Abstract
In April 2014, the American Journal of Transplantation published a report on the first lung transplant in the United States recovered from an uncontrolled donation after circulatory determination of death donor (uDCDD), assessed by ex vivo lung perfusion (EVLP). The article identified logistical and ethical issues related to introduction of lung transplant from uDCDDs. In an open clinical trial, we have Food and Drug Administration and Institutional Review Board approval to transplant lungs recovered from uDCDDs judged suitable after EVLP. Through this project and other experiences with lung recovery from uDCDDs, we have identified solutions to many logistical challenges and have addressed ethical issues surrounding lung transplant from uDCDDs that were mentioned in this case report. Here, we discuss those challenges, including issues related to recovery of other solid organs from uDCDDs. Despite logistical challenges, uDCDDs could solve the critical shortage of lungs for transplant. Furthermore, by avoiding the deleterious impact of brain death and days of positive pressure ventilation, and by using opportunities to treat lungs in the decedent or during EVLP, lungs recovered from uDCDDs may ultimately prove to be better than lungs currently being transplanted from conventional brain-dead organ donors.
Introduction
In the American Journal of Transplantation, Suzuki et al (1) described the first lung transplant performed in the United States after recovery from a Maastricht Category 2 uncontrolled donation after circulatory determination of death (uDCDD) donor assessed by ex vivo lung perfusion (EVLP). The authors posit that the potential role of uDCDD donors (uDCDDs) as a donor source should be “reconsidered,” and pose logistical and ethical hurdles that require attention. We are actively working to perform lung transplants from uDCDDs. In September 2013, we began an NIH-funded Phase II clinical trial, “Ex-vivo Perfusion and Ventilation of Lungs Recovered from Non-Heart-Beating Donors to Assess Transplant Suitability” (www.clinical-trials.gov, NCT01615484). Based on our experience with lung recovery from uDCDDs, we offer solutions to the ethical and logistical issues cited in the case report.
Limitations of Conventional Donors to Provide Enough Lungs
The number of conventional brain-dead donors is inadequate to meet the need for all patients with end-stage lung disease who could benefit from lung transplant. Despite efforts to increase donor numbers, numbers of brain-dead donors have decreased in many countries. Aspiration at the time of brain injury leads to pneumonia in many conventional brain-dead donors. Without an immune system, EVLP is unlikely to resolve pneumonia in a donor lung. Neurogenic pulmonary edema is common after severe brain injury (2); this might be improved with EVLP. Although controlled donation after circulatory determination of death (cDCDD) donors have not been well utilized for lung transplant, the number of cDCDD donors is relatively small.
Potential of EVLP to Further Increase Lung Donors
EVLP has been used to increase the yield of lungs from conventional brain-dead organ donors (3), but its impact will be limited by the size of this donor pool, and the pathophysiology of lung injury in these donors. EVLP could substantially increase the number of available lungs if used to assess suitability of lungs for transplant recovered from uDCDDs, both Category 1 (sudden deaths outside of hospital) and Category 2 (sudden deaths within the hospital). The number of these potential lung donors has not been well studied. The AmericanHeart Association estimates thereare over 200 000 cardiac arrests outside of hospital treated by Emergency Medical Services (EMS) personnel, with only 12% obtaining a rhythm and being transported. The median age is 60. How many of these over 80 000 potential uDCDDs would be suitable for lung transplant is unknown. There are almost as many in-hospital cardiac arrests; likely a smaller percentage of hospitalized patients would be suitable lung donor candidates. However, uDCDDs might provide thousands more lung donors. This contrasts with the 1800 lung transplants currently performed annually in the United States. Lung transplant is expensive, as are many life-prolonging therapies, including other organ transplants and cancer therapies. However, long-term survival and improved quality of life after lung transplant exceed many therapies for cancer patients. Ultimately, choices about resource allocation in health care may be necessary.
uDCDDs in Spain
Based on “presumed consent,” organ recovery from uDCDDs has been routine in Spain for over a decade. All Spanish citizens are presumed organ donors unless they have specifically declined participation. Consent is still sought from next-of-kin (NOK). According to Rafael Matesanz, M.D., Director of Spain’s Organización Nacional de Trasplantes (ONT), 93% of NOK consent to organ donation (Dr. Matesanz, personal communication, Madrid, Spain, March 19, 2014).
In some Spanish cities, after cardiopulmonary resuscitation (CPR) is determined to be futile, potential uDCDDs are placed on a CPR machine, ventilated and transported to a hospital with organ procurement surgeons. At the hospital, CPR and ventilation are stopped for 5 min, and death is declared by emergency department (ED) physicians. Then, physicians place cannulae via groin vessels to initiate perfusion and cooling of the abdominal organs, an occluding balloon in the descending thoracic aorta to prevent cerebral blood flow, and chest tubes to instill cold solutions into the pleural spaces to cool the lungs. NOK are contacted; they have the opportunity to deny organ recovery, but usually agree and organ recovery proceeds. In Spain, this has resulted in 67 lung transplants from uDCDDs since 2002 (Elisabeth Coll, personal communication, ONT, Madrid, October 13, 2014). Lung assessment techniques have been variable, as has been the use of EVLP.
uDCDDs in the United States
One ethical concern is the possibility of cerebral perfusion by CPR during transportation of potential uDCDDs to the hospital before death is declared. This is a substantial challenge to use of uDCDDs for organ recovery in the United States. Because death is defined as irreversible cessation of respiratory and circulatory functions (4), a panel convened by the Health Resources and Services Administration (HRSA) concluded that resumption of circulation by any means, including CPR to maintain integrity of organs for transplant, could not be sanctioned at any time (5). This dilemma could be resolved by changing either the notion that death must occur before organ recovery (6) or the notion of irreversibility (7) or functions, particularly from individuals who provide first-person consent.
Potential of uDCDD Donors in Lung Transplant
Aside from increasing the number of lungs for transplant, uDCDDs may offer other advantages. Because brain death is associated with up-regulation of innate immunity in the donor and a “cytokine storm” (8,9), and brain-dead donors require prolonged positive pressure ventilation, which contributes to lung injury, lungs recovered from uDCDDs might function better than lungs recovered from brain-dead donors, currently being transplanted. Furthermore, EVLP offers opportunities to treat donor lungs, to allow recovery from the antecedent warm ischemic insult and possibly modify the lung to reduce IRI (10). We and others showed that ventilation of rat lungs in an NHBD with nitric oxide (11) or carbon monoxide (500 ppm) (12,13) improved lung function after transplant.
Logistical Challenges to Lung Recovery From uDCDDs
In their case report, Suzuki et al allude to logistical and ethical challenges that require address before lung recovery from uDCDDs can become established practice, stating that “[…] expansion of the lung allograft pool through uDCDDs is perceived to be associated with nonquantifiable risks” (14), and “The hesitation for use of lungs through uDCDDs stems mainly from medical concerns over secondary injury from prolonged warm ischemia […].” Many of these challenges have already been identified and addressed. As confidence in EVLP as a method to assess suitability of lungs for transplant accumulates, we believe that we have a tool to assess suitability of lungs recovered from uDCDDs. EVLP allows assessment of gas exchange and compliance over time, palpation, bronchoscopy and radiography.
The authors comment that topical cooling was a “critically important adjunct to restrict warm ischemic time.” It is not clear whether topical cooling is critical. In a porcine study, Rega et al (15) showed that topical cooling for 3 h was superior to ventilation for 3 h, when lungs were perfused in a circuit, but both interventions were instituted after 1 h of nonventilated warm ischemia. So for the relatively short time before hypothermic Perfadex flush to cool the lungs, static inflation or continued ventilation may have maintained lung viability. Based on our animal data (16–18), we believe that 1 h of nonventilated and 2 h of ventilated ischemia would be well tolerated. Nakajima et al (19) showed in a canine model that EVLP for 3.5 h with Steen solution resulted in excellent lung function after transplant following 4 h of nonventilated warm ischemia.
We agree that organ recovery from uDCDDs has substantial logistical challenges. After an earlier uDCDD project, we focused on cooperation from EMS workers (20). As first responders, EMS professionals are gatekeepers to uDCDDs. In the last decade, there has been an important shift in EMS responsibilities, with death declaration in the field by trained EMS staff. Medical examiners are contacted for permission to move the body, but currently there is no urgency. Police have become accustomed to viewing the deceased at the scene, rather than finding out about failed resuscitation after a decedent was taken to a hospital.
When we started recovering lungs from uDCDDs for this project, Deborah Radisch, M.D., medical examiner for Wake County, NC (also state medical examiner and a UNC faculty member), was enthusiastically supportive. She instructed her staff to authorize immediate movement of the potential uDCDD unless EMS suspected foul play and requested law enforcement review of the scene. Because they were accustomed to viewing the deceased at the scene, police objected to this “new” practice. This led to a hiatus until we met with leadership in the Wake County district attorney’s office. That office also supported the project, and agreed that in years past, decedents had been moved from the scene to EDs, where they were pronounced dead if CPR was unsuccessful. The Wake County district attorney’s office, with the state medical examiner’s assistance, wrote a memorandum to all police precincts in Wake County, authorizing rapid transportation of the deceased. Our medical examiner has authorized removal of decedents from motor vehicle crashes provided that blood and urine are collected for toxicology. We routinely recover and store this on all uDCDD cases. The remaining logistical hurdle is identifying and informing NOK of motor vehicle crash victims. We agree with Suzuki et al that “it is important that medical examiners and coroners are educated regarding uDCDD and protocols are put in place for the preservation of any needed forensic evidence.” This can be done and has been done here.
Through interaction with EMS staff in several North Carolina (NC) counties, we learned that EMS staff were not comfortable approaching NOK about organ donation, but were very engaged in facilitating contact between the NOK and the organ procurement organization (OPO) (20). Accordingly, when CPR is terminated and death is declared, NOK is informed of the death and then told to expect a few phone calls, including from Carolina Donor Services (CDS). Minimizing warm ischemia is critically important. There are many logistical hurdles to address that can help achieve acceptable warm ischemic times. Although blood type, serologies, and HLA are not available at the time of lung recovery from uDCDDs, blood withdrawn during lung recovery can be sent for expeditious typing. EVLP can be started at a time to coincide with its termination when serology results are available.
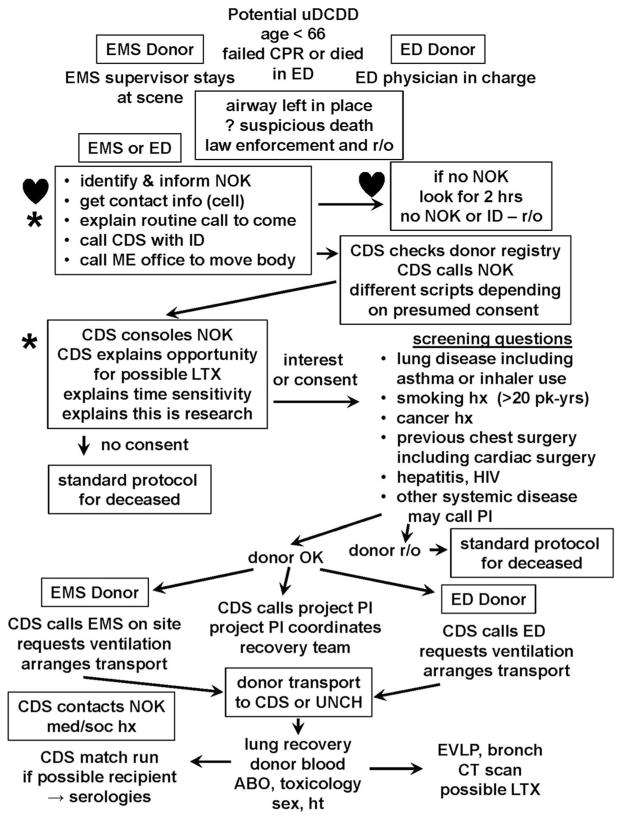
Our protocol for lung recovery is outlined in Figure 1. CDS is called for decedents in Wake County or participating EDs. NOK is identified by EMS or the ED, informed of the death, and told to expect some routine phone calls. A trained CDS requestor contacts NOK, using one of two scripts, depending on donor first-person consent status. If NOK are not available or cannot be located, the case is abandoned after 2 h. When NOK are contacted, they are consoled and asked if, or informed that, the decedent wanted to donate organs. They are told that there is an NIH-sponsored study to evaluate lungs recovered after death and that suitable lungs could be transplanted. If NOK assent, screening questions are asked as depicted in Figure 1. If suitable, CDS explains they will call back later for a more detailed medical/social history (med/soc). CDS calls EMS or ED to initiate ventilation and arranges transport to CDS’ operating room or to UNC Hospitals, where the first available OR is made available. The project PI is called. A donor surgeon is located and a team is mobilized to recover the lungs. CDS call center staff obtain a detailed med/soc from NOK. Lungs are recovered in the standard fashion. Subcarinal nodes are recovered for HLA typing. Donor blood is aspirated from the right atrium/IVC for blood type, culture, HLA, toxicology and serologies. After ABO typing, a match list is run by CDS. EVLP is performed in a Class II hood, begun so that 4-h EVLP will be completed when serology results are available. After EVLP, lungs are cooled, flushed with cold Perfadex, packaged as lungs are from a conventional donor, and an ex vivo CT scan is performed. Suitable lungs are transplanted into consented recipients.
Figure 1. Schema for current clinical trial at UNC (NCT01615484) to evaluate lungs recovered from uDCDDs.
Eligible individuals are under age 66 not successfully resuscitated after CPR or died in the emergency department (ED). Circumstances that require law enforcement investigation are ruled out (r/o). Many potential donors are r/o for medical reasons. After EVLP and ex vivo CT scan, suitable lungs are offered to consented recipients. Procedures that would reduce ischemic time if first-person consent is honored. ♥, Procedures where improved logistics will reduce ischemic time.
Impact of First-Person Consent
First-person authorization via the establishment of online state donor registries puts an entirely new slant on the process of organ recovery from uDCDDs. Forty-eight states have now revised their Uniform Anatomical Gift Act (UAGA) to allow first-person authorization to be a legal consent to donate organs. Although many OPOs are reluctant to proceed with organ recovery without NOK assent, it is not legally required if first-person authorization is in place. The NC Attorney General’s office agrees that our OPO does not require NOK consent to proceed with lung recovery from registered organ donors. The NC law states “If an anatomical gift has been or might be made of a body part of a decedent […] the medical examiner and procurement organization shall cooperate in the timely removal of the body part from the decedent for the purpose of transplantation, therapy, research, or education” (21). According to Sheldon Kurtz, JD, who wrote the first draft of the UAGA and was reporter for the committee that finalized the draft circulated to states for approval, the act requires the OPO to recover solid organs from consented decedents if the intent is transplant. NOK consent is not required, and delay to find NOK is unnecessary (Sheldon Kurtz, personal communication, University of Iowa, December 2, 2014). The NC Attorney General’s office is considering this interpretation. CDS remains unwilling to recover lungs without NOK consent. Some OPOs (Ohio and New Jersey) have proceeded with organ recovery from brain-dead donors under first-person consent, over NOK objections. The New Jersey OPO resorted to obtaining a court order authorizing organ recovery. Several eye banks, including NC’s, do not require NOK consent. If NOK cannot be located within 6 h, corneas are recovered from registered organ and tissue donors.
Suzuki et al are concerned that “families may be mistrustful of the health care team if they are notified of the patient’s death after the organs have already been retrieved.” As a practical matter, NOK assent can be obtained before transplant or even before evaluation occurs. However, the law requires timely removal of organs for the purpose of preservation for transplant, and transplantation if the organs are suitable and there is a potential recipient. If explained in this light, and that recovery was performed to honor the wishes of the decedent, we believe that the public will accept the concept. Perhaps additional public education is required. NOK will need to provide essential information regarding details of a med/soc history, so their participation in the process is necessary. Thus, NOK would have an opportunity to refuse transplantation, even if the decedent consented and organs were recovered. A recent study suggests that the vast majority of OPOs in the United States believe that first-person authorization should be honored (22). The UAGA requires lung recovery from consented donors, even before NOK are found and informed of the death.
Ethical Issues in uDCDDs
Some of the concerns raised by Suzuki et al related to ethical issues are perhaps misplaced. There is no concern about the dead donor rule (23) in the scenario of lung-only recovery from an uDCDD, because the individual’s death has been determined before any intervention begins.
Lung ventilation in an uDCDD poses no risk for auto-resuscitation or restoration of brain stem function, because systemic blood flow is not restored. Ventilation will not interfere with the permanence of cessation of circulation, a point of contention for recovery of other organs from uDCDDs (5). Suzuki et al reflect that “greater ethical consensus on how and when death may be declared may be needed before the pool of potential uDCDDs can be utilized in the United States.” While this may be true for resuming circulation to preserve abdominal organs, this concern does not exist when a decedent’s body is ventilated for lung recovery.
Ethical concerns also emerge from consideration of interventions upon the body after death. The passage of laws that address recovery with first-person consent indicates a growing consensus about the acceptability of the practice, so ethical concerns about ventilation after death may abate. Intent to seek consent from NOK before recovery may further reassure the public about a trustworthy practice. Mechanical ventilation is a routine part of CPR. The decision to terminate CPR and ventilation is based on a decision that death has occurred. Even without first-person authorization, continued mechanical ventilation postmortem until NOK is contacted may not pose an ethical dilemma. Our research project/clinical trial adheres to accepted principles of ethical conduct of research on the recently deceased (24).
Summary
The logistical issues surrounding the potential use of uDCDDs as lung donors have been addressed in our local area. Improvement to minimize ischemic time is still possible. Because jurisdiction over sudden deaths, EMS response, and medical examiner practices vary within states and even within counties and cities, the widespread practice of lung recovery from uDCDDs will require education of various stakeholders. The remaining ethical issues relate to consideration of how we respect the preferences of the decedent and of NOK regarding organ donation in a process that engenders societal trust. There are still substantial obstacles to timely recovery of lungs for transplant evaluation. Many decedents lack identification, or NOK is hard to locate, or too grief-stricken to discuss donation. We are learning that many potential donors have co-existing medical conditions that preclude safe lung transplant. In 15 months, we have recovered 16 lung blocks; 8 had EVLP. Seven were not suitable for transplant; one was suitable, but no consented appropriate recipient was available. We are continuing to encounter and address obstacles to timely uDCDD recovery and are convinced that uDCDDs are a potentially important source of lungs for transplant. Recovery of other organs for transplantation from uDCDDs in the United States may require changes in law or policy with regard to the dead donor rule and definition of death. This is appropriate, given that half of US adults have indicated a desire to donate their organs for transplant. There is a moral obligation to attempt to honor that wish.
Acknowledgments
The authors are grateful for the expert editorial assistance of Margaret Alford Cloud, UNC Division of Cardiothoracic Surgery, and for the review and thoughtful input of Arlene Davis, BSN, JD, Associate Professor in the Department of Social Medicine, University of North Carolina at Chapel Hill. Lessons learned concerning resolution of logistical issues relate to Dr. Egan’s participation in 1R38OT03593 (HRSA), 1RC2HL101641 (NIH) and Dr. Egan and Mr. Requard’s participation in 1UM1HL113115 (NIH).
Abbreviations
- scDCDD
controlled donation after circulatory determination of death
- CDS
Carolina Donor Services (organ procurement organization)
- CPR
cardiopulmonary resuscitation
- EMS
emergency medical services
- EVLP
ex vivo lung perfusion
- HRSA
Health Resources and Services Administration
- IRI
ischemia-reperfusion injury
- med/soc
medical/social history
- NC
North Carolina
- NOK
next-of-kin
- ONT
Organización Nacional de Trasplantes (Spain)
- OPO
organ procurement organization
- UAGA
Uniform Anatomical Gift Act
- uDCDD
uncontrolled donation after circulatory determination of death
- uDCDDs
uncontrolled donation after circulatory determination of death donors
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Suzuki Y, Tiwari JL, Lee J, et al. Should we reconsider lung transplantation through uncontrolled donation after circulatory death? Am J Transplant. 2014;14:966–971. doi: 10.1111/ajt.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers FB, Shackford SR, Trevisani GT, Davis JW, Mackersie RC, Hoyt DB. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma Inj Infec Crit Care. 1995;39:860–867. doi: 10.1097/00005373-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 4.National Conference of Commissioners on Uniform State Laws. Uniform Determination of Death Act. National Conference of Commissioners on Uniform State Laws; 1980; Available from: http://www.uniformlaws.org/Act.aspx?title=Anatomical%20Gift%20Act%20%282006%29. [Google Scholar]
- 5.Bernat JL, Bleck TP, Blosser SA, et al. Circulatory death determination in uncontrolled organ donors: A panel viewpoint. Ann Emerg Med. 2014;63:384–390. doi: 10.1016/j.annemergmed.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Halpern SD. Donation after circulatory determination of death: Time for transparency. Ann Emerg Med. 2014;63:401–403. doi: 10.1016/j.annemergmed.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Wall SP, Munjal KG, Dubler NN, Goldfrank LR N.Y.C. uDCDD Study Group. Uncontrolled organ donation after circulatory determination of death: US policy failures and call to action. Ann Emerg Med. 2014;63:392–400. doi: 10.1016/j.annemergmed.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Avlonitis VS, Fisher AJ, Kirby JA, Dark JH. Pulmonary transplantation: The role of brain death in donor lung injury. Transplantation. 2003;75:1928–1933. doi: 10.1097/01.TP.0000066351.87480.9E. [DOI] [PubMed] [Google Scholar]
- 9.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53:425–435. doi: 10.1111/j.1399-6576.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Liu M, Yeung J, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 11.Dong BM, Abano JB, Egan TM. Nitric oxide ventilation of rat lungs from non-heart-beating donors improves post-transplant function. Am J Transplant. 2009;9:2707–2715. doi: 10.1111/j.1600-6143.2009.02840.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong B, Stewart PW, Egan TM. Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non–heart-beating donors. J Thorac Cardiovasc Surg. 2013;146:429.e1–436e1. doi: 10.1016/j.jtcvs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Kohmoto J, Nakao A, Stolz DB, et al. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279–2290. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 14.Wigfield CH, Love RB. Donation after cardiac death lung transplantation outcomes. Curr Opin Organ Transplant. 2011;16:462–468. doi: 10.1097/MOT.0b013e32834a99ac. [DOI] [PubMed] [Google Scholar]
- 15.Rega FR, Jannis NC, Verleden GM, Flameng WJ, Lerut TE, Van Raemdonck DE. Should we ventilate or cool the pulmonary graft inside the non-heart-beating donor? J Heart Lung Transplant. 2003;22:1226–1233. doi: 10.1016/s1053-2498(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 16.Egan TM, Lambert CJ, Jr, Reddick RL, Ulicny KS, Jr, Keagy BA, Wilcox BR. A strategy to increase the donor pool: The use of cadaver lungs for transplantation. Ann Thorac Surg. 1991;52:1113–1121. doi: 10.1016/0003-4975(91)91290-c. [DOI] [PubMed] [Google Scholar]
- 17.Ulicny KS, Jr, Egan TM, Lambert CJ, Jr, Reddick RL, Wilcox BR. Cadaver lung donors: Effect of preharvest ventilation on graft function. Ann Thorac Surg. 1993;55:1185–1191. doi: 10.1016/0003-4975(93)90031-c. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CS, D’Armini AM, Egan TM. Canine double-lung transplantation with cadaver donors. J Thorac Cardiovasc Surg. 1996;112:577–583. doi: 10.1016/S0022-5223(96)70038-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima D, Chen F, Yamada T, et al. Reconditioning of lungs donated after circulatory death with normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2012;31:187–193. doi: 10.1016/j.healun.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Burker EJ, Fingerhut D, Ebneter D, et al. Emergency medical services knowledge and attitudes about non-heart-beating donors: Effect of an educational intervention. J Heart Lung Transplant. 2015;34:204–212. doi: 10.1016/j.healun.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revised Uniform Anatomical Gift Act, N.C. Gen. Stat. § 130A-402 et seq, Stat. 130A, Article 16. 2007. Available from: http://www.ncga.state.nc.us/EnactedLegislation/Statutes/HTML/ByArticle/Chapter_130A/Article_16.html.
- 22.Chon WJ, Josephson MA, Gordon EJ, et al. When the living and the deceased cannot agree on organ donation: A survey of US Organ Procurement Organizations (OPOs) Am J Transplant. 2014;14:172–177. doi: 10.1111/ajt.12519. [DOI] [PubMed] [Google Scholar]
- 23.Truog RD, Miller FG, Halpern SD. The dead-donor rule and the future of organ donation. N Engl J Med. 2013;369:1287–1289. doi: 10.1056/NEJMp1307220. [DOI] [PubMed] [Google Scholar]
- 24.Pentz RD, Cohen CB, Wicclair M, et al. Ethics guidelines for research with the recently dead. Nat Med. 2005;11:1145–1149. doi: 10.1038/nm1105-1145. [DOI] [PubMed] [Google Scholar]