Abstract
AIM
To identify risk factors for the occurrence of acute kidney injury (AKI) in the postoperative period of partial hepatectomies.
METHODS
Retrospective analysis of 446 consecutive resections in 405 patients, analyzing clinical characteristics, preoperative laboratory data, intraoperative data, and postoperative laboratory data and clinical evolution. Adopting the International Club of Ascites criteria for the definition of AKI, potential predictors of AKI by logistic regression were identified.
RESULTS
Of the total 446 partial liver resections, postoperative AKI occurred in 80 cases (17.9%). Identified predictors of AKI were: Non-dialytic chronic kidney injury (CKI), biliary obstruction, the Model for End-Stage Liver Disease (MELD) score, the extent of hepatic resection, the occurrence of intraoperative hemodynamic instability, post-hepatectomy haemorrhage, and postoperative sepsis.
CONCLUSION
The MELD score, the presence of non-dialytic CKI and biliary obstruction in the preoperative period, and perioperative hemodynamics instability, bleeding, and sepsis are risk factors for the occurrence of AKI in patients that underwent partial hepatectomy.
Keywords: Kidney injury, Hepatectomy, Postoperative, Liver, Resection
Core tip: Acute kidney injury (AKI) is a serious complication after partial hepatectomy. This research aims to identify risk factors for the occurrence of AKI in the postoperative period of partial hepatectomies. The Model for End-Stage Liver Disease score, the presence of non-dialytic chronic kidney injury and biliary obstruction in the preoperative period, and perioperative hemodynamics instability, bleeding, and sepsis are risk factors for the occurrence of AKI in patients that underwent partial hepatectomy.
INTRODUCTION
Despite of the limited data regarding the occurrence of acute kidney injury (AKI) after partial hepatectomy, the reported incidence ranges from 0.9% to 15.1%[1-4]. A comprehensive analysis of the scarce data[5] is also hampered by the lack of consensus in the exact definition of AKI after liver resection.
Candidates for liver resections often present with multiple potential risk factors regarding postoperative AKI, such as excessive bleeding during the hepatectomy, and the occurrence of post-hepatectomy liver failure (PLF)[2,3,5-7]. Eventually, patients can have a combination of insults, that can be aggravated by distributive circulatory derangements by sepsis[2,3,5-8] or exposure to nephrotoxic drugs[9].
The hemodynamic changes in patients after major liver resections, mainly in patients with underlying chronic liver injury, may simulate those of patients with acute liver failure or cirrhosis[10]. Thus, the current criteria suggested by the International Club of Ascites (ICA) for definition of AKI would be the most appropriate criteria for these patients[11], since urine output measurement and static serum creatinine (sCr) levels are not included in ICA criteria.
Assuming post-operative AKI as primary endpoint, the aim of the present report was to identify the risk factors for the occurrence of this serious complication after partial hepatectomies.
MATERIALS AND METHODS
This report is based on a historical cohort study of patients who underwent partial hepatectomy from January 2008 to July 2016 at the Hepatobiliary Surgery Department of Cancer Hospital-UOPECCAN. Patients with evidence of dialytic chronic renal dialysis at the time of surgery, the need of emergency hepatectomy or patients who died at the intraoperative or immediate postoperative period (within the first 24 h after the procedure) were excluded. The study was approved by the Research Ethics Board at West Parana University (No. 1.665.135; July 2016), and the need for informed written consent was waived. The study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Preoperative data
The data collected included: Patient demographic data, preoperative use of nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme and inhibitors, the presence of comorbidities including: Non-dialytic chronic kidney disease (CKI), defined as estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2[12], liver cirrhosis with Model End- Liver Disease (MELD) score calculation[13], biliary obstruction and prior exposure to chemotherapy.
Preoperative baseline laboratory tests values were obtained from the patient electronic charts in the previous 3 mo, and in patients with more than one value, the value closest to the hospitalar admission date were selected. Laboratory tests included: Serum dosages of urea, creatinine, sodium, potassium, bilirubin, and albumin, International Normalized Ratio value, serum platelet count and eGFR value calculation according to the formula[14]:
eGFR: mL/min per 1.73 m2 = k × 186 × (sCr)-1.15 × (age)-0.203;
K = 1 (if male) or 0.72 (if female)
Intraoperative and surgical data
The surgical and anesthesic covariates recorded were: Open or laparoscopic resection, extent of liver resection (major hepatectomy was defined as resection of at least three Couinaud liver segments), resection modalities according to Brisbane nomenclature[15], type of vascular clamping of the liver (intermittent Pringle maneuver[16], continuous Pringle maneuver[17] or total vascular exclusion[18]), segment I resection, two-stage resection[19], associated extrahepatic resection, complex vascular reconstruction (portal vein, hepatic artery or hepatic veins, with or without protesis), regional lymphadenectomy (hepatic pedicle lymph nodes[20]), intraoperative transfusions of red blood cells, and intraoperative hemodynamic instability, defined as a sustained systolic blood pressure less than 90 mmHg or more than 40 mmHg below the patient’s usual systolic blood pressure during 30 min.
Postoperative data and complications
Similarly to the preoperative laboratory blood tests, we retrieved its values in the postoperative period, including the most altered values in the first 30 postoperative days.
Postoperative complications the first 30 postoperative days recorded were: Post-hepatectomy haemorrhage (PHH)[21], post-hepatectomy liver failure (PHLF)[22], biliary fistula[23], postoperative ascites, wound infection[24], pulmonary complications, including pulmonary infection[25], acute respiratory distress syndrome and acute lung injury[26], cardiovascular complications, including coronary insufficiency, cardiac arrhythmias, peripheral thrombosis, thromboembolism, and stroke[5].
The occurrence and staging of AKI were defined according to the ICA[11] criteria, although the RIFLE[27] and AKIN[28] criteria were used for comparative purposes (Table 1). The use of aminoglycosides, renal replacement therapy (hemodyalisis), the occurrence of hepatorenal syndrome (HRS)[11] and hospitalization time in days were recorded. The overall complications were classified according do Clavien-Dindo classification for postoperative complications[29].
Table 1.
Postoperative overall complications and acute kidney injury staging according to International Club of Ascites[11], risk, injury, failure, loss, end-stage[27] and Acute Kidney Injury Network[28] criteria (n = 446) n (%)
| Overall complications | 113 (25.3) |
| Overall complications (Clavien-Dindo classification) | |
| I | 46 (10.3) |
| II | 25 (5.6) |
| III a/b | 18 (4.0) |
| IV a/b | 7 (1.6) |
| V (death) | 17 (3.8) |
| AKI (ICA) | 80 (17.9) |
| I | 26 (5.8) |
| II | 21 (4.7) |
| III | 33 (7.4) |
| AKI (RIFLE) | 70 (15.7) |
| Risk | 16 (3.6) |
| Injury | 21 (4.7) |
| Failure | 33 (7.4) |
| AKI (AKIN) | 80 (17.9) |
| I | 26 (5.8) |
| II | 21 (4.7) |
| III | 32 (7.2) |
| HRS | 11 (2.5) |
| RRT (hemodyalises) | 9 (2.0) |
AKI: Acute kidney injury; ICA: International Club of Ascites; RIFLE: Risk, injury, failure, loss, end-stage; AKIN: Acute Kidney Injury Network; HRS: Hepatorenal syndrome; RRT: Renal replacement therapy.
Statistical analysis
To ensure the stability of our multivariate model, the sample size of the study was determined based on the results of a historical cohort not published in our Hepatobiliary Surgery Department, with an incidence of ARF after partial hepatectomies fixed at 18%, ensuring the adequate number of events per variable[30]. Categorical variables were expressed in absolute numbers and percentages were compared by the χ2 test or Fisher’s exact test when indicated. Continuous variables were expressed as absolute and mean ± SD, and the comparison by the Student’s t-test or non-parametric Mann-Whitney test after checking the normality assumptions by the Shapiro-Wilk test. The variables selected in the univariate model (P < 0.05) were tested in the multiple logistic regression model to identify independent binary predictors on the occurrence of postoperative AKI. The results of the model were expressed by means of the odds ratio, together with the corresponding 95%CIs and the p values of the Wald test. A value of P < 0.05 (two-tailed) was considered significant. Statistical calculations were made with the software GPower 3.0.10 and SPSS 16.0 package for Windows.
RESULTS
During the period from January 2008 to July 2016, 436 patients underwent liver resection surgery, of which 31 patients were excluded, with 405 included patients in the study for the final analysis (Figure 1).
Figure 1.
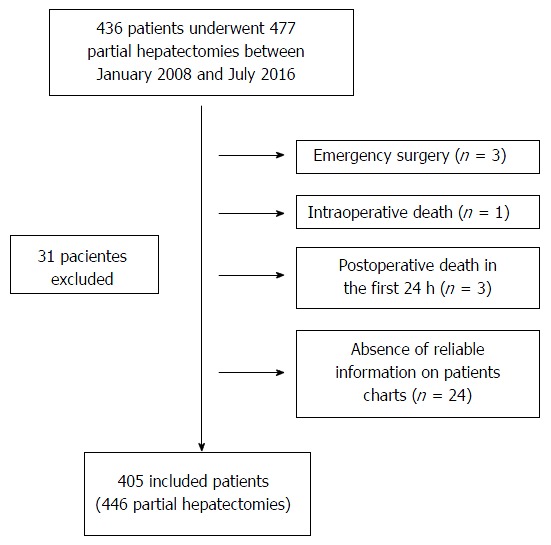
Flow chart outlining the included and excluded patients in the study.
Of the total of included patients, 271 underwent minor partial hepatectomies (60.7%) and 175 patients (39,3%) underwent major resections, and the most common resection modalities according to Brisbane nomenclature[14] were bisegmentectomy in 105 patients, segmentectomy in 103 patients, right hepatectomy in 85 patients, non-anatomical resections in 63 patients and left hepatectomy in 45 patients. The segment I were resected in 31 patients.
The most common indications for partial hepatectomy in patients with malignant tumors were colorectal cancer metastases 183 patients (41%) and hepatocellular carcinoma in 75 patients (16.8%), and patients with benign tumors were hepatic adenoma in 35 patients (7.8%) and hepatic hemangioma in 15 patients (3.4%).
Table 2 shows the clinical data of the patients prior the 466 partial hepatectomies according to the occurrence of AKI. It is observed that in the AKI group the prevalence of non-dialytic CKI and cirrhosis were higher, as well as higher MELD scores and biliary obstruction prior to partial hepatectomy. Regarding preoperative laboratory tests, the AKI group had higher bilirubin levels than non-AKI group, 2.84 ± 3.95 mg/dL vs 1.63 ± 3.05 mg/dL, respectively.
Table 2.
Preoperative patient characteristics according to the occurrence of postoperative acute kidney injury in 466 partial hepatectomies n (%)
| No AKI (n = 366) | AKI (n = 80) | P | |
| Gender, male | 180 (49.2) | 43 (53.8) | 0.269 |
| Age (years), mean (SD) | 54.6 (16.57) | 57.4 (16.10) | 0.842 |
| ACE inhibitors | 19 (5.2) | 10 (12.5) | 0.210 |
| NSAIDs | 26 (7.1) | 10 (12.5) | 0.082 |
| Non-dialytic CKI | 1 (0.27) | 8 (10.0) | < 0.001 |
| Diabetes mellitus | 44 (12.0) | 14 (17.5) | 0.121 |
| Systemic arterial hypertension | 70 (19.1) | 15 (18.8) | 0.467 |
| Preoperative chemotherapy | 85 (23.2) | 24 (30.0) | 0.402 |
| Cirrhosis | 23 (6.3) | 10 (12.5) | 0.042 |
| MELD score, mean (SD) | 7.67 (1.15) | 8.05 (1.05) | 0.020 |
| Biliary obstruction | 6 (1.6) | 13 (16.2) | < 0.001 |
| Baseline laboratory tests | |||
| Serum urea (mg/dL), mean ± SD | 31.45 ± 10.71 | 35.63 ± 23.77 | 0.021 |
| Serum creatinine (mg/dL), mean ± SD | 0.90 ± 0.71 | 0.98 ± 0.62 | 0.229 |
| eGFR (mL/min per square meter), mean ± SD | 98.38 ± 51.32 | 89.86 ± 35.46 | 0.944 |
| Sodium (mEq/L), mean ± SD | 135.67 ± 3.25 | 134.25 ± 3.00 | 0.350 |
| Potassium (mEq/L), mean ± SD | 4.44 ± 0.63 | 4.34 ± 0.75 | 0.697 |
| INR, mean ± SD | 1.13 ± 0.45 | 1.14 ± 0.20 | 0.912 |
| Bilirrubin (mg/dL), mean ± SD | 1.63 ± 3.05 | 2.84 ± 3.95 | 0.002 |
| Albumin (g/dL), mean ± SD | 3.61 ± 0.87 | 3.41 ± 0.92 | 0.505 |
| Platelets (mm3), mean ± SD | 211869.55 ± 103744.67 | 215522.81 ± 115186.57 | 0.129 |
AKI: Acute kidney injury; ACE: Angiotensin conversion enzyme; NSAIDs: Non-steroidal anti-inflamatory drugs; CKI: Chronic kidney injury; MELD: Model for End-Stage Liver Disease; INR: International normalized ratio.
Overall and renal postoperative complications rates are shown in Table 1. A total of 113 patients (25.3%) presented some type of complication, and according to the Dindo-Clavien scale, the complications grade I were the most common, occurring in 46 patients (10.3%). According to ICA criteria, AKI occurred in 80 patients (17.9%), as well as by the AKIN criteria. A slight difference in the incidence of AKI was observed according to RIFLE criteria (15.7%).
Regarding surgical and intraoperative information, patients with AKI underwent more extensive surgical procedures (major hepatectomies), and especially, had significantly higher rates of hemodynamic instability and red blood cell transfusion during liver resections than non-AKI patients, 31.2% vs 7.1% and 28.8% vs 8.5%, respectively, with P < 0.001 for both variables (Table 3).
Table 3.
Intraoperative characteristics of the 446 liver resections according to the occurrence of postoperative acute kidney injury n (%)
| No AKI (n = 366) | AKI (n = 80) | P | |
| Surgical approach | 0.071 | ||
| Open | 343 (93.7) | 79 (98.8) | |
| Laparoscopic | 23 (6.3) | 1 (1.2) | |
| Extention of resection | 0.002 | ||
| Major resection | 128 (35.2) | 45 (56.2) | |
| Minor resection | 238 (64.8) | 35 (43.8) | |
| Tumoral histology | 0.134 | ||
| Benign | 70 (19.1) | 6 (7.5) | |
| Malignant | 296 (80.9) | 74 (92.5) | |
| Segment I resection | 23 (6.3) | 8 (10.0) | 0.098 |
| Two-stage hepatectomy | 26 (7.1) | 6 (7.5) | 0.439 |
| Intermitent Pringle maneuver (15’\5’) | 124 (33.9) | 31 (38.8) | 0.438 |
| Continuous Pringle maneuver | 26 (7.1) | 10 (12.5) | 0.254 |
| Total vascular exclusion | 6 (1.6) | 2 (2.5) | 0.212 |
| Complex vascular reconstruction | 6 (1.6) | 1 (1.2) | 0.634 |
| Regional lymphadenectomy | 86 (23.5) | 25 (31.2) | 0.155 |
| Associated extrahepatic resection | 27 (7.4) | 10 (12.5) | 0.103 |
| Intraoperative instability | 26 (7.1) | 25 (31.2) | < 0.001 |
| Red blood cell transfusion | 31(8.5) | 23 (28.8) | < 0.001 |
AKI: Acute kidney injury.
According to the postoperative laboratory tests (Table 4), patients with AKI had significantly higher levels of urea and creatinine after surgery, with a significant lower eGFR, 53.73 ± 34.38 mL/min per square meter vs 83. 24 ± 60.04 mL/min per square meter (P < 0.001).
Table 4.
Postoperative laboratory tests values and complications after 466 partial hepatectomies according to the occurrence of postoperative acute kidney injury n (%)
| No AKI (n = 366) | AKI (n = 80) | P | |
| Laboratoty tests | |||
| Serum urea (mg/dL), mean ± SD | 47.61 ± 49.36 | 82.19 ± 77.45 | < 0.001 |
| Serum creatinine (mg/dL), mean ± SD | 1.29 ± 1.16 | 2.29 ± 2.21 | < 0.001 |
| eGFR (ml/min/m2), mean ± SD | 83.24 ± 60.04 | 53.73 ± 34.38 | < 0.001 |
| Sodium (mEq/L), mean ± SD | 132.88 ± 4.27 | 132.29 ± 5.55 | 0.385 |
| Potassium (mEq/L), mean ± SD | 4.86 ± 0.81 | 5.16 ± 0.94 | 0.013 |
| INR, mean ± SD | 1.82 ± 2.46 | 2.08 ± 1.161 | 0.438 |
| Bilirrubin (mg/dL), mean ± SD | 3.46 ± 4.54 | 4.54 ± 6.84 | 0.001 |
| Albumin (g/dL), mean ± SD | 2.58 ± 0.62 | 2.36 ± 0.59 | 0.069 |
| Platelets (mm3), mean ± SD | 144101.93 ± 120446.829 | 132906.89 ± 113193.18 | 0.518 |
| Aminoglycosides | 7 (1.9) | 3 (3.8) | 0.341 |
| PHLF | 7 (1.9) | 21 (26.3) | < 0.001 |
| A | 4 (1.1) | 3 (3.8) | |
| B | 3 (0.8) | 10 (12.5) | |
| C | 0 (0) | 8 (10.0) | |
| PHH | 1 (0.3) | 9 (11.3) | < 0.001 |
| A | 0 (0) | 2 (2.5) | |
| B | 0 (0) | 4 (5.0) | |
| C | 1 (0.3) | 3 (3.8) | |
| Biliary fistula | 25 (6.8) | 10 (12.5) | 0.086 |
| A | 15 (4.1) | 6 (7.5) | |
| B | 7 (1.9) | 3 (3.8) | |
| C | 3 (0.8) | 1 (1.2) | |
| Postoperative ascites | 58 (15.9) | 23 (28.8) | 0.059 |
| Wound infection | 13 (3.6) | 7 (8.8) | 0.062 |
| Pulmonary complications | 15 (4.1) | 6 (7.5) | 0.177 |
| Cardiovascular complications | 7 (1.9) | 2 (2.5) | 0.501 |
| Sepsis | 2 (0.5) | 13 (16.2) | < 0.001 |
| Hospital stay (d), mean ± SD | 6.68 ± 3.65 | 12.20 ± 9.41 | 0.008 |
AKI: Acute kidney injury; eGFR: Estimated glomerular filtration rate; PHLF: Post-hepatectomy liver failure; PHH: Post-hepatectomy haemorrhage.
In the postoperative evolution, patients with AKI had higher rates of IHPH (25%), PHH (11.2%), sepsis (16.2%) and longer hospital stay (12.20 ± 9.41 d) (Table 4). According to the univariate model (Table 5), six covariates were statistically more frequent in the AKI group and the six were confirmed in the multiple logistic regression model as predictors: MELD score, the presence of biliary obstruction and non-dialytic CKI in the preoperative period, intraoperative hemodynamic instability, and finally PHH and sepsis in the postoperative period.
Table 5.
Univariate and logistic regression analyses of risk factors for acute kidney injury
| Univariate analyses |
Multiple logistic regression |
||||
| P | OR | 95%CI | P | ||
| Extent of resection | 0.002 | 2.249 | 1.217 | 4.156 | 0.010 |
| Biliary obstruction | < 0.001 | 10.240 | 3.094 | 33.891 | < 0.001 |
| Hemodynamics instability | < 0.001 | 5.244 | 1.337 | 20.568 | 0.017 |
| Red blood cell transfusion | < 0.001 | 0.244 | |||
| Cirrhosis | 0.042 | 0.241 | |||
| MELD score | 0.020 | 4.342 | 1.347 | 15.654 | 0.046 |
| Sepsis | < 0.001 | 11.609 | 3.185 | 39.911 | < 0.001 |
| Posthepatectomy haemorrhage | < 0.001 | 12.652 | 7.769 | 53.612 | < 0.001 |
| CKI | < 0.001 | 8.975 | 1.533 | 44.675 | 0.022 |
AKI: Acute kidney injury; OR: Odds ratio; MELD: Model for End-Stage Liver Disease; CKI: Chronic kidney injury.
DISCUSSION
This study aimed to identify the main risk factors for AKI in the postoperative period of partial hepatectomies. There is a certain disparity of the available criteria for postoperative AKI definition in these situations, thus, we adopted the current criteria suggested by the ICA[11] for definition of AKI in cirrhotic patients. In patients eligible for partial hepatectomy with underlying liver diseases or who underwent major liver resections, often the both, the ICA criteria[11] do not include unreal measurements for these patients, such as static sCr measurements and urine output.
The incidence of AKI in the present study according to ICA and AKIN criteria was 17.9%, and according to RIFLE criteria was 15.7%. These AKI incidence were higher than other publications on the subject[1-5]. The AKIN and RIFLE criteria were applied for comparison, and this slight underestimation of AKI by RIFLE criteria can be probably explained by the fact that the ICA and AKIN criteria consider as stage I AKI a small increase of 0.3 mg/dL in sCr.
Including AKI, the overall complication rate in this study was 25.3%, and the mortality rate was 3.8%, that is comparable to the results of two large retrospective studies evaluating morbidity and mortality of partial hepatectomies[31,32].
The present study did not neglect the analysis of the two main AKI risk factors after partial hepatectomies, which would be perioperative bleeding and PHLF[6]. Perioperative haemorrhage with renal hypoperfusion[6], with or without the deleterious effects of blood transfusion[3], was a strong predictor of postoperative AKI in this study, reflected by intraoperative hemodynamic instability and posthepatectomy haemorrhage. An increased renal susceptibility to the perioperative renal ischemia[22-25], such as in CKI, was a predictor in the authors’ series.
Additionally, it is expected that major resections may have larger blood losses during operation and higher incidence of PHLF as well, it was corroborated by the significant influence of major resections on AKI occurrence, according to our logistic regression model. In a recent report of a large series of liver resections for hepatocellular carcinoma, major liver resection was a predictor for postoperative AKI[4].
For prevention of intraoperative bleeding, there are intraoperative maneuvers that may be crucial, such as vascular control of the liver[2] and LCVP anesthesia[1,33,34], preventing the back bleeding from hepatic veins,. The Pringle maneuver (intermittent[16] or continuous[17]) is routinely applied in liver resections at the authors’ Department, thus there was no difference between the groups, and LCVP anesthesia parameters were not evaluated.
Second factor relates to the occurrence of PLF with its distributive circulatory changes, which is a major cause of death after hepatic resection, and eventually can progress to HRS[11]. Similar to the results from a previous report[4], the MELD score[13], a usefully and extensively validated tool for predicting liver failure progression, was a predictor of postoperative AKI, and the most important, it can be applied in the preoperative period.
The presence of biliary obstruction was an independent predictor of postoperative AKI according to the authors’ results, and the mechanism by which bilirubin may be toxic to the kidneys seems to be inflammatory as well as obstructive[35], and hemodynamic changes may also play a role in biliary cast nephropathy[36]. In addition to the aforementioned effects, patients who are candidates for surgery in the presence of biliary obstruction with congestive cholestasis in the liver[37,38] may undergo major hepatic resections, with consequent decrease in the volume of a functionally deficient liver parenchyma, predisposing for PHLF.
Eventually, patients can have combinations of renal insults that can be aggravated by sepsis[2,3,5,6], which was an independent predictor in the authors’ analysis. The septicemia and its hemodynamic and systemic repercussions may eventually coexist with liver failure, often being the final event of PHLF[5].
The shortcomings of the current study, besides its retrospective nature, were the non-inclusion of anesthesic maneuvers among covariates, such was LCVP anesthesia, and the non-inclusion of hepatic steatosis, since it is a determinant of the functional quality of the parenchyma[39,40]. As mentioned, the retrospective nature of the study did not allow the authors to include non-standardized non-reliable data.
In order to reduce the incidence of postoperative AKI after partial hepatectomy, a careful patient selection and preoperative resection planning are mandatory, specially in the case of predisposing CKI, biliary obstruction and underlying cirrhosis, in which MELD score calculation can be extremely worthfull[41-43]. Measures for preventing sustained intraoperative hypotension and postoperative bleeding must be undertaken, as well as prevention and prompt treatment of sepsis. In the case of high risk patients for postoperative AKI, the nephrologist must be promptly involved in multidisciplinary discussions.
COMMENTS
Background
Acute kidney injury (AKI) is an serious complication after partial hepatectomy, however, there are limited published data regarding this subject, in addition, there is no concensus about the definition of AKI in these patients.
Research frontiers
The present study did not neglect the analysis of the two main AKI risk factors after partial hepatectomies, which would be perioperative bleeding, with or without the deleterious effects of blood transfusion, and post-hepatectomy liver failure.
Innovations and breakthroughs
The hemodynamic changes in patients after major liver resections may simulate those of patients with acute liver failure or cirrhosis. Thus, the current criteria suggested by the International Club of Ascites (ICA) for definition of AKI would be the most appropriate criteria for these patients.
Applications
In order to reduce the incidence of postoperative AKI after partial hepatectomy, a careful patient selection and preoperative resection planning are mandatory, specially in the case of predisposing CKI, biliary obstruction and underlying cirrhosis, in which Model for End-Stage Liver Disease score calculation can be extremely worthfull.
Terminology
Candidates for liver resections often present with multiple potential risk factors regarding postoperative AKI, such as excessive bleeding during the hepatectomy, and the occurrence of post-hepatectomy liver failure (PLF). The current criteria suggested by theICA for definition of AKI would be the most appropriate criteria for these patients. For prevention of intraoperative bleeding, there are intraoperative maneuvers that may be crucial, such as vascular control of the liver and low central venous pressure anesthesia. Second factor relates to the occurrence of PLF with its distributive circulatory changes, that eventually can progress to hepatorenal syndrome.
Peer-review
This paper was retrospectively analyzed the clinical data, and found some risk factors of acute kidney injury. The material was rich, the result was reasonable, and the discussion did have some valuable information.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: The study was approved by the Research Ethics Board at West Parana University (number. 1.665.135; July 2016). The study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent statement: All authors (Bredt LC, Peres LAB) declare that all involved persons (subjects or legally authorized representative) gave their informed verbal consent (in person or by phone, as appropriate) prior to study inclusion.
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Data sharing statement: All authors (Bredt LC, Peres LAB) declare that the original anonymous dataset is available on request from the corresponding author (lcbredt@gmail.com).
Peer-review started: February 17, 2017
First decision: April 14, 2017
Article in press: May 24, 2017
P- Reviewer: Chuang WL, Morales-Gonzalez JA, Prieto J, Xu Z S- Editor: Song XX L- Editor: A E- Editor: Li D
References
- 1.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB (Oxford) 2009;11:622–628. doi: 10.1111/j.1477-2574.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753–761. doi: 10.1007/s12630-015-0397-9. [DOI] [PubMed] [Google Scholar]
- 4.Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC, Compagnon P, Dhonneur G, Feray C, Azoulay D. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford) 2016;18:540–548. doi: 10.1016/j.hpb.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg. 2009;250:720–728. doi: 10.1097/SLA.0b013e3181bdd840. [DOI] [PubMed] [Google Scholar]
- 6.Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40:1221–1224. doi: 10.1016/j.transproceed.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Peres LA, Bredt LC, Cipriani RF. Acute renal injury after partial hepatectomy. World J Hepatol. 2016;8:891–901. doi: 10.4254/wjh.v8.i21.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 9.Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 10.Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, Fard N, Raisi H, Edalatpour A, Mehrabi A. Small for Size and Flow (SFSF) syndrome: An alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol. 2016;40:267–275. doi: 10.1016/j.clinre.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 12.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, Burns K, Manns B, White C, Madore F, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 14.Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, Morere JF, Beuzeboc P, Deray G; Renal Insufficiency and Cancer Medications (IRMA) Study Group. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 15.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2: 333-39. HPB (Oxford) 2002;4:99; author reply 99–100. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li AK, Mok SD. Simplified hepatectomy: the tourniquet method. Aust N Z J Surg. 1989;59:161–163. doi: 10.1111/j.1445-2197.1989.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 18.Delva E, Nordlinger B, Parc R, Lienhart A, Hannoun L, Huguet C. Hepatic vascular exclusion (HVE) for major liver resections. Int Surg. 1989;72:78–81. [PubMed] [Google Scholar]
- 19.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeck D. The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann Surg Oncol. 2003;10:1007–1011. doi: 10.1245/aso.2003.09.903. [DOI] [PubMed] [Google Scholar]
- 21.Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, Fan ST, Nimura Y, Figueras J, Vauthey JN, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB (Oxford) 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 25.Kelly E, MacRedmond RE, Cullen G, Greene CM, McElvaney NG, O’Neill SJ. Community-acquired pneumonia in older patients: does age influence systemic cytokine levels in community-acquired pneumonia? Respirology. 2009;14:210–216. doi: 10.1111/j.1440-1843.2008.01423.x. [DOI] [PubMed] [Google Scholar]
- 26.Raghavendran K, Napolitano LM. Definition of ALI/ARDS. Crit Care Clin. 2011;27:429–437. doi: 10.1016/j.ccc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 31.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708-710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–1529. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 34.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Ozturk H, Terzi A, Ozturk H, Kukner A. Effect of sirolimus on renal injury induced by bile duct ligation in rats. Acta Cir Bras. 2010;25:401–406. doi: 10.1590/s0102-86502010000500004. [DOI] [PubMed] [Google Scholar]
- 36.Padillo FJ, Cruz A, Espejo I, Barcos M, Gómez-Alvarez M, Muntané J. Alteration of the renal regulatory hormonal pattern during experimental obstructive jaundice. Rev Esp Enferm Dig. 2009;101:408–412. doi: 10.4321/s1130-01082009000600006. [DOI] [PubMed] [Google Scholar]
- 37.Cohnert TU, Rau HG, Buttler E, Hernandez-Richter T, Sauter G, Reuter C, Schildberg FW. Preoperative risk assessment of hepatic resection for malignant disease. World J Surg. 1997;21:396–400; discussion 401. doi: 10.1007/pl00012260. [DOI] [PubMed] [Google Scholar]
- 38.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 40.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 42.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 43.Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, Majno P, Mira P, Rubbia-Brandt L, Ferrero A, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointest Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]


