Highlights
-
•
Biohydrogen Production Processes.
-
•
Microorganisms involved in biohydrogen production processes.
-
•
Immobilization methods of microalgae.
-
•
Bioreactors for biohydrogen production process.
Keywords: Hydrogen, Algae, Biofuels, Immobilization, Bioreactors
Abstract
Multifariousness of biofuel sources has marked an edge to an imperative energy issue. Production of hydrogen from microalgae has been gathering much contemplation right away. But, mercantile production of microalgae biofuels considering bio-hydrogen is still not practicable because of low biomass concentration and costly down streaming processes. This review has taken up the hydrogen production by microalgae. Biofuels are the up and coming alternative to exhaustible, environmentally and unsafe fossil fuels. Algal biomass has been considered as an enticing raw material for biofuel production, these days photobioreactors and open-air systems are being used for hydrogen production from algal biomass. The formers allow the careful cultivation control whereas the latter ones are cheaper and simpler. A contemporary, encouraging optimization access has been included called algal cell immobilization on various matrixes which has resulted in marked increase in the productivity per volume of a reactor and addition of the hydrogen-production phase.
1. Introduction
Forthwith, the world is bearing the challenges of high energy demands as well as escalating fuel prices because of breakneck growth of world population and hasty industrialization. So there is a need of an hour to cope up with such challenges and for this researchers are now paying much appreciable attention by recommending sustainable and cost-effective methods for energy production [1], [2]. Fossil fuels are associated with the environmental pollution and thus, more efforts have been evolved in renewable energy sources being economic and environmental friendly [2]. Governments are now proactive in addressing secured supply of raw materials with limiting climate change and many potential candidate fuels have been studied in the energy area, subsequently [3], [4]. Biomass is one of the most encouraging renewable resources being used to bring about different types of biofuels, serving as biodiesel, bioethanol, biogas and biohydrogen. Energy from biomass would contribute to a stable energy supply and to local society due to an increase in commercial activities [5]. Biomass can be derived from cultivation of dedicated energy crops; by harvesting forestry and other plant residues; and from biomass wastes [6]. Hydrogen is extensively being seen as a clean fuel, environmentally safe, renewable energy resource and an excellent substitute of fossil fuels and a potential candidate with highest energy density with many of the technical, socio-economic and environmental benefits to its credence among all other known fuels (143 GJ per tonne) and is the only acknowledged fuel that does not produce carbon dioxide as a by-product when used in fuel cells for electricity generation [2], [6]. The bulkiest users of hydrogen are the fertilizer and petroleum industries with approximately 50% and 37% respectively [6]. Hydrogen production has been determined only at the laboratory scale with yield still low for commercial application and so, the optimization of design and operating parameters for maximum hydrogen production is a must step while addressing the subject of hydrogen production rate. The optimization basically counts on the microalgae strain along with the available growth conditions [7]. For biofuels to be broadly authorized in the energy merchandise, spotlight must be on acclimatizing and improving photosynthetic organisms for biofuel production [8].
By the time mentioned, the sucrose and starch crops, for instance, sugarcane and corn as well as lignocellulosic materials like rice straw and switchgrass are being used as biofuel feedstock’s. But, high cost in the hydrolysis of lignocellulosic materials is a matter of concern. Sugars come in several forms, containing approximately four calories per gram. Simple sugars like monosaccharides like glucose, fructose and galactose. Biohydrogen production offers a sustainable alternate and by utilizing renewable carbon sources can be considered as carbon dioxide offset. This can utilize various carbon sources including wastewater. Glucose, sucrose are readily degradable and hence preferred as model substrates for hydrogen production. Because of complex composition and polymeric structure complex carbon must released or converted to simple sugars. Complex polymer consists of tightly bound lignin, cellulose and hemicelluloses. Cellulose and hemicelluloses can be degraded under same conditions and add up the cost factor which is a matter of concern [2], [9]. A lot of microorganisms are involved in the production of biofuels like hydrogen, but most accepted are cyanobacteria and green microalgae freshly considered as third generation feedstock’s being more efficient at converting sunlight into the chemical energy and require a smaller footprint and less water for cultivation [7], [10], [11].
Diverse pretreatment methods and physic-chemical pretreatments have been revised for the hydrogen production. It is necessary step to breach the algal cell wall along with the complex carbohydrate to release simple sugars. Pretreatment methods such as physical (sonication, milling, grinding, pyrolysis), chemical (acid, alkali, thermal) and biological methods (enzymatic) is being employed to break algal cell wall, to hydrolyze the complex carbohydrates and to release fermentable sugars [9].
An immobilized cell means a cell by natural or artificial paths is being prevented independent movement from its neighbouring environment to all parts of the system which is under consideration [12]. Basically, there are six different types of cell immobilization methods. They are covalent coupling, affinity immobilization, adsorption, confinement in the liquid–liquid emulsion, capture behind semi-permeable membrane and entrapment. Utilization of immobilization technique contributes more resilience while designing a reactor comparing conventional suspension systems. Furthermore, increase in cell density, increase in cell wall permeability, no washout of cells and better system stability are certain additional merits of cell immobilization technique. Out of all, entrapment of cell within polymeric matrices and self adhesive attachment of cells onto surfaces of solid support are usually more common. Important criteria for successful entrapment are to set algal cells from within their partition, while pores inside gel matrix allow diffusion of substrates and metabolic products towards and from cells [13].
In these, bioreactors which are being considered for hydrogen production from algal biomass are matter of concern. Biohydrogen production by microorganisms has attracted our increasing worldwide attention, having its potential for inexhaustible, low-cost and a renewable source of energy. They are categorically pre-requisite for large scale hydrogen production by microorganisms. Certain microorganisms have been evolved for hydrogen production either from organic materials like sugar or biomass. Bioreactors are closed systems that have varied size from the small (5 mL–10 mL) to the larger scale or more than 500,000 L industrial scale. Photobioreactors are made up of an array of tubes, tanks bags, where photosynthetic microorganisms including algae are being cultivated and later monitored as light is the essential component for growing photosynthetic microorganisms. Bioreactors that have been mentioned later in this are photobioreactors, continuous stirred tank reactor (CSTR), fixed bed bioreactors, membrane bioreactors, multi-stage bioreactors and hybrid bioreactors [69].
2. Hydrogen production
2.1. Principle
Hydrogen makes up about three quarters of all matter and thus the most plentiful element of universe. Compelling hydrogen sources includes fossil fuels (95–99%) and water [14]. Dealing with future hydrogen demands independent of fossil fuels, it is necessary to appreciate all available renewable resources [15].
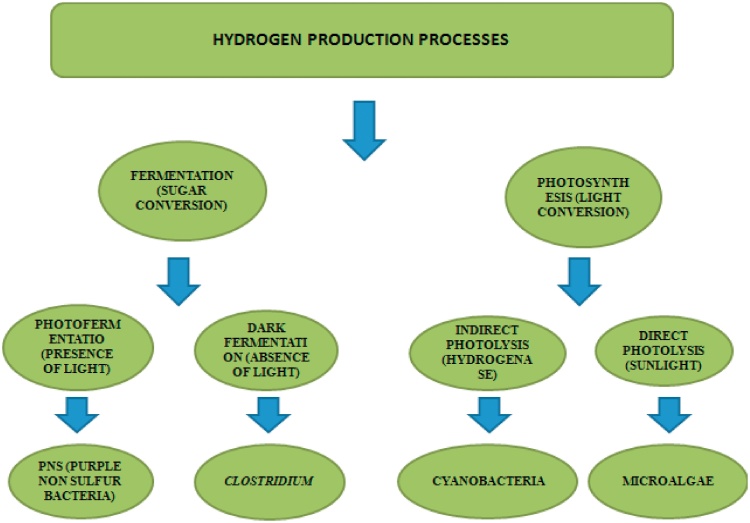
The classical methods for hydrogen production consists of steam reforming of natural gases, coal gasification and electrolysis of water are energy intensive processes that requires high temperatures (>840 °C) and are not environmental friendly as such. Electrolysis of water being the cleanest technology for hydrogen production, can only used in sectors with cheap electricity as adds up to 80% of the operating cost [3]. Fig. 1 explains hydrogen production processes with examples.
Fig. 1.
Hydrogen production methods.
2.2. Mechanism
With the noteworthy merits, the low production rates, low substrate conversion efficiencies are certain practical hindrances need to overcome for the successful hydrogen production.
2.2.1. Direct photolysis
There is a dissociation of water into hydrogen and oxygen in the presence of light, that is, H2O → H2 + ½O2 [16], [17]. Green microalgae can use light to carry out photosynthesis as they possess chlorophyll a and the photosynthetic systems: Photosystem (PS) II and Photosystem (PS) I, respectively [18], [19]. Disadvantages are the enzyme hydrogenase is very sensitive to oxygen so when a certain amount of oxygen is present, will inhibit hydrogenase activity and will stop it from producing hydrogen. Also, it requires high intensity of light. The advantages include tenfold more solar conversion in green microalgae.
2.2.2. Indirect photolysis
There is two step processes, firstly there is a splitting of water molecules in the presence of sunlight and protons and oxygen is formed. Secondly carbon dioxide fixation occurs storage carbohydrate is being produced, followed by the production of hydrogen gas by hydrogenase [20].
| 12H2O + 6CO2 + light energy → C6H12O6 + 6O2 |
| C6H12O6 + 12H2O + light energy → 12H2 + 6CO2 |
Blue-green algae (cyanobacteria) are promising microorganisms for this. Advantages are hydrogen evolution is separated from oxygen evolution. It can also produce relatively higher hydrogen yields. Furthermore, by-products can be efficiently converted to hydrogen. Disadvantages are like significant adenosine triphosphate (ATP) requirement of nitrogenase. Also, this requires continuous light source which is difficult for large scale processes [21], [22], [23], [24].
2.2.3. Dark fermentation
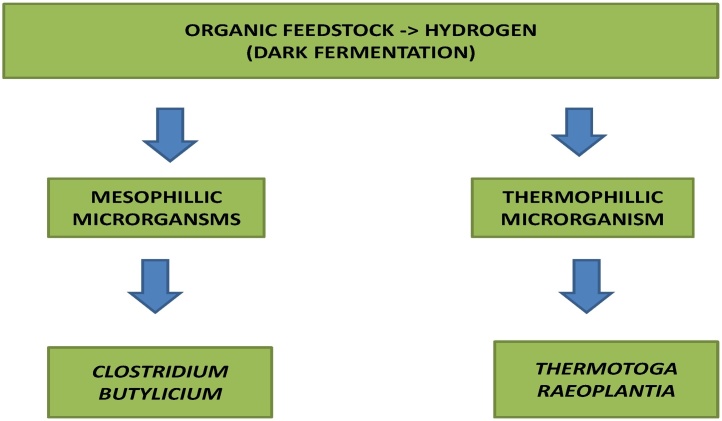
Hydrogen production in a dark environment without the presence of sunlight, water and oxygen. Fermentative microorganisms hydrolyze complex organic polymers to monomers that are further converted to a mixture of lower molecular weight organic acids and alcohols by necessary hydrogen producing bacteria [24], [25], [26]. Advantages consist of use of a variety of carbon sources and production of hydrogen without light. It produces valuable by-products like butyric acid, lactic acid and acetic acid etc. Disadvantages are relatively lower hydrogen yields. Also the product gas mixture contains carbon dioxide which has to be separated [3]. Fig. 2 explains different types of microorganisms capable of dark fermentation with examples.
Fig. 2.
Dark fermentation with different type of microorganisms.
2.2.4. Photo-fermentation
It is a fermentative conversion of organic substrates into hydrogen and carbon dioxide by use of sunlight as an energy source.
| CH3COOH + 2H2O + light → 4H2 + 2CO2 |
Using light as the energy source, the organic acid substrates are oxidized using the tricarboxylic acid cycle (TCA), producing electrons, protons and carbon dioxide. Example includes purple non sulfur bacteria (PNS) [11], [17]. Advantages in removal of environmental pollutants, use of industrial waste and use of organic acids produced from dark fermentation. Disadvantages are need to nitrogen limit condition and pretreatment of industrial effluent as it may be toxic [23].
3. Hydrogen production from algal biomass
The renewable energy sources play crucial role in decreasing the greenhouse effect but also provides an alternative approach regarding increasing global energy demands resulting into depletion of energy reserves [27]. Many algal species show potential to produce hydrogen under certain conditions [28]. Nonetheless, certain technical barriers like developing low-energy methods to harvest microalgal cells, difficulties in continuously producing biomass at a large scale, the presence of invasive species in large-scale ponds, low light penetrance in dense microalgal cultures, and the lack of cost-effective bioenergy carrier extraction techniques, are required to overcome before using microalgae as an economically viable biofuel feedstock [29].
3.1. Microrganisms
Algae are the association of organisms similar in their morphological and physiological features, that is, ability of photoautotrophic metabolic pathways, exists predominantly in water etc. Microalgae can fix carbon dioxide from the atmosphere and with this fact; they are positively characterized in terms of biomass cultivation convenience [29].
Over 40,000 species of algae have been described, and this is likely only a small fraction of the total number of available species. The U.S. Department of Energy’s Aquatic Species Program analyzed approximately 3000 different microalgae for their potential to produce biofuels [30]. Table 1 explains numerous microorganisms involved in hydrogen production from algal biomass with their processes.
Table 1.
Microorganisms used in hydrogen production.
| Microrganism | Mode of Operation | References |
|---|---|---|
| C. reinhardtii Phaeodactylum tricornutum | Genetic engineering using expressed sequence tags (ESTs) | [30] |
| Thalassiosira pseudonana | ||
| Cyanidioschyzon merolae | ||
| Ostreococcus lucimarinus | ||
| Ostreococcus tauri | ||
| Micromonas pusilla | ||
| Fragilariopsis cylindrus | ||
| Pseudo-nitzschia | ||
| Thalassiosira rotula | ||
| Chlorella vulgaris | ||
| Dunaliella salina | ||
| Micromonas pusilla | ||
| Galdieria sulphuraria | ||
| Porphyra purpurea | ||
| Volvox carteri | ||
| Aureococcus anophageferrens | ||
| Chlorella pyrenoidosa | Lipid Biosynthesis | [31] |
| Chlamydomonasmoewusii | Anaerobic Fermentation | [32] |
| Scenedesmus oblique | Anaerobic Fermentation | [33] |
| Anabaena variabilis | Photo-Fermentation | [34] |
| Rhodobactersphaeroides | Transcriptional analysis | [29] |
| Enterobacter aerogenes | Batch Fermentation | [35] |
| Clostridium butyricum | Anaerobic Fermentation | [36] |
| Bacillus coagulans | Anaerobic Fermentation | [10] |
| Clostridium acetobutylicum ATCC 824 | Anaerobic Fermentation | [37] |
| Laminaria japonica | Anaerobic Fermentation | [38] |
| Gelidium amansii | Dark Fermentation | [39] |
3.1.1. Culture conditions
Inescapably, accomplishments of hydrogen-producing bioreactor systems are governed by various factors like:
3.1.2. pH
pH has been sanctioned as one of the most important environmental factors that affects metabolic pathways and yields hydrogen. Comparative studies have shown that the ideal pH range should be around 5.2 and 6.0 [39], [40]. The effect of pH in a CSTR must be in the range of 4.0–7.0, optimum at a pH of 5.5 [41].
It has been observed hydrogen production from glucose using continuous cultures under identical conditions except pH values, and concluded that higher hydrogen yield and production rate are attained at a pH of 5.7 compared with pH 6.4 [6]. More emphasis was on the maximum hydrogen yield which was achieved only when microbial reactions followed an ethanol fermentation type that occurred at a pH of about 4.5 [42].
3.1.3. Hydraulic retention time (HRT)
Hydraulic retention time is required to prefer microbial populations with growth rates being able to catch up with mechanical dilution set up by continuous volumetric flow. It was found that shortening the hydraulic retention time from 8 h to 6 h would reduce microbial diversity leading to an increase in the hydrogen yield [43].
It was found that the methane concentration ranged between 0.0011 and 0.0058 mol l−1 at low dilution rates (D = 0.002–0.0167 h−1) was hardly measureable at higher dilutions (D > 0.075 h−1), indicating negligible methanogenic activity at high dilution rates. This means that hydraulic retention time is capable of inhibiting or terminating methanogenesis in hydrogen production via anaerobic fermentation [44].
3.1.4. Hydrogen partial pressure
The dissolved hydrogen concentration adding to the hydrogen partial pressure is the key factors affecting microbial pathways. Hydrogen production is less favourable as the hydrogen partial pressure rises. Thus it is mandatory to remove excess hydrogen from the system for maintaining hydrogen production [43], [45].
It was found an increase in the hydrogen yield from 0.85 to 1.43 mol mol−1 hexose when the reactor is sparged with nitrogen at fifteen times hydrogen produced rate [46].
3.1.5. Nutrients
For accessing optimal cell cultivation and hydrogen production nitrogen, phosphate and other inorganic trace minerals are imperative supplements for carbohydrate based feedstocks. Organic nitrogen is more favourable compared to inorganic one. Phosphate is required for optimal hydrogen production. The elements like Mg, Na, Zn and Fe are crucial supplements and have suggested an optimum nutrient formulation. Iron is of utmost importance in the enzymatic activity of hydrogen production that is [NiFe]-hydrogenases, [FeFe]-hydrogenases, and [Fe]-hydrogenase [47], [48], [49], [50], [51], [52], [53], [54].
The apical level production from sweet potato starch residue by a repeated batch culture containing C. butyricum IFO13949 when 1.0% polypepton was added as nitrogen source was reported. In contradiction, addition of urea (NH4)2SO4 or NH4Cl resulted in the absence of hydrogen evolution by the same culture [47].
3.1.6. Temperature
Microbes are competent in a temperature range of 15–85 °C but may vary under temperatures from 15 to 34 °C for mixed cultures [6], [27].
It has been considered that the hydrogen production capacity of a mixed culture under varying temperatures from 15 to 34 °C and found that hydrogen yield and specific hydrogen production rate increased with temperature, achieving respective maximum values of 359 mmol l−1 d−1 and 1.42 mol H2 mol−1 glucose at 30–34 °C and 28–32 °C respectively [6].
3.1.7. Substrate concentration
This factor, moreover, has been a point of debate. Recent studies have found that hydrogen yield to increase with increasing glucose concentration [55]. Along with substrate concentration other operating conditions like hydraulic retention time, composition of microbial cultures also affects the same [56], [57].
It was found that hydrogen yield to increase with increasing glucose concentration from 10 to 35 g l−1at a hydraulic retention time of 12 h [58] when in fact it was investigated that there is continuous hydrogen production at 12 h hydraulic retention time on 10–50 g l−1 sucrose and found that the hydrogen yield decreased from 1.7 ± 0.2 mol H2 mol−1 hexose added at 10 g l−1 sucrose to 0.8 ± 0.1 mol H2 mol l−1 hexose added at 50 g l−1 [58]. These considerations indicated that besides substrate concentration conditions as in hydraulic retention time and composition of microbial cultures also influences hydrogen production.
3.1.8. Seed culture
Clostridium and Enterobacter are extensively used as inoculums for fermentative hydrogen production [59]. A lot of studies have shown that using pure cultures of bacteria for fermentative hydrogen production were conducted in batch mode and used glucose as substrate [60], [61]. Mixed bacterial cultures from anaerobic sludge, compost and soil is used as inoculums for fermentative hydrogen production.
3.1.9. Feedstock
Simple sugars such as glucose, sucrose and lactose being biodegradable are preferred as model substrates for hydrogen production. But, the costs for pure carbohydrate sources are high on practical-scale production that is feasible on renewable and low cost sources [62], [63], [64], [65].
Proclaimed by plentiful studies of hydrogen fermentative processes, carbohydrates are the essential source of hydrogen. Along the lines, wastes and biomass rich in sugars and complex carbohydrates turn out to be the most apt feedstocks for biohydrogen generation [66].
3.2. Immobilization
The usefulness of microalgae in biotechnology has been heightened in fresh years. These organisms being involved in food, cosmetic, aquaculture, pharmaceutical industries and various other industries but small size poses an obstacle in the biotechnological applications. Cell immobilization techniques have been refined to solve above mentioned complications [4]. Immobilized algae have been used for biomass obtain and macronutrient removal. The extremely high accumulation capacity of some of these organisms for potentially dangerous substances has been also exploited for bioremediation techniques applied on polluted waters especially involving metals [67].
Environmental benefits of immobilized algal cells are not confined to pollutant removal level only. These techniques have been currently being used in the area of toxicity measurement experiments. Utmost, the immobilization techniques being devised for microorganisms in general can also be applied for microalgae, with the check of light transmission if living cells are intended to be immobilized [68]. Table 2 give examples of immobilization method and their matrix uses.
Table 2.
Immobilization methods.
| Method | Matrix | Remarks | Refrences |
|---|---|---|---|
| Encapsulation | Alginate beads | Initial cell concentration of 100 × 106 cells ml−1 of alginate and 1 × 106 cells ml−1 could be entrapped. | [69] |
| Encapsulation | Calcium alginate beads | With apt light intensity and pH of the medium for optimal values for the suspension culture the immobilized culture was evolving hydrogen for approximately three weeks of S depletion. | [70] |
| Entrapment | Alginate films | It has been observed that there were higher cell densities and a specific hydrogen production rates after the immobilization process. | [71], [72], [73], [74], [75] |
| Binding | Glass beads | Bound cells are more easily cycled between growth mode and hydrogen production mode. | [76] |
| Fumed silica is an appropriate solid support for the cells. | |||
| Neither growth nor hydrogen production is inhibited by the presence of the silica, and the cells are shown to bind to the particles. |
3.3. Bioreactor
Traditional industrial methods are quite costly. It is imaginable to design bioreactors on large scale using microorganisms as bioreactors are categorically a pre-requisite for large-scale hydrogen production by microorganisms [77], [78].
3.3.1. Photo-bioreactors
Design depends on microbiological processes linked with microalgae or cyanobacteria. With differing photochemical efficiency, absorption coefficient and size, the light regime including light and dark cycles is hypothetically is much more determining than biological factors [71]. Therefore, productivity of a photo-bioreactor is light-dependent with large surface to volume ratio as a pre-requisite. Photo-bioreactors have been designed in such a way to access an economical, rapid multiplication and high density of the microalgae culture [66], [80], [81].
3.3.2. Continuous stirred tank reactor (CSTR)
Continuous stirred tank reactors are frequently being used for hydrogen production. In a continuous stirred tank reactor, hydrogen-producing microbes are thoroughly mixed and suspended in the reactor liquid from the mixing pattern. Under this, a good substrate-microbes contact and mass transfer can be absolutely accomplished while on the other hand, the continuous stirred tank reactor is not be able to maintain high levels of fermentative biomass because of the rapid mixing operating pattern. Biomass washout is possible to occur at short hydraulic retention times (HRTs), and thus the hydrogen production rates are noticeably restricted [66], [74], [78], [79].
3.3.3. Fixed-bed bioreactor
This is negotiated with support carriers packed within the tank. The hydraulic mixing regime is less turbulent comparing with the continuous stirred tank reactor with a consequence of higher mass transfer resistance along with lowered rates of substrate conversion and hydrogen production. High hydrogen yield could not be cultivated persistently in a fixed-bed reactor as pH gradient distribution along the reactor column will cause a heterogeneous distribution of microbial activity. So, to overcome this, recirculation flow was recommended [43], [82].
3.3.4. Membrane bioreactor
Membrane bioreactor (MBR) is basically used to control biomass concentration. MBR did not exhibit superiority other high-rate hydrogen production systems. Membrane fouling and high operating cost also limit the use of membrane bioreactor process in bio hydrogen fermentation [68].
3.3.5. Multi-stage bioreactors
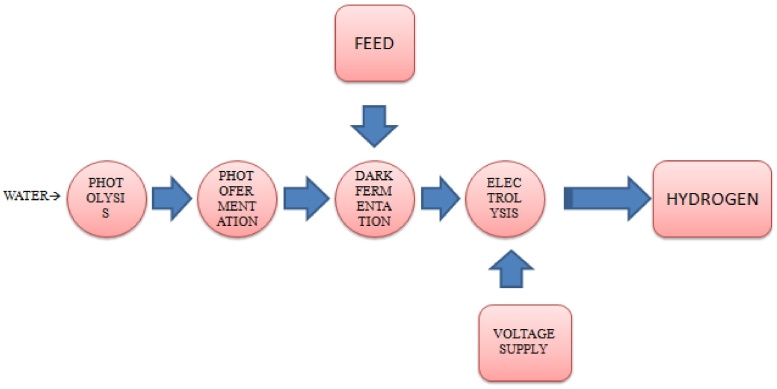
Multi-stage bioreactors consisting of three four stages have been prospective to maximize the production from the substrate [66], [75]. Sunlight is first percolated via the first stage called direct photolysis reactor where the visible light will be exploited by blue green algae whereas the unfiltered infrared light is used by photo-synthetic microbes in the second stage called photo-fermentative reactor. The effluent from the second stage along with feedstock is stuffed into a third stage called dark fermentation reactor where the bacteria convert the substrate to hydrogen and organic acids. The fourth stage is the use of a MEC which uses organic acids being generated from the dark fermentation under light-independent process. So it can be regulated during night or in low light condition [76]. Fig. 3 explains the setup of multi-stage bioreactor system.
Fig. 3.
Multi-stage bioreactor system.
3.3.6. Hybrid bioreactors
Principle of the hybrid two-stage bioreactors is bio fermentation of substrate to hydrogen and organic acids that takes place in a conventional reactor in a primary stage of process, and other gaseous energy in the mode of methane or hydrogen that is being extricated from the second stage. While optimizing gas production, a different reactor for the second stage is being operated under different conditions like higher pH and longer hydraulic retention times and is later is applied. Non-sulphur photosynthetic microbes are capable of converting organic acids to hydrogen. Combination of dark and photo-fermentation in a hybrid two-stage system can be helpful in improving the overall hydrogen yield. The synergism lies in the maximal pursuit of the substrate due to improved thermodynamics. In the first stage, the biomass is fermented to acetate, carbon dioxide and hydrogen in a thermophilic dark fermentation and in the second stage photobioreactor, acetate is converted into hydrogen and carbon dioxide [69], [83].
4. Conclusion
The fossil fuel holds back the dearth towards the 21st century due to increase in energy demand and increase in greenhouse gas emission makes it important to develop alternative energy carriers that are renewable, clean and environmentally friendly. Hydrogen holds a promising role as a future fuel and renewable energy source whereas the current classical methods of producing hydrogen are energy intensive, costly and are not environmentally friendly. Major technical objection in accomplishing practical applications of bio-hydrogen includes lowering the cost of production, delivery, storage, conversion and practical applications. Other objection of the bio-hydrogen production is unstable hydrogen production that possibly attributes towards the metabolic shift of hydrogen producing organisms. The optimization of key experimental factors, genetic modification and metabolic engineering of microalgae are the eventual approaches that make hydrogen production cost-effective and sustainable. The marine algae are considered an important biomass source; however, their utilization as an energy source is still quite low. Large number of diverse works is carried out in this area which indicates that currently algal energy is intensively developing in all directions: increase in growth rate, improving of harvesting methods, the genetic engineering of crops, optimization of chemical and thermal methods for producing biofuels due to the urgency of the energy problem: the environmental risks are associated with the use of fossil fuels and disadvantages of first and second generation biofuels. But a lot of work on improving of algae cultivation and processing mechanisms is being done to safeguard commercialization of microalgal biofuels. Jeopardy of undesirable mutations is mitigated with cell immobilization. Genetic alterations of immobilized microalgae could also help in improving hydrogen generation processes. The leakage complication is of utmost concern in cell immobilization as it forestalls the fundamental objective of demarcating viable cells in a circumscribed matrix. Research efforts have been concentrated around the design of photobioreactors with the incorporation green algae and cyanobacteria. The traditional industrial methods of hydrogen production are quite costly and the problem is to find a cheaper and easiest way for production of hydrogen.
Conflict of interest
The authors with listed names are immediately certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter mentioned above.
References
- 1.Mallick N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. BioMetals. 2002;15:377–390. doi: 10.1023/a:1020238520948. [DOI] [PubMed] [Google Scholar]
- 2.Azwar M.Y., Hussain M.A., Abdul-Wahab A.K. Development of biohydrogen production by photobiological: fermentation and electrochemical processes: a review. Renew. Sustain. Energy Rev. 2014;31:158–173. [Google Scholar]
- 3.Saifuddin N., Priatharsini P. Developments in bio-hydrogen production from algae: a review. Res. J. Appl. Sci. Eng. Technol. 2016;12:968–982. [Google Scholar]
- 4.Chaumont D. Biotechnology of algal biomass production: a review of systems for outdoor mass culture. J. Appl. Phycol. 1993;5:593–604. [Google Scholar]
- 5.Sherrif S.A., Barbir F.A., Veziroglu T.N. Principles of hydrogen energy production, storage and utilization. J. Sci. Ind. Res. 2003;62(January–Feburary):46–63. [Google Scholar]
- 6.Chang F.Y., Lin C.Y. Biohydrogen production using an up-flow anaerobic sludge blanket reactor. Int. J. Hydrogen Energy. 2004;29:33–39. [Google Scholar]
- 7.Moreno-Garrido I. Microalgae immobilization: current techniques and uses. Bioresour. Technol. 2008;99:3949–3964. doi: 10.1016/j.biortech.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Dincer I. Green methods for hydrogen production. Int. J. Hydrogen Energy. 2012;37(2):1954–1971. [Google Scholar]
- 9.Behera S., Singh R., Arora R., Sharma N.K., Shukla M., Kumar S. Scope of algae as third generation biofuels Frontiers in bioengineering and biotechnology. Mar. Biotechnol. 2015;90(2):1–13. doi: 10.3389/fbioe.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotay S.M., Das D. Microbial hydrogen production with Bacillus coagulans IIT-BT S1 isolated from anaerobic sewage sludge. Bioresour. Technol. 2007;98(6):1183–1190. doi: 10.1016/j.biortech.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Manish S., Banerjee R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy. 2008;33(1):279–286. [Google Scholar]
- 12.Kaparapu J., Geddada M.N.R. Applications of immobilized algae. J. Algal Biomass Util. 2016;7(2):122–128. [Google Scholar]
- 13.Brouers M., Shi D.J., Hall D.O. Immobilization methods for cyanobacteria in solid matrices. Methods Enzymol. 1988;167:629–636. [Google Scholar]
- 14.Wang J., Wan W. Factors influencing fermentative hydrogen production: a review. Int. J. Hydrogen Energy. 2009;34:799–811. [Google Scholar]
- 15.Rozendala R.A., Hamelersa H.V.M., Euverinkb G.J.W., Metzb S.J., Buismana C.J.N. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy. 2006;31:1632–1640. [Google Scholar]
- 16.Johnston B., Mayo M.C., Khare A. Hydrogen: the energy source for the 21st century. Technovation. 2005;25:569–585. [Google Scholar]
- 17.Akkerman I., Janssen M., Rocha J., Wijffels R.H. Photobiological hydrogen production: photochemical efficiency and bioreactor design. Int. J. Hydrogen Energy. 2002;27:1195–1208. [Google Scholar]
- 18.Ghirardi M.L., Zhang L., Lee J.W., Flynn T., Seibert M., Greenbaum E., Melis A. Microalgae: a green source of renewable hydrogen. Trends Biotechnol. 2000;18:506–511. doi: 10.1016/s0167-7799(00)01511-0. [DOI] [PubMed] [Google Scholar]
- 19.Das D., Veziroglu T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy. 2008;33:6046–6057. [Google Scholar]
- 20.Prince R.C., Kheshgi H.S. The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit. Rev. Microbiol. 2005;31:19–31. doi: 10.1080/10408410590912961. [DOI] [PubMed] [Google Scholar]
- 21.Melis A., Melnicki M.R. Integrated biological hydrogen production. Int. J. Hydrogen Energy. 2006;31:1563–1573. [Google Scholar]
- 22.Momirlan M., Veziroglu T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner. Int. J. Hydrogen Energy. 2005;30:681–808. [Google Scholar]
- 23.Mathews J., Wang G. Metabolic pathway engineering for enhanced biohydrogen production. Int. J. Hydrogen Energy. 2009;34:7404–7416. [Google Scholar]
- 24.Das D., Veziroglu T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy. 2008;33:6046–6057. [Google Scholar]
- 25.Lin C.Y., Jo C.H. Hydrogen production from sucrose using an anaerobic sequencing batch reactor process. J. Chem. Technol. Biotechnol. 2003;78:678–684. [Google Scholar]
- 26.Schara V., Maeda G.T., Wood T.K. Metabolically engineered bacteria for producing hydrogen via fermentation. Microb. Biotechnol. 2008;1(2):107–125. doi: 10.1111/j.1751-7915.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai T., Imanaka H., Nakajima A., Uwamori K., Omori Y., Fukui T., Atomi H., Imanaka T. Continuous hydrogen production by the hyperthermophilic archaeon: thermococcus kodakaraensis KOD1. J. Biotechnol. 2005;116:271–282. doi: 10.1016/j.jbiotec.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Li C.L., Fang H.H.P. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit. Rev. Environ. Sci. Technol. 2007;37:1–39. [Google Scholar]
- 29.Radakovits R., Jinkerson R.E., Darzins A., Posewitz M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell. 2010;9:486–501. doi: 10.1128/EC.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kars G., Gündüz U., Yücel M., Türker L., Eroglu I. Hydrogen production and transcriptional analysis of Nifd, Nifk and hups genes in Rhodobacter sphaeroides O.U.001 grown in media with different concentrations of molybdenum and iron. Int. J. Hydrogen Energy. 2006;31:1536–1544. [Google Scholar]
- 31.Voloshin R.A., Rodionova M.V., Zharmukhamedov S.K., Veziroglu T.N., Allakhverdiev S.I. Int. J. Hydrogen Energy. 2016;41:17257–17273. [Google Scholar]
- 32.Greenwell H.C., Laurens L.M.L., Shields R.J., Lovitt R.W., Flynn K.J. Placing microalgae on the biofuels priority list: a review of the technological challenges. J. R. Soc. Interface. 2010;7:703–726. doi: 10.1098/rsif.2009.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler M., Hemschemeier A., Gotor C., Melis A., Happe T. [Fe]-hydrogenases sin green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int. J. Hydrogen Energy. 2002;27:1431–1439. [Google Scholar]
- 34.Liu J., Bukatin V.E., Tsygankov A.A. Light energy conversion into H2 by Anabaena variabilis mutant PK84 dense cultures exposed to nitrogen limitations. Int. J. Hydrogen Energy. 2006;31:1591–1596. [Google Scholar]
- 35.Fabiano B., Perego P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy. 2002;27:149–156. [Google Scholar]
- 36.Fang H.H.P., Zhu H., Zhang T. Phototrophic hydrogen production from glucose by pure and co-cultures of Clostridium butyricum and Rhodobactersphaeroides. Int. J. Hydrogen Energy. 2006;31:2223–2230. [Google Scholar]
- 37.Zhang H., Bruns M.A., Logan B.E. Biological hydrogen production by Clostridium acetobutylicum in an unsaturated flow reactor. Water Res. 2006;40:728–734. doi: 10.1016/j.watres.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Shi X., Jung K.W., Kim D.H., Ahn Y.T., Shin H.S. Direct fermentation of Laminaria japonica for biohydrogen production by anaerobic mixed cultures. Int. J. Hydrogen Energy. 2006;36:5857–5864. [Google Scholar]
- 39.Park J.H., Yoon J.J., Park H.D., Kim Y.J., Lim D.J., Kim S.H. Feasibility of biohydrogen production from Gelidium amansii. Int. J. Hydrogen Energy. 2006;36:13997–14003. [Google Scholar]
- 40.Oh Y.K., Kim S.H., Kim M.S., Park S. Thermophilic biohydrogen production from glucose with trickling biofilter. Biotechnol. Bioeng. 2006;88:690–698. doi: 10.1002/bit.20269. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z.P., Adav S.S., Show K.Y., Tay J.H., Liang D.T., Lee D.J. Characteristics of rapidly formed hydrogen-producing granules and biofilms. Biotechnol. Bioeng. 2008;101:926–936. doi: 10.1002/bit.21956. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z.P., Show K.Y., Tay J.H., Liang T.D., Lee D.J. Enhanced continuous biohydrogen production by immobilized anaerobic microflora. Energy Fuels. 2008;22:87–92. [Google Scholar]
- 43.Fang H.H.P., Liu H. Effect of pH on hydrogen production from glucose by a mixed culture. Bio Resour. Technol. 2002;82:87–93. doi: 10.1016/s0960-8524(01)00110-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang R.N., Cao A.G., Xu J., Gao L. Bioconversion of lignocellulosic biomass to hydrogen: potential and challenges. Biotechnol. Adv. 2009;27:1051–1060. doi: 10.1016/j.biotechadv.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z.P., Show K.Y., Tay J.H., Liang D.T., Lee D.J., Jiang W.J. Effect of hydraulic retention time on biohydrogen production and anaerobic microbial community. Process Biochem. 2006;41:2118–2123. [Google Scholar]
- 46.Lo Y.C., Su Y.C., Chen C.Y., Chen W.M., Lee K.S., Chang J.S. Biohydrogen production from cellulosic hydrolysate produced via temperature-shift enhanced bacterial cellulose hydrolysis. Bioresour. Technol. 2009;100:5802–5807. doi: 10.1016/j.biortech.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 47.Tiwari M.K., Guha S., Harendranath C.S., Tripathi S. Influence of extrinsic factors on granulation in UASB reactor. Appl. Microbiol. Biotechnol. 2006;71:145–154. doi: 10.1007/s00253-006-0397-3. [DOI] [PubMed] [Google Scholar]
- 48.Mizuno O., Ohara T., Shinya M., Noike T. Characteristics of hydrogen production from bean curd manufacturing waste by anaerobic microflora. Water Sci. Technol. 2000;42:345–350. [Google Scholar]
- 49.Yokoi H., Saitsu A., Uchida H., Hirose J., Hayashi S., Takasaki Y. Microbial hydrogen production from sweet potato starch residue. J. Biosci. Bioeng. 2001;91:58–63. doi: 10.1263/jbb.91.58. [DOI] [PubMed] [Google Scholar]
- 50.Lin C.Y., Lay C.H. Effects of carbonate and phosphate concentrations on hydrogen production using anaerobic sewage sludge microflora. Int. J. Hydrogen Energy. 2004;29:275–281. [Google Scholar]
- 51.Lin C.Y., Lay C.H. A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int. J. Hydrogen Energy. 2004;30:285–292. [Google Scholar]
- 52.Shima S., Pilak O., Vogt S., Schick M., Stagni M.S., Klaucke W.M., Warkentin E., Thauer R.K., Ermler U. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science. 2008;321:572–575. doi: 10.1126/science.1158978. [DOI] [PubMed] [Google Scholar]
- 53.Bleijlevens B., Buhrke T., Linden E.V.D., Friedrich B., Albracht S.P. The auxiliary protein HypX provides oxygen tolerance to the soluble [NiFe]- hydrogenase of Ralstonia eutropha H16 by way of a cyanide ligand to nickel. J. Biol. Chem. 2004;279:46686–46691. doi: 10.1074/jbc.M406942200. [DOI] [PubMed] [Google Scholar]
- 54.Frey M. Hydrogenases: hydrogen-activating enzymes. Chem. Biochem. 2002;3:153–160. doi: 10.1002/1439-7633(20020301)3:2/3<153::AID-CBIC153>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 55.Adams M.W., Hall D.O. Purification of the membrane-bound hydrogenase of Escherichia coli. J. Biol. Chem. 1979;183:11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishihara H., Miyashita Y., Aoyama K., Kodama T., Igarashi Y., Takamura Y. Characterization of an extremely thermophilic and oxygen-stable membrane bound hydrogenase from a marine hydrogen-oxidizing bacterium Hydrogeno vibrio marinus. Biochem. Biophy. Res. Commun. 1997;232:766–770. doi: 10.1006/bbrc.1997.6369. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.H., Han S.K., Shin H.S. Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter. Process Biochem. 2006;41:199–207. [Google Scholar]
- 58.Ginkel S.V., Logan B.E. Inhibition of biohydrogen production by un-dissociated acetic and butyric acids. Environ. Sci. Technol. 2005;39:9351–9356. doi: 10.1021/es0510515. [DOI] [PubMed] [Google Scholar]
- 59.Ginkel S.W.V., Oh S.E., Logan B.E. Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy. 2005;30:1535–1542. [Google Scholar]
- 60.Kyazze G., Perez N.M., Dinsdale R., Premier G.C., Hawkes F.R., Guwy A.J., Hawkes D.L. Influence of substrate concentration on the stability and yield of continuous biohydrogen production. Biotechnol. Bioeng. 2006;93:971–979. doi: 10.1002/bit.20802. [DOI] [PubMed] [Google Scholar]
- 61.Wang J., Wan W. Factors influencing fermentative hydrogen production: a review. Int. J. Hydrogen Energy. 2009;34:799–811. [Google Scholar]
- 62.Zhang H.S., Bruns M.A., Logan B.E. Biological hydrogen production by Clostridium acetobutylicum in an unsaturated flow reactor. Water Res. 2006;40:728–734. doi: 10.1016/j.watres.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z.P., Show K.Y., Tay J.H., Liang T.D., Lee D.J. Enhanced continuous biohydrogen production by immobilized anaerobic microflora. Energy Fuels. 2008;22:87–92. [Google Scholar]
- 64.Lo Y.C., Bai M.D., Chen W.M., Chang J.S. Cellulosic hydrogen production with a sequencing bacterial hydrolysis and dark fermentation strategy. Bioresour. Technol. 2008;99:8299–8303. doi: 10.1016/j.biortech.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Lo Y.C., Su Y.C., Chen C.Y., Chen W.M., Lee K.S., Chang J.S. Biohydrogen production from cellulosic hydrolysate produced via temperature-shift enhanced bacterial cellulose hydrolysis. Bioresour. Technol. 2009;100:5802–5807. doi: 10.1016/j.biortech.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 66.Luo G., Talebnia F., Karakashev D., Xie L., Zhou Q., Angelidaki I. Enhanced bioenergy recovery from rapeseed plant in a biorefinery concept. Bioresour. Technol. 2011;102:1433–1439. doi: 10.1016/j.biortech.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 67.Nayak K.B., Roy S., Das D. Biohydrogen production from algal biomass (Anabaena sp. PCC 7120) cultivated in airlif. Photobio. Int. J. Hydrogen Energy. 2014;39:7553–7560. [Google Scholar]
- 68.Ntaikou I., Antonopoulou G., Lyberatos G. Biohydrogen production from biomass and wastes via dark fermentation: a review. Waste Biomass Valoriz. 2010;1:21–39. [Google Scholar]
- 69.Show K.Y., Lee D.J., Chang J.S. Bioreactor and process design for biohydrogen production. Bioresour. Technol. 2011;102:8524–8533. doi: 10.1016/j.biortech.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 70.Hahn J.J., Ghirardi M.L., Jacoby W.A. Immobilized algal cells used for hydrogen production. Biochem. Eng. J. 2007;37:75–79. [Google Scholar]
- 71.Laurinavichene T.V., Kosourov S.N., Ghirardi M.L., Seibert Michael, Anatoly A. Prolongation of H2 photoproduction by immobilized, sulfur-limited Chlamydomonas reinhardtii cultures. J. Biotechnol. 2008;134:275–277. doi: 10.1016/j.jbiotec.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Melis A., Zhang L., Forestier M., Ghirardi M., Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antal T.K., Kukarskikh G.P., Volgusheva A.A., Krendeleva T.E., Tyystjärvi E., Rubina A.B. Hydrogen photoproduction by immobilized S-deprived Chlamydomonas reinhardtii: Effect of light intensity and spectrum, and initial medium pH. Algal Res. 2016;17:38–45. [Google Scholar]
- 74.Kosourov S.N., Seibert M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol. Bioeng. 2009;102:50–58. doi: 10.1002/bit.22050. [DOI] [PubMed] [Google Scholar]
- 75.Kosourov S.N., Batyrova K., Petushkova E., Tsygankov A., Ghirardi M., Seibert M. Maximizing the hydrogen photoproduction yields in Chlamydomonas reinhardtii cultures: he effect of the H2 partial pressure. Int. J. Hydrogen Energy. 2012;37:8850–8858. [Google Scholar]
- 76.Moreira S.M., Santos M.M., Guilhermino L., Ribeiro R. Immobilization of the marine microalga Phaeodactylum tricornutum in alginate for in situ experiments: bead stability and suitability. Enzyme Microb. Technol. 2006;38:135–141. [Google Scholar]
- 77.Akkerman I., Janssen M., Rocha J., Wijffels R.H. Potobiological hydrogen production: photochemical efficiency and bioreactor design. Int. J. Hydrogen Energy. 2002;27:1195–1208. [Google Scholar]
- 78.Younesi H., Najafpour G., Ismail K.S.K., Mohamed A.R., Kamaruddin A.H. Biohydrogen production in a continuous stirred tank bioreactor from synthesis gas by anaerobic photosynthetic bacterium: Rhodopirillum rubrum. Bioresour. Technol. 2008;99(7):2612–2619. doi: 10.1016/j.biortech.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 79.Ding J., Wang X., Zhou X.F., Renu N.Q., Guo W.Q. CFD optimization of continuous stirred-tank (CSTR) reactor for biohydrogen production. Bioresour. Technol. 2010;101(18):7005–7013. doi: 10.1016/j.biortech.2010.03.146. [DOI] [PubMed] [Google Scholar]
- 80.Evens T.J., Chapman D.J., Robbins R.A., D’Asaro E.A. An analytical pat-plate photobioreactor with a spectrally attenuated light source for the incubation of phytoplankton under dynamic light regimes. Hydrobiologia. 2000;434:55–62. [Google Scholar]
- 81.Doenitz W.Z., Dietrich E.E., Streicher R. Electrochemical high technology for hydrogen production or direct electricity generation. Int. J. Hydrogen Energy. 1988;13:283–287. [Google Scholar]
- 82.Wang A., Sun D., Cao G., Wang H., Ren N.Q., Wu W.M., Logan B.E. Integrated hydrogen production process from cellulose by combining dark fermentation microbial fuel cells, and a microbial electrolysis cell. Bioresour. Technol. 2011;102:4137–4143. doi: 10.1016/j.biortech.2010.10.137. [DOI] [PubMed] [Google Scholar]
- 83.Markov S.A. Hydrogen production in bioreactors: current trends. Energy Procedia. 2012;29:394–400. [Google Scholar]