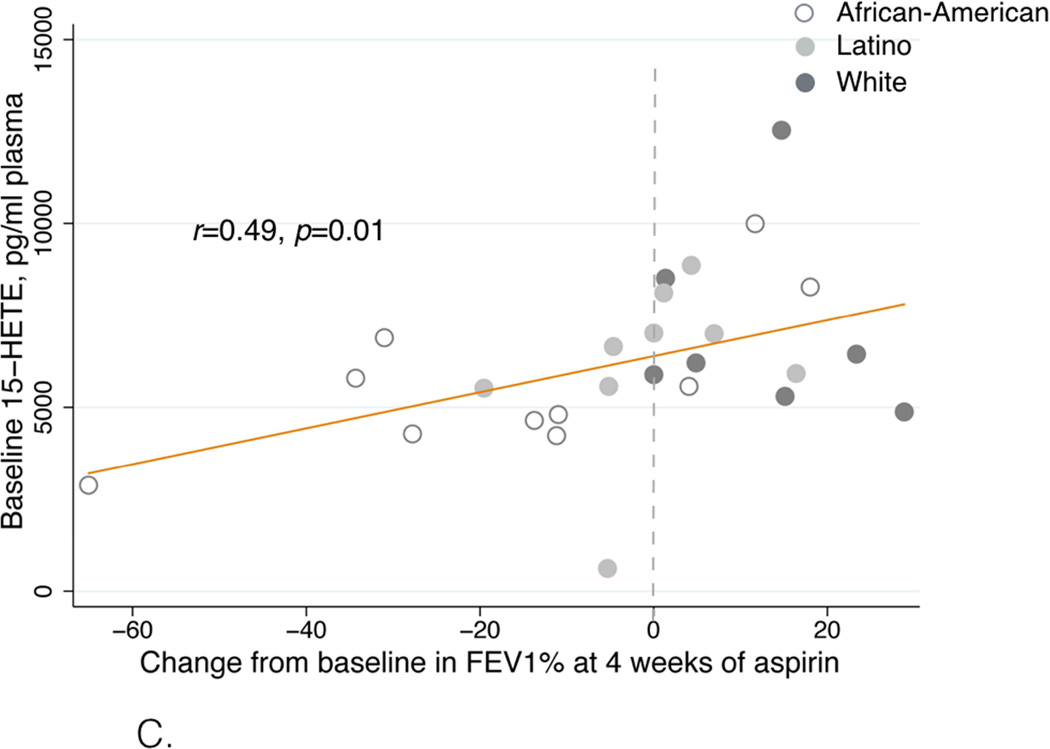
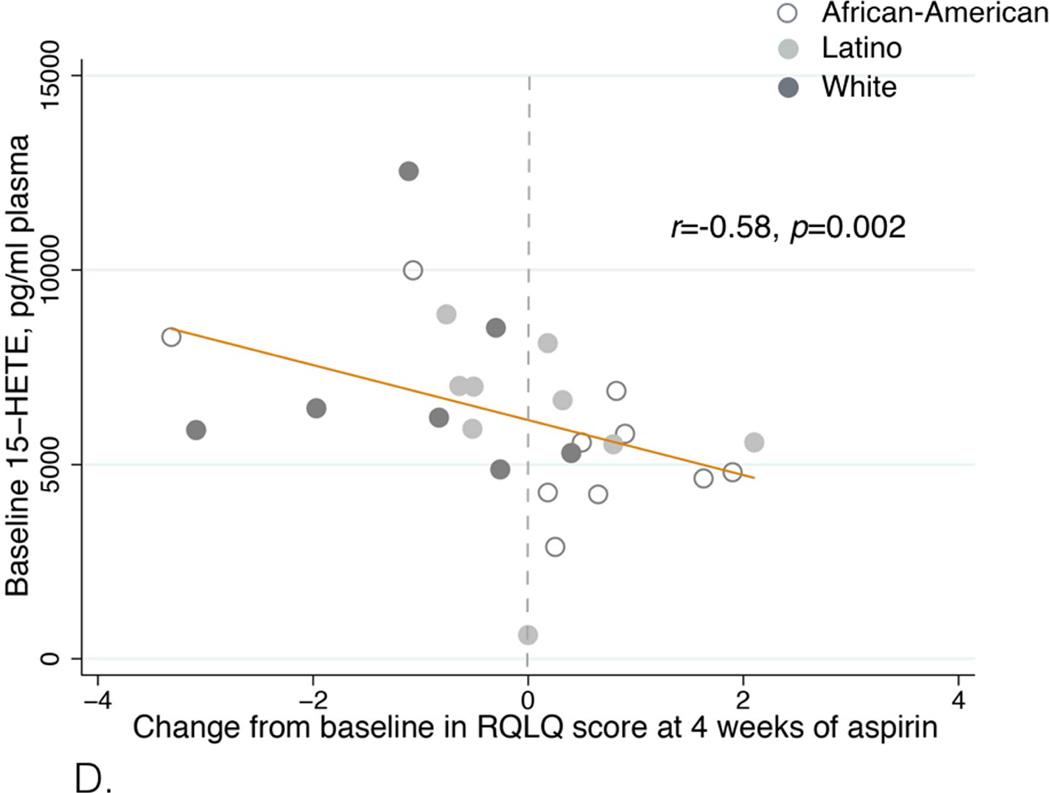
After aspirin treatment, respiratory symptoms improved in sixteen patients, worsened in twelve patients, and did not change in four. Seven patients were unable to complete the desensitization protocol. Patients with symptom improvement had higher baseline plasma 15-hydroxyeicosatetraenoic acid (15-HETE) levels than patients with symptom worsening: 7006 (IQR 6056-8688) vs. 4800 (IQR 4238-5575) pg/ml, p=0.0005. Baseline 15-HETE plasma levels positively correlated with the change in ACT score (r=0.61, p=0.001) and in FEV1 after four weeks of aspirin treatment (r=0.49, p=0.01). It inversely correlated with RQLQ score (r=−0.58, p=0.002). African-American and Latino patients were more likely to have symptom worsening on aspirin or fail to complete the initial desensitization than White, Non-Latino patients (p=0.02).
Conclusions
In AERD patients, low baseline 15-HETE plasma levels and African-American or Latino ethnicity are associated with worsening of respiratory symptoms during aspirin treatment.
Keywords: aspirin-exacerbated respiratory disease, 15-hydroxyeicosatetraenoic acid, eicosanoids, eosinophils, aspirin desensitization
Introduction
Aspirin-exacerbated respiratory disease (AERD) affects 7% of adult asthma patients.1 AERD appears in adulthood, manifesting as asthma, nasal polyps, and hypersensitivity reactions, such as acute bronchospasm, rhinorrhea, and conjunctivitis, to aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs).2,3 Aspirin desensitization has been shown to be an effective treatment for many AERD patients.4–8 Aspirin desensitization and long-term aspirin therapy have been reported to improve asthma control, and reduce emergency room visits and hospitalizations for asthma exacerbations, decrease oral steroid use, and reduce nasal polyp recurrence.6–10
Changes in several biomarkers in plasma, urine, or sputum have been evaluated during aspirin administration in AERD patients. Peripheral blood and tissue eosinophilia is a prominent feature of AERD, and alterations in eosinophil counts were observed during aspirin challenges and after several weeks of aspirin treatment.11–13 After long-term aspirin treatment in AERD patients, respiratory symptoms improved while peripheral eosinophil counts either increased12 or did not change.8 After several weeks of high-dose aspirin therapy, there was no change in urinary leukotriene E4 (LTE4) or 9α,11β-prostaglandin F2 levels,8,12 while serum levels of interleukin (IL) 4 and sputum levels of matrix metalloproteinase-9 both decreased.13 Urinary thromboxane, prostacyclin, prostaglandin D metabolite (PGDM), and prostaglandin E metabolite (PGEM) levels decreased after eight weeks of successful aspirin treatment.12
Eosinophilia in AERD and in other forms of asthma has been linked to levels of one eicosanoid, 15-hydroxyeicosatetraenoic acids (15-HETE).14,15 15-HETE is derived from arachidonic acid via the 15-lipoxygenase (15-LO) pathway and eosinophils have a high capacity to produce this eicosanoid.16 In asthma patients, most of the 15-HETE comes from eosinophils although it is also produced by bronchial epithelium.14 In patients with severe eosinophilic asthma compared to patients with severe asthma without airway eosinophilia, higher levels of 15-HETE were observed in bronchoalveolar lavage fluid (BALF) and were associated with airway fibrosis.15 On the other hand, inhalation of external 15-HETE results in reduced bronchial responsiveness to methacholine.17 In addition, 15-HETE production increased in peripheral blood lymphocytes, eosinophils, and in cells from nasal polyp tissue in response to stimulation with aspirin in aspirin-sensitive and in aspirin-tolerant severe asthma patients.18–21 Although 15-HETE is associated with eosinophilic inflammation in asthmatic and AERD patients, it is unknown whether 15-HETE is affected by aspirin desensitization and whether its levels change during aspirin treatment in AERD. Changes in the levels of some other HETE’s after aspirin have been studied. For instance, 5-HETE levels do not change, while 12-HETE levels increase after aspirin administration in both AERD patients and healthy volunteers.22 Other HETE metabolites, including 8-HETE, 19-HETE, and 20-HETE, and other eicosanoids have not been studied in AERD, specifically in relation to the outcomes of aspirin treatment.
In addition to arachidonic acid metabolites, derivatives from linoleic acid play an important role in asthma. For example, linoleate 13-hydroxyoctadecadineoic acids (13-HODE) is formed from linoleic acid through lipoxygenation by 15-LO, like 15-HETE.23 13-HODE has been shown to induce airway epithelial apoptosis and is associated with features of severe airway obstruction, lung remodeling, and increase in epithelial stress-related proinflammatory cytokines.24 The influence of 13-HODE on AERD treatment outcomes has not been studied.”
We investigated associations between clinical outcomes of aspirin desensitization, peripheral blood eosinophilia, and circulating levels of several oxylipins, in an ethnically diverse group of AERD patients from the Bronx, NY.
Methods
Patients
Patients with suspected AERD aged 18 years or older were recruited into the study. All patients signed an informed consent at the time of enrollment, which was approved by the Institutional Review Board at Albert Einstein College of Medicine.
Study protocol
All patients underwent a standard oral graded aspirin challenge to diagnose aspirin sensitivity using a previously described protocol.25 All patients were requested to take 10 mg of montelukast daily starting one week prior to the challenge to enhance safety of aspirin challenges.26 None of the patients were taking 5-lipoxygenase inhibitors during the study. All patients continued to take their prescribed inhaled corticosteroids (ICS) with or without long-acting beta-agonists (LABA) at the time of the study. On the mornings of the study visits, ICSs and both LABA and short-acting beta-agonists were withheld. All visit were conducted starting 7:30 am. Patients were asked not to have breakfast or drinks past midnight and were given a meal after aspirin challenge at the study site.
For the graded challenge, 40 mg of aspirin (dissolved Original Alka-Seltzer®) was initially administered and then the dose was doubled every 90 minutes (80, 160, and 325 mg).25 The diagnosis of AERD was confirmed by the presence of a positive aspirin challenge response, defined as a hypersensitivity reaction with at least one of the following: a) ≥20% decline in FEV1; b) decline in FEV1 of < 20% combined with naso-ocular reactions; c) isolated naso-ocular reactions. Patients who experienced hypersensitivity reactions were treated based on their specific symptoms. After symptoms resolved, the provoking aspirin dose was repeated and the dose escalation continued until the 325 mg aspirin dose was reached. Patients were then placed on 650 mg of aspirin twice daily.
Baseline data included spirometry and nasal inspiratory peak flow (NPF). Spirometry was performed according to the Puritan Bennett Renaissance® II spirometer guidelines (Pleasanton, CA). NPF was measured following manufacturer guidelines with an In-Check™ Nasal inspiratory flow device (Clement International, Ltd., Essex, UK). The best of three efforts was recorded for spirometry and NPF. Scores from the Asthma Control Test (ACT) and Rhino-Conjunctivitis Quality of Life Questionnaire (RQLQ) were recorded for each patient. Blood was collected at baseline for measurement of the complete blood count (CBC) and eosinophil count (Sysmex XN-Series Automated Hematology Analyzer, Lincolnshire, IL).
Patients were reevaluated after four weeks of aspirin treatment. Each of the parameters described above was recorded at the follow-up visit. All follow-up visits were conducted at the same time of the day and in the manner described for the baseline visit. Having patients take all their medications including 650 mg of aspirin at the study site during the follow-up visit insured compliance with aspirin. While all patient had a hypersensitivity reaction at the baseline visit, none of the patients had a reaction to 650 mg of aspirin at the follow up visit after four weeks.
Plasma eicosanoid measurements
Blood samples were collected into EDTA-containing collection tubes. Plasma was separated and frozen at –80°C. 250 µl plasma was mixed with 250 uL of 0.1% acetic acid in 5% methanol and 3ng of each internal standard (d4-PGE2, 10,11-epoxyhepoxylin, and 10,11-dihydroxynonadecanoic acid). Samples were randomized; eicosanoids were extracted by blinded technicians.
Eicosanoids were isolated by liquid:liquid extraction as previously described.27,28 Online liquid chromatography of extracted samples was performed with an Agilent 1200 Series capillary HPLC (Agilent Technologies, Santa Clara, CA, USA). 28 Concentrations of eicosanoids were measured and are reported as picograms per milliliter (pg/mL). The following eicosanoid levels were possess potent leukotriene B4 (LTB4); thromboxane (TXB) metabolite – 11-dehydro-TXB2; prostaglandin D2 (PGD2); prostaglandin E2 (PGE2), prostacyclin metabolite – 6-keto-PGF1α; several HETE metabolites – 5-HETE, 8-HETE, 11-HETE, 12-HETE, 15-HETE, 19-HETE, and 20-HETE; trihydroxyoctadecamonoenoic acid (THOME) metabolites – 9,10,13-THOME, 9,12,13-THOME; dihydroxyoctadecenoic acid metabolites (DHOME) – 9,10-DHOME, 12,13-DHOME; epoxyeicosatrienoic acid metabolites (EET) – 11,12-EET, 14–15-EET; dihydroxyeicosatrienoic acid metabolites (DHET) – 5,6-DHET, 8,9-DHET, 11,12-DHET, 14,15-DHET, 17,18-DHET; hydroxyoctadecadienoic acid metabolites (HODE) – 9-HODE, 13-HODE; epoxyoctadecenoic acid metabolites (EpOME) – 9,10-EpOME, 12,13-EpOME.
Statistical Analysis
After four weeks of aspirin therapy, change in symptoms was diagnosed in patients who reported either an ACT score change of at least 3 points29 or an RQLQ score change of at least 0.5 points.30 If the change in both questionnaire scores was less the symptoms were described as unchanged.
Baseline characteristics were compared between four groups of AERD patients: those who were unable to complete the desensitization protocol, patients with symptom improvement, patients with symptom worsening, and patients with no change in symptoms after four weeks of aspirin treatment. One-way ANOVA or Kruskal-Wallis rank test (if data were non-normally distributed) were used for analysis. For comparison of outcomes between patients with symptom improvement and patients with symptom worsening a t-test or Mann Whitney test (if data were non-normally distributed) were used. All summary statistics were expressed as means ± standard error of the means (SEM) or as medians and interquartile ranges (IQR). Paired data were analyzed by using paired t-test or Wilcoxon signed-rank test, as appropriate. Categorical data were analyzed by chi-square or Fisher’s exact tests, as appropriate. Pearson or Spearman rank correlation analyses were used, as appropriate. Since multiple comparisons of plasma eicosanoid levels were performed, Bonferroni correction was applied to the eicosanoid analyses. Associations between eicosanoids that were significant on bivariate association analysis (15-HETE) and success of aspirin desensitization were analyzed in a logistic regression model. The model was adjusted for potential confounders (age, gender, and race/ethnicity). Due to a small sample size we did not assess for interactions. All statistical analyses were performed with STATA 14.0 software (StataCorp, College Station, TX). P-values of <0.002 were considered significant for eicosanoid analyses and <0.05 for all other analyses.
Results
Thirty-nine AERD patients were recruited into the study. All patients underwent aspirin challenges. Seven patients were unable to complete the aspirin desensitization protocol due to persistent gastrointestinal or respiratory symptoms. Patients who completed the aspirin desensitization protocol continued taking aspirin 650 mg orally twice daily and were scheduled for a follow-up visit four weeks later.
Clinical responses to aspirin desensitization after four weeks
After four weeks of daily aspirin treatment, patients fell into four distinct categories of patient-reported clinical outcomes: 1) Improvement in respiratory (asthma and/or nasal) symptoms; 2) worsening of respiratory symptoms; 3) no change in symptoms, or 4) inability to complete the initial aspirin desensitization protocol due to either continuous symptoms of nausea and vomiting or prolonged bronchospasm, or both.
Patient demographics and characteristics categorized by these aspirin treatment outcomes are presented in Table I. There was no difference in treatment outcomes by age or gender, but there was a significant difference in responses among different ethnic groups. Among patients who reported worsening of respiratory symptoms at four weeks, 75% were African-Americans and 25% were Latinos; among those who failed to complete the initial desensitization, 85.7% were African-Americans and 14.3% were Latinos. In contrast, none of the white, non-Latino patients either worsened or failed to complete the initial desensitization (p=0.02 for outcomes by race/ethnicity). In addition, Lund-Mackay sinus scores were significantly higher in patients who could not complete the desensitization protocol (p=0.02), suggesting that these patients had more severe sinus disease.
Table 1.
Baseline participants’ characteristics by aspirin treatment outcomes at four weeks
| All participants (N=39) |
Baseline values by aspirin desensitization outcomes* | |||||
|---|---|---|---|---|---|---|
| Improvement in symptoms (N=16) |
No change in symptoms (N=4) |
Worsening of symptoms (N=12) |
Desensitization not completed (N=7) |
P-value for comparison between four groups |
||
| Age, years (±SEM) | 41.7 (±1.7) | 42.3 (±2.8) | 43.5 (±2.5) | 42.2 (±3.3) | 48.3 (±4.7) | 0.8 |
| Women, N (%) | 26 (66.7) | 10 (62.5) | 3 (75.0) | 9 (75.0) | 4 (57.14) | 0.9 |
| Race, N (%) | 0.02 | |||||
| African-American | 20 (51.3) | 4 (25.0) | 1 (25.0) | 9 (75.0) | 6 (85.7) | |
| Latino | 12 (30.7) | 6 (37.5) | 2 (50.0) | 3 (25.0) | 1 (14.3) | |
| White, non-Latino | 7 (18.0) | 6 (37.5) | 1 (25.0) | 0 | 0 | |
| Asthma duration, years (IQR) | 9.0 (3–19) | 13.0 (5–19.5) | 5.0 (3–7.5) | 5.0 (3–23) | 7 (3–22) | 0.4 |
| Rhinitis duration, years (IQR) | 7.0 (4–13) | 8.5 (4.5–17) | 7.5 (4.5–10) | 10.0 (3–17) | 5.0 (2–12) | 0.6 |
| Nasal polyp duration, years (IQR) | 5.0 (2–7) | 5.5 (3–12.5) | 7.0 (4.5–8.5) | 3.5 (1–7) | 2.0 (1–5) | 0.2 |
|
Aspirin hypersensitivity duration years (IQR) |
5.0 (1–11) | 4.5 (2–18) | 3.0 (1–5.5) | 5.0 (1–10) | 6.0 (1–11) | 0.7 |
| History of ESS, N (%) | 33 (84.6) | 15 (94) | 4 (100) | 8 (67) | 6 (86) | 0.3 |
|
Time since last ESS before desensitization, months (IQR) |
32.0 (9–72) | 32.0 (6–72) | 78 (37–90) | 19.5 (9.5–66) | 36.0 (20–72) | 0.9 |
| Use of high-dose ICS, N (%) | 29 (74.4) | 11 (69) | 2 (50) | 10 (83.3) | 6 (86) | 0.5 |
| Use of omalizumab, % | 7 (18) | 3 (19) | 0 | 2 (17.0) | 2 (29.0) | 0.8 |
| Chronic use of oral prednisone, N (%) | 9 (23.1) | 3 (18.75) | 1 (25.0) | 1 (8.3) | 4 (57.1) | 0.1 |
| FEV1, % predicted (±SEM) | 84.4 (±3.1) | 84.3 (±4.9) | 79.5 (±11.8) | 88.8 (±5.0) | 80.0 (±8.6) | 0.8 |
| NPF, L/min (IQR) | 95 (55–130) | 100 (80–135) | 140 (80–160) | 100 (50–125) | 60 (50–90) | 0.4 |
| Lund-Mackay score (IQR) | 19 (14–21) | 20 (17–21) | 13 (12–14) | 16 (14–20) | 22 (21–24) | 0.02 |
|
Peripheral blood eosinophil count k/µL (±SEM) |
0.7 (±0.07) | 0.6 (±0.08) | 0.6 (±0.09) | 0.7 (±0.14) | 1.0 (±0.2) | 0.2 |
| ACT score (±SEM) | 18 (±0.7) | 19 (±1.0) | 16 (±1.7) | 18 (±1.2) | 19 (±2.2) | 0.5 |
| RQLQ score (IQR) | 2.5 (1.8–4.2) | 2.9 (1.5–4.2) | 2.8 (1.5–4.5) | 2.4 (1.9–3.7) | 3.5 (2.3–4.2) | 0.9 |
| 15-HETE, pg/mL plasma (IQR) | 5850 (4825–7000) | 7006 (6056–8688) | 5969 (5081–7381) | 4800 (4238–5575) | 5663 (5325–6363) | 0.0005** |
ESS – endoscopic sinus surgery
ICS – inhaled corticosteroids
FEV1 – forced expiratory value in 1 second
NPF – nasal peak flow
ACT score – asthma control test, higher value corresponds to better asthma control
RQLQ score – rhinoconjunctivitis quality of life questionnaire, lower value corresponds to better ocular-nasal symptom control
15-HETE – 15-hydroxyeicosatetraenoic acid
Treatment outcomes were evaluated using the change from baseline in ACT and RQLQ scores
p-value for comparison between patients with improvement of symptoms vs. with worsening of symptoms
Values are expressed as means (± standard error of the means, SEM) or medians (interquartile range, IQR)
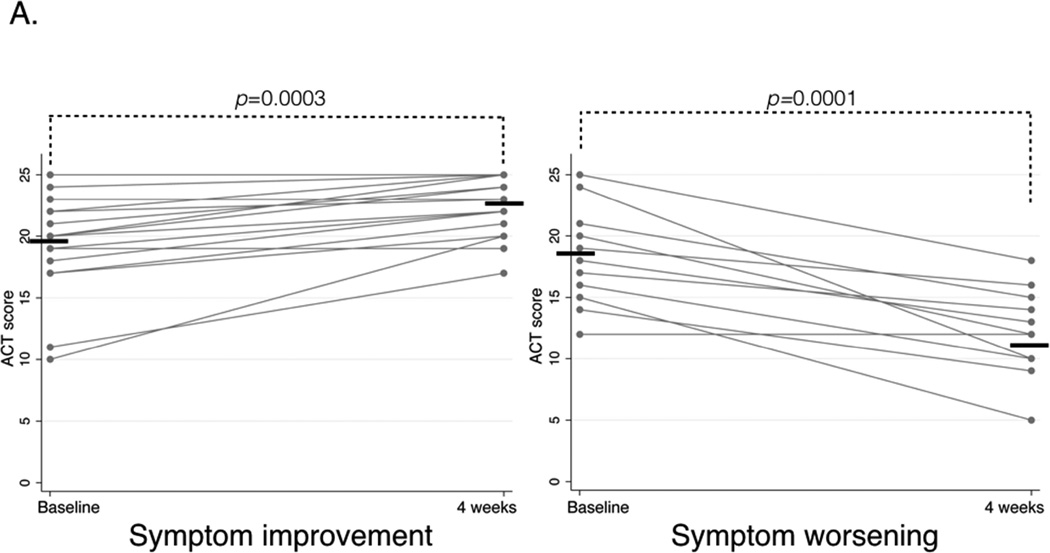
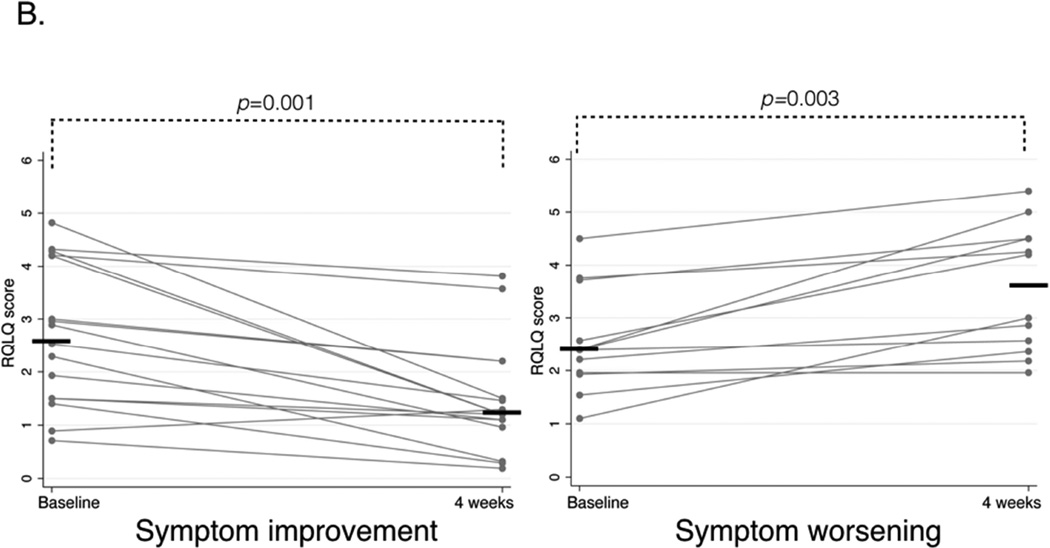
In patients with symptom improvement, mean ACT score improved from 19.25 (±1.0) to 22.3 (±0.6), p=0.0003, and mean RQLQ score improved from 2.7 (IQR 1.5–4.2) to 1.2 (IQR 1.0–1.9), p=0.001. In patients with symptom worsening, mean ACT score worsened from 17.9 (±1.2) to 11.9 (±1.0), p=0.0001, and mean RQLQ score worsened from 2.4 (IQR 1.9–3.1) to 3.6 (IQR 2.5–4.5), p=0.003 (Figure 1A and B).
Figure 1.
A. Asthma Control Test (ACT) questionnaire scores before and after four weeks of aspirin treatment for four weeks in patients with symptom improvement (left panel) and symptom worsening (right panel). Higher score corresponds to better asthma control, horizontal bars represent mean values.
B. Rhinoconjunctivitis Quality Of Life Questionnaire (RQLQ) scores before and after aspirin treatment for four weeks in patients with symptom improvement (left panel) and symptom worsening (right panel). Lower score corresponds to better symptom control, horizontal bars represent median values.
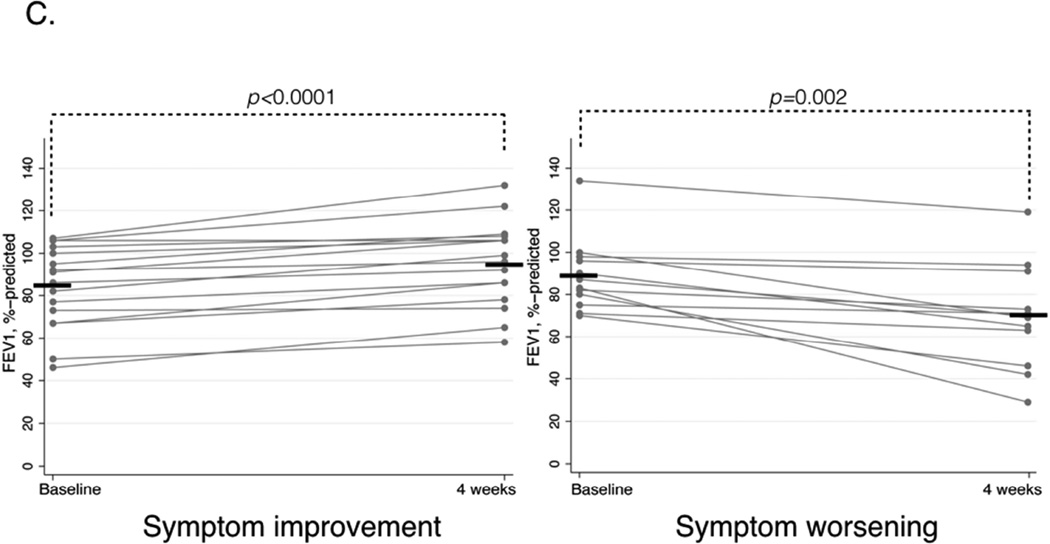
C. FEV1%-predicted before and after aspirin treatment for four weeks in patients with symptom improvement (left panel) and symptom worsening (right panel). Horizontal bars represent mean values.
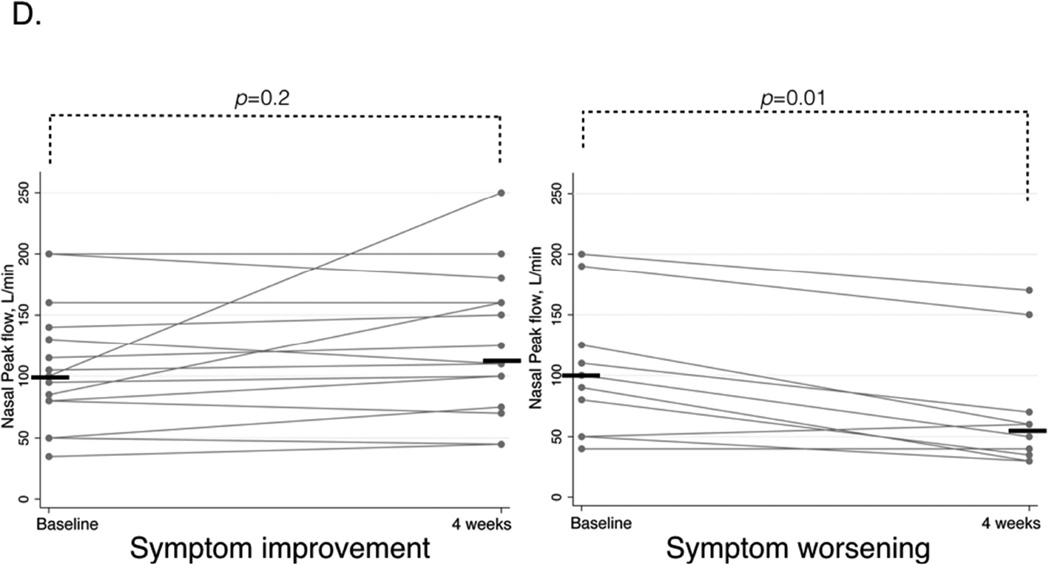
D. NPF before and after aspirin treatment for four weeks in patients with symptom improvement (left panel) and symptom worsening (right panel). Horizontal bars represent median values.
In patients with symptom improvement mean FEV1 significantly increased from 84.3%-predicted (±4.9) to 95.2%-predicted (±5.0), p<0.0001, but NPF did not significantly change. In patients with symptom worsening, mean FEV1 significantly decreased from 88.8%-predicted (±5.0) to 70.0%-predicted (±7.3), p=0.002, and median NPF value also significantly decreased from 100 (IQR 50–125) L/min to 55 (IQR 35–70), p=0.01 (Figure 1C and D).
Plasma eicosanoid analysis
Patients with symptom improvement after four weeks of aspirin therapy had higher baseline plasma 15-HETE levels than patients with symptom worsening: 7006 (IQR 6056–8688) pg/ml vs. 4800 (IQR 4238–5575) pg/ml (p=0.0005, Table I and Figure 2A). Baseline 15-HETE plasma levels positively correlated with the ACT score change after four weeks (r=0.61, p=0.001, Figure 2B), and with the FEV1 change after four weeks of aspirin treatment (r=0.49, p=0.01, Figure 2C). Baseline 15-HETE plasma levels inversely correlated with RQLQ score change at four weeks (r=−0.58, p=0.002, Figure 2D). The worsening in ACT and RQLQ scores and in FEV1 was observed in non-Caucasian patients (Figures 2B–D). There was no significant correlation between baseline 15-HETE plasma levels and NPF.
Figure 2.
A. Differences in baseline plasma 15-HETE levels between patients with symptom improvement (left) and symptom worsening (right) after four weeks of aspirin therapy. Horizontal bars represent median values.
B. Correlation between baseline plasma 15-HETE levels and ACT score change after four weeks of aspirin treatment. The effect size was determined by using Spearman correlation coefficient and denoted as an r-value.
C. Correlation between baseline plasma 15-HETE levels and FEV1 change after four weeks of aspirin treatment. The effect size was determined by using Spearman correlation coefficient and denoted as an r-value.
D. Correlation between baseline plasma 15-HETE levels and RQLQ score change after four weeks of aspirin treatment. The effect size was determined by using Spearman correlation coefficient and denoted as an r-value.
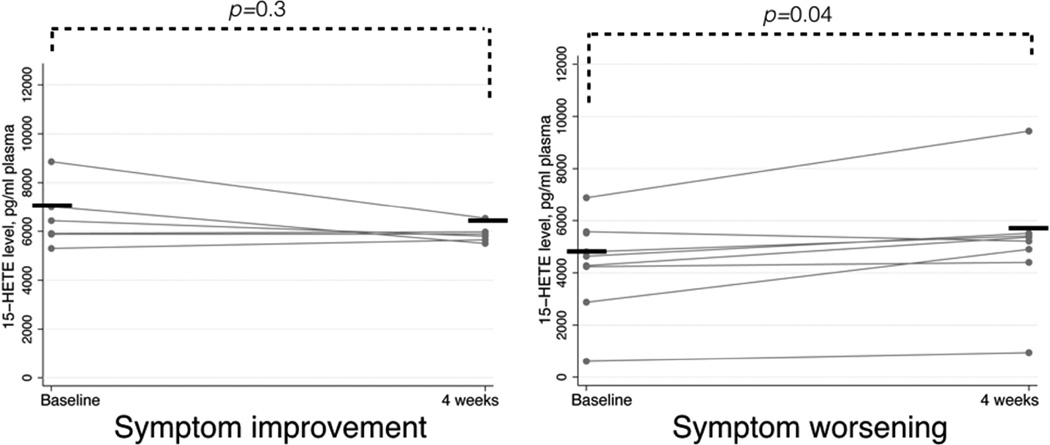
After aspirin desensitization, plasma 15-HETE significantly changed from baseline only in the patients whose symptoms worsened. These patients had an increase in plasma 15-HETE levels from 4800 (IQR 4238–5575) pg/ml at baseline to 5288 (IQR 4650–5463) pg/ml after four weeks of aspirin treatment (p=0.04, Figure 3).
Figure 3.
Change in plasma 15-HETE levels after four weeks of aspirin therapy in patients with symptom improvement and symptom worsening. Horizontal bars represent median values.
The baseline plasma levels of all of the other (twenty six) measured eicosanoids were not significantly different between patients with symptom improvement and patients with symptom worsening (Figure E1). There was also no significant change in the plasma levels of all the other eicosanoids after four weeks of aspirin treatment in either group (Figure E2.).
Changes in peripheral eosinophil counts
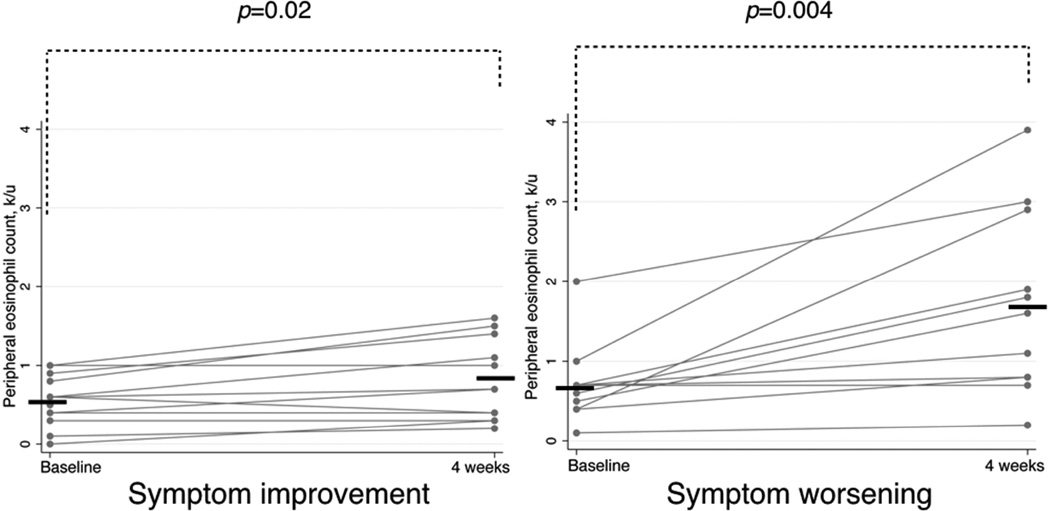
Mean peripheral eosinophil counts increased in all patients after four weeks of aspirin treatment, from 0.7 (±0.07) to 1.1 (±0.18) k/µL (p=0.001). However, the eosinophil count increases were not uniform in these patient groups. Mean eosinophil counts increased from 0.6 (±0.08) to 0.8 (±0.15) k/µL (p=0.02) in patients with symptom improvement. In contrast, mean eosinophil counts increased from 0.7 (±0.14) to 1.7 (±0.35) k/µL (p=0.004) in patients with symptom worsening (Figure 4.). The change from baseline in eosinophil counts was greater in patients with symptom worsening than in patients with symptom improvement: 210% (±67.0) vs. 37% (±13.0), respectively (p=0.006, data not shown). African-American and Latino patients were more likely to have higher peripheral eosinophil counts and a decrease in ACT score and in FEV1 after 4 weeks of aspirin treatment (Figure 5).
Figure 4.
Change in absolute eosinophil count after four weeks of aspirin treatment therapy in patients with symptom improvement (left panel) and symptom worsening (right panel). Horizontal bars represent mean values.
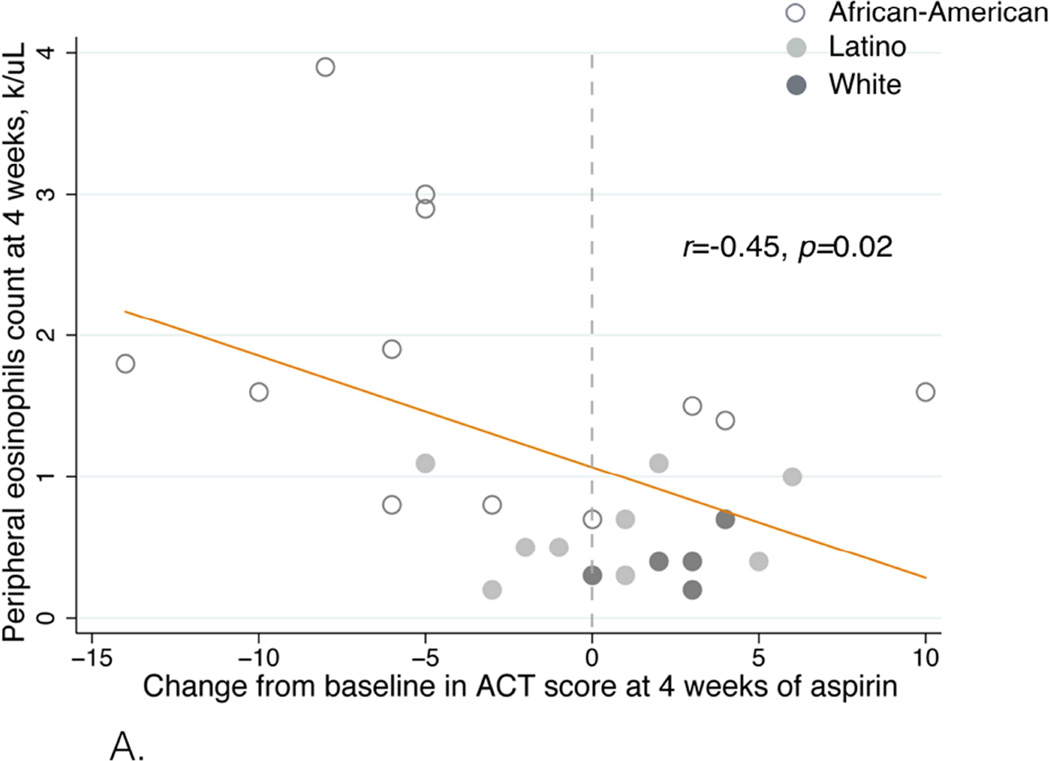
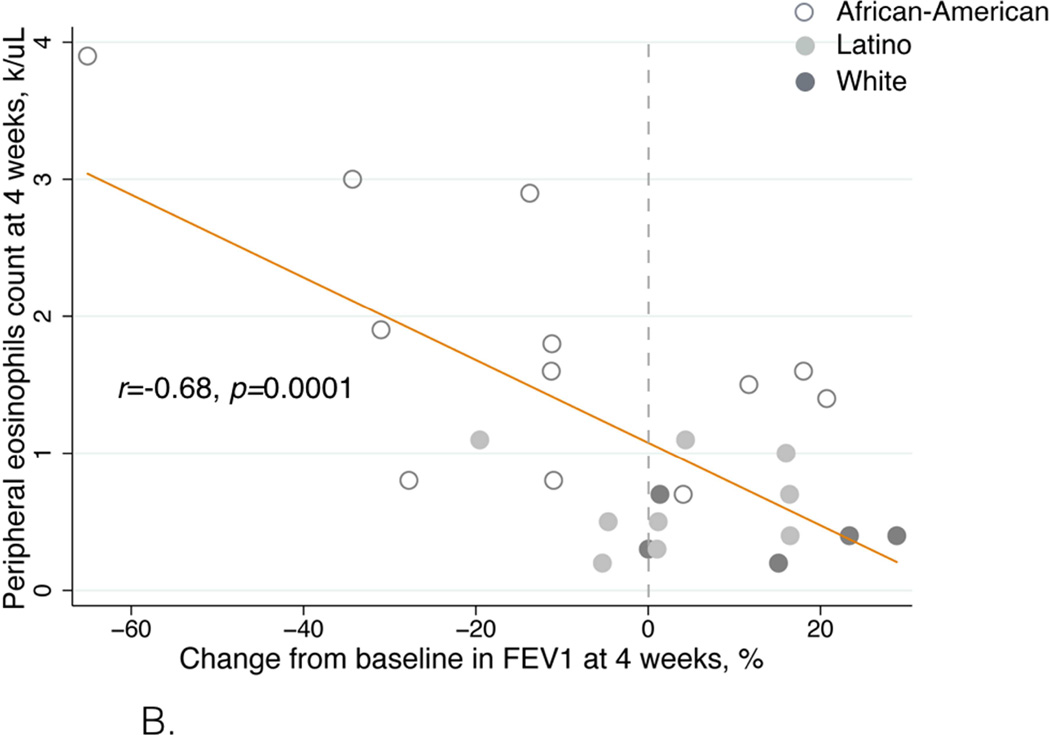
Figure 5.
A. Eosinophil count after four weeks of aspirin treatment correlated with ACT score change. The effect size was determined by using Pearson correlation coefficient and denoted as an r-value.
B. Eosinophil count after four weeks of aspirin treatment correlated with FEV1 change. The effect size was determined by using Pearson correlation coefficient and denoted as an r-value.
The eosinophil count after four weeks of aspirin treatment inversely correlated with the change in ACT score (r=−0.45, p=0.02, Figure 5A) and change in FEV1 (r=−0.68, p=0.0001, Figure 5B).
There was no significant correlation between eosinophil counts after four weeks of aspirin treatment and RQLQ score or NPF.
Relationship between 15-HETE and peripheral eosinophils
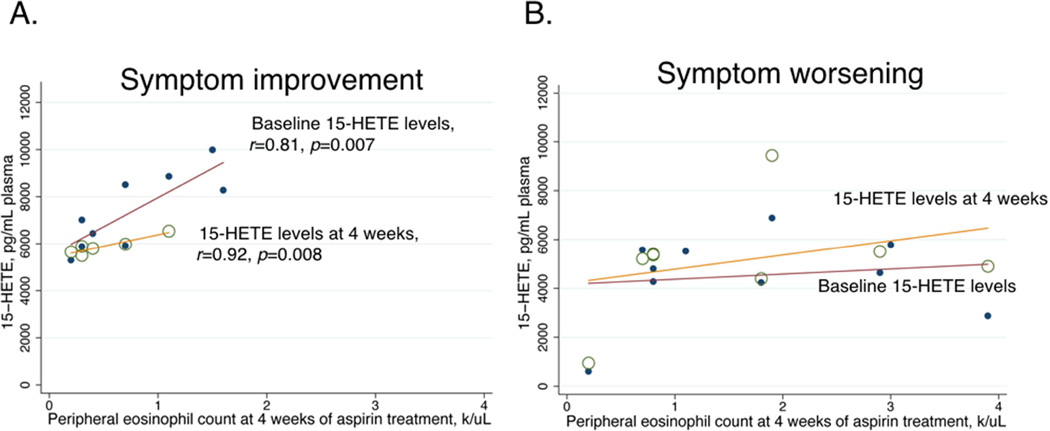
In patients with symptom improvement, eosinophil counts after four weeks were positively correlated with plasma 15-HETE levels both at baseline (r=0.81, p=0.007) and at four weeks of aspirin treatment (r=0.92, p=0.008) (Figure 6A.). In contrast, in patients with symptom worsening after four weeks of aspirin treatment, there was no significant correlation between eosinophil counts and plasma 15-HETE levels (Figure 6B).
Figure 6.
A. Correlation between eosinophil count after four weeks of aspirin treatment and plasma 15-HETE levels at baseline (closed circles) and at four weeks (open circles) in patients with symptom improvement. The effect size was determined by using Pearson correlation coefficient and denoted as an r-value.
B. Correlation between eosinophil count after four weeks of aspirin treatment and plasma 15-HETE levels at baseline (closed circles) and at four weeks (open circles) in patients with symptom worsening.
Multivariable analysis of factors associated with improved symptom score after aspirin desensitization
The unadjusted odds ratio (OR) for improvement in symptom score during aspirin treatment was 1.12 (95%CI 1.02–1.22) for every 100 pg/mL of baseline plasma 15-HETE levels. Adjustment for age, gender, and race/ethnicity did not substantially alter the association between baseline plasma 15-HETE levels and improvement in symptom score during aspirin treatment (OR: 1.18 (95%CI 1.02–1.36)). Additional adjustment for chronic oral prednisone use also did not alter this association (OR 1.2 (95%CI 1.01–1.42)). Adjusting for Lund-Mackay scores did not substantially alter the association between baseline plasma 15-HETE levels and improvement in symptom scores during aspirin treatment (OR: 1.18 (95%CI 1.03–1.36)).
Discussion
Long-term aspirin treatment is a common adjunct in AERD care and its benefits have been reported in several studies.5,31,32 In this study we investigated associations of plasma eicosanoids with outcomes of aspirin treatment in AERD patients. Similar to previous reports, some patients reported symptom improvement.7–9,12 However, a substantial fraction of our AERD patients had worsening of respiratory symptoms in response to high-dose aspirin treatment. To our knowledge, this is the first report on aspirin treatment outcomes in a predominantly minority AERD population, on the association of the treatment outcomes with 15-HETE plasma levels, and on the unusual and unexpected outcome of high-dose aspirin treatment in AERD patients – worsening of respiratory symptoms and a decline in FEV1.
While aspirin desensitization and high-dose long-term aspirin treatment are helpful for symptom control in most AERD patients, not all patients benefit from this treatment. To date, studies reported several reasons for discontinuation of aspirin treatment, such as aspirin-induced gastrointestinal (GI) side effects,6,8,9 followed by desensitization failure,12 and a lack of improvement.9 One study reported discontinuation of long-term aspirin treatment due to asthma or nasal symptoms in four out of ninety-three patients.9 We observed worsening of respiratory symptoms and an FEV1 decline in some of the desensitized patients. Worsening was not uniform among all demographic groups. Among African-American patients, 64% had worsening in respiratory symptoms after aspirin treatment. In Latino patients, 27% experienced worsening in respiratory symptoms. In contrast, no White, non-Latino patients had worsening in respiratory symptoms on aspirin therapy. It is possible that also White, non-Latino AERD patients experience worsening of respiratory symptoms on aspirin therapy. However, due to a small number of these patients in our study we did not observe this outcome.
We observed that lower baseline 15-HETE plasma levels were associated with worsening in respiratory symptoms in response to aspirin therapy and higher baseline 15-HETE plasma levels were associated with improvement in respiratory symptoms and function (FEV1) after aspirin treatment. In addition, 15-HETE plasma levels significantly correlated with peripheral eosinophilia in patients with an FEV1 increase, while there was a lack of correlation in patients with an FEV1 decline on aspirin therapy. Therefore, it is not only the baseline 15-HETE levels that are predictive of response to aspirin treatment, but perhaps more importantly, its relationship with eosinophilia. Having both a higher baseline 15-HETE plasma levels and a lower eosinophil count during aspirin treatment seem to be associated with improvement in respiratory symptoms and function after four weeks of aspirin treatment.
This observation raises the question as to how aspirin affects 15-HETE and eosinophilia in general, and more specifically in AERD patients. Previous studies reported that there was higher production of 15-HETE by eosinophils after aspirin stimulation of peripheral blood leukocytes and in nasal polyps of AERD patients. 19–21
Aspirin-induced acetylation of human cyclooxygenases (COX-1 and COX-2) in vitro causes a complete inhibition of COX-1 activity. In contrast, aspirin inhibits COX-2 by only about 50% and converts COX-2 to a form that catalyzes production of 15-HETE instead of prostaglandin H2 (the precursor of prostaglandins D2 and E2) from arachidonic acid.33 The produced 15-HETE is primarily of the R- and not the S-stereoconfiguration that is typical of 15-HETE produced by 15-LO.33 Our detection method does not distinguish the two 15-HETE stereoisomers. However, such analysis could be performed in a future study.
Native 15(S)-HETE is metabolized into the lipoxins (LX) A2 and B2. Lipoxin generation is initiated in two ways. The first pathway involves conversion of LTA4 by 12-LO to lipoxin A2 and B2 in leukocytes and does not require 15-HETE.34 The second pathway involves 15-LO, which converts arachidonic acid to 15(S)-HETE. 15(S)-HETE is then converted to lipoxins by 5-LO.35
In the presence of aspirin, a third pathway of lipoxin production is initiated: COX-2-generated 15(R)-HETE is converted by 5-LO to the aspirin-triggered lipoxins, 15-epimer-lipoxins A4 and B4.36 Lipoxins possess potent anti-inflammatory effects, including inhibition of eosinophil chemotaxis and a decrease in IL-5 and eotaxin secretion.37
Interestingly, 15-LO products possess both pro- and anti-inflammatory effects. For example, its derivatives can serve as precursors to the above-mentioned anti-inflammatory lipoxins or can be metabolized to pro-inflammatory 14,15-leukotriene derivatives – eoxins.38,39 They increase the permeability of the endothelial cells in vitro.38 Eosinophils and nasal polyps are an abundant source of eoxins.
We observed a higher level of 15-HETE at baseline in patients whose symptoms improved on aspirin. While we did not measure lipoxins, it is possible that more 15(S)-HETE was available to be converted into lipoxins in patients who were successfully desensitized and treated.
Previous studies reported that high 15-HETE levels are mainly indicative of pro-inflammatory responses in asthma.15,23,40,41 High 15-HETE levels have also been shown in the sputum of patients with chronic bronchitis compared to control subjects.42
We observed a significant increase in peripheral eosinophilia in most of the study patients after four weeks of aspirin treatment. We noticed that the increase in eosinophilia was inversely correlated with ACT score change and FEV1 change at four weeks. There were no significant differences in baseline eosinophil count by treatment outcomes, but patients with worsening in respiratory symptoms and function had a greater increase in eosinophil counts at four weeks. In addition, an improvement in respiratory symptoms and function (FEV1) was observed in patients who had a strong positive correlation between the eosinophil count and 15-HETE plasma levels while there was no significant correlation between eosinophil counts and 15-HETE levels in patients whose respiratory symptoms and FEV1 worsened. This observation suggests that plasma 15-HETE may regulate peripheral blood eosinophilia and its deficit results in a disproportional increase in eosinophils during aspirin treatment. The increase in peripheral blood eosinophilia after aspirin treatment could be due to the previously reported decrease in a PGD2 metabolite, which otherwise helps recruit eosinophils into the respiratory tissue.11 The decrease in PGD2 metabolite production after aspirin treatment diminishes its chemotactic shunting of eosinophils into the respiratory tissue thereby releasing eosinophils into the bloodstream.12
Unexpectedly, all patients who had worsening of respiratory symptoms after four weeks of aspirin treatment were ethnic minority individuals. The question remains as to why minority patients had lower 15-HETE plasma levels at baseline and a significantly greater increase in eosinophil counts after four weeks of aspirin.
Disparities in asthma prevalence, severity, and morbidity among minority populations have been documented by previous research.43,44 African-American patients also seem to have more severe sinus disease.45 Our study indicates that similar disparities exist in AERD, specifically with regard to aspirin treatment outcomes.
In summary, this study suggests that aspirin treatment outcomes are not uniform across all AERD patients. A disproportionate increase in eosinophils as a consequence of aspirin treatment in AERD can be associated with worsening of lung function. Higher 15-HETE level at baseline and its strong correlation with eosinophilia at four weeks of aspirin therapy is characteristic for patients who benefited from this treatment. This indicates a possible role of baseline 15-HETE as predictor of aspirin treatment outcomes in AERD patients. Additional studies are needed to investigate the mechanism and additional treatment options for AERD in a larger group of ethnically diverse patients.
Supplementary Material
Highlights Box.
What is already known about this topic? High-dose daily aspirin provides therapeutic benefits to patients with AERD.
What does this article add to our knowledge? Low baseline plasma 15-HETE levels and increased eosinophilia during aspirin treatment may predict worsening respiratory function during high-dose aspirin therapy in AERD patients.
How does this study impact current management guidelines? These findings need to be confirmed in a larger study. If confirmed, baseline 15-HETE plasma levels could be used to select AERD patients who are most likely to benefit from aspirin treatment.
Acknowledgments
The authors would like to thank Dr. Thomas Aldrich and Dr. Peter F. Weller for their review and comments on the manuscript, and Dr. Aileen McGinn for her help with statistical analysis. The authors also thank Derek Wagner from StataCorp LP for his help in creating figures for this article.
Declaration of funding sources:
This work was supported by CTSA grant number 5KL2TR001071 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Portions of this work were funded by the Stony Wold-Herbert Fund research grant (to EJ) and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025034 to DCZ). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- ACT
Asthma Control Test
- AERD
aspirin-exacerbated respiratory disease
- BALF
bronchoalveolar lavage fluid
- COX
cyclooxygenase
- DHET
dihydroxyeicosatrienoic acid
- DHOME
dihydroxyoctadecenoic acid
- EET
epoxyeicosatrienoic acid
- EpOME
epoxyoctadecenoic acid
- FeNO
fraction of exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- IL
interleukin
- IQR
interquartile range
- LTE4
leukotriene E4
- LO
lypoxygenase
- LX
lipoxin
- NPF
nasal inspiratory peak flow
- NSAIDs
non-steroidal anti-inflammatory drugs
- OR
odds ratio
- PGDM
prostaglandin D metabolite
- PGEM
prostaglandin E metabolite
- RQLQ
Rhino-Conjunctivitis Quality of Life Questionnaire
- SEM
standard error of the mean
- THOME
trihydroxyoctadecamonoenoic acid
- TXB
11-dehydrothromboxane B2
- 95%CI
95% confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681. e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–983. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- 3.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 4.Kowalski Ml, Makowska JS, Blanca M, et al. Hypersensetivity to non-steroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA and GA2LEN/HANNA. Allergy. 2011;66:818–829. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol. 2006;118:801–804. doi: 10.1016/j.jaci.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003;111:180–186. doi: 10.1067/mai.2003.7. [DOI] [PubMed] [Google Scholar]
- 7.Berges-Gimeno MP, Simon RA, Stevenson DD. Early effects of aspirin desensitization treatment in asthmatic patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2003;90:338–341. doi: 10.1016/S1081-1206(10)61803-0. [DOI] [PubMed] [Google Scholar]
- 8.Swierczynska-Krepa M, Sanak M, Bochenek G, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol. 2014;134:883–890. doi: 10.1016/j.jaci.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Ta V, White AA. Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3:711–718. doi: 10.1016/j.jaip.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 10.White AA, Stevenson DD. Aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:211–222. doi: 10.1016/j.iac.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D: A dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katial RK, Strand M, Prasertsuntarasai T, Leung R, Zheng W, Alam R. The effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory diseases. J Allergy Clin Immunol. 2010;126:738–744. doi: 10.1016/j.jaci.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Bradding P, Redington AE, Djukanovic R, Conrad DJ, Holgate ST. 15-lipoxygenase immunoreactivity in normal and in asthmatic airways. Am J Respir Crit Care Med. 1995;151:1201–1204. doi: 10.1164/ajrccm/151.4.1201. [DOI] [PubMed] [Google Scholar]
- 15.Chu HW, Balzar S, Westcott JY, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 16.Morita E, Schroder JM, Christophers E. Production of 15-hydroxyeicosatetraenoic acid by purified human eosinophils and neutrophils. Scand J Immunol. 1990;32:497–502. doi: 10.1111/j.1365-3083.1990.tb03190.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai CK, Phillips GD, Jenkins JR, Holgate ST. The effect of inhaled 15-(s)-hydroxyeicosatetraenoic acid (15-HETE) on airway calibre and non-specific responsiveness in normal and asthmatic human subjects. Eur Respir J. 1990;3:38–45. [PubMed] [Google Scholar]
- 18.Kowalski ML, Ptasinska A, Bienkiewicz B, Pawliczak R, DuBuske L. Differential effects of aspirin and misoprostol on 15-hydroxyeicosatetraenoic acid generation by leukocytes from aspirin-sensitive asthmatic patients. J Allergy Clin Immunol. 2003;112:505–512. doi: 10.1016/s0091-6749(03)01716-0. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski ML, Ptasinska A, Jedrzejczak M, et al. Aspirin-triggered 15-HETE generation in peripheral blood leukocytes is a specific and sensitive Aspirin-Sensitive Patients Identification Test (ASPITest) Allergy. 2005;60:1139–1145. doi: 10.1111/j.1398-9995.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewandowska-Polak A, Jedrzejczak-Czechowicz M, Makowska JS, Jarzebska M, Jankowski A, Kowalski ML. Lack of association between aspirin-triggered 15-hydroxyeicosatetraenoic acid release and mast cell/eosinophil activation in nasal polyps from aspirin-sensitive patients. J Investig Allergol Clin Immunol. 2011;21:507–513. [PubMed] [Google Scholar]
- 21.James A, Daham K, Backman L, et al. The influence of aspirin on release of eoxin C4, leukotriene C4 and 15-HETE, in eosinophilic granulocytes isolated from patients with asthma. Int Arch Allergy Immunol. 2013;162:135–142. doi: 10.1159/000351422. [DOI] [PubMed] [Google Scholar]
- 22.Nizankowska E, Michalska Z, Wandzilak M, et al. An abnormality of arachidonic acid metabolism is not a generalized phenomenon in patients with aspirin-induced asthma. Eicosanoids. 1988;1:45–48. [PubMed] [Google Scholar]
- 23.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, Dahlen SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Curr Pharm Biotechnol. 2011;12:1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 24.Mabalirajan U, Rehman R, Ahmad T, et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep. 2013;3:1349. doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macy E, Bernstein JA, Castells MC, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 26.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann Allergy Asthma Immunol. 2006;97:688–693. doi: 10.1016/S1081-1206(10)61101-5. [DOI] [PubMed] [Google Scholar]
- 27.Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- 28.Zha W, Edin ML, Vendrov KC, et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res. 2014;55:2124–2136. doi: 10.1194/jlr.M053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124:719–723. e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 30.ATS. Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) American Thoracic Society; 2015. http://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/rqlq.php: [Google Scholar]
- 31.Chen JR, Buchmiller BL, Khan DA. An Hourly Dose-Escalation Desensitization Protocol for Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3:926–931. e1. doi: 10.1016/j.jaip.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006;118:773–786. doi: 10.1016/j.jaci.2006.07.024. quiz 87-8. [DOI] [PubMed] [Google Scholar]
- 33.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 34.Romano M, Serhan CN. Lipoxin generation by permeabilized human platelets. Biochemistry. 1992;31:8269–8277. doi: 10.1021/bi00150a021. [DOI] [PubMed] [Google Scholar]
- 35.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano M, Cianci E, Simiele F, Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 38.Feltenmark S, Gautam N, Brunnstrom A, et al. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci U S A. 2008;105:680–685. doi: 10.1073/pnas.0710127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundstrom SL, Yang J, Kallberg HJ, et al. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One. 2012;7:e33780. doi: 10.1371/journal.pone.0033780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C, Xu D, Liu L, et al. 15-Lipoxygenase-1 induces expression and release of chemokines in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L196–L203. doi: 10.1152/ajplung.00036.2008. [DOI] [PubMed] [Google Scholar]
- 41.Shannon VR, Chanez P, Bousquet J, Holtzman MJ. Histochemical evidence for induction of arachidonate 15-lipoxygenase in airway disease. Am Rev Respir Dis. 1993;147:1024–1028. doi: 10.1164/ajrccm/147.4.1024. [DOI] [PubMed] [Google Scholar]
- 42.Profita M, Sala A, Riccobono L, et al. 15(S)-HETE modulates LTB(4) production and neutrophil chemotaxis in chronic bronchitis. Am J Physiol Cell Physiol. 2000;279:C1249–C1258. doi: 10.1152/ajpcell.2000.279.4.C1249. [DOI] [PubMed] [Google Scholar]
- 43.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: a multilevel challenge. J Allergy Clin Immunol. 2009;123:1209–1217. doi: 10.1016/j.jaci.2009.02.043. quiz 18–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pino-Yanes M, Thakur N, Gignoux CR, et al. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol. 2015;135:228–235. doi: 10.1016/j.jaci.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahdavinia M, Benhammuda M, Codispoti CD, et al. African American Patients With Chronic Rhinosinusitis Have a Distinct Phenotype of Polyposis Associated With Increased Asthma Hospitalization. J Allergy Clin Immunol Pract. 2016 doi: 10.1016/j.jaip.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.