Abstract
Objective
To assess whether a spot urinary albumin:creatinine ratio (ACR) measured before 20 weeks of gestation can predict subsequent development of preeclampsia.
Methods
The ACR was determined from midstream urine sample taken between 17 and 20 weeks of gestation. Urine albumin was measured by immunoturbidimetric method using commercially available kit (Beckman Coulter) through Beckman AU 480 fully automated biochemistry analyzer. Urine creatinine was measured by modified kinetic Jaffe reaction without deproteinization.
Participants were then followed until delivery. Primary outcome measure was preeclampsia, secondary outcome measures were gestational hypertension, gestational diabetes mellitus, IUGR, and normal range estimate of urinary albumin-to-creatinine ratio was established.
Result
The median spot urinary albumin-to-creatinine ratio measured between 17 and 20 weeks of gestation was 5.2 mg/g of creatinine (2.5–9.6). Women who subsequently developed preeclampsia had higher spot urinary albumin-to-creatinine ratio (median 30.795 [9.7–92.8]) in comparison with women who developed gestational hypertension (median 5.2 [0.7–7.2]) and unaffected women (median 5.2 [2.5–9.6]). The urinary albumin-to-creatinine ratio of the mother who developed IUGR was significantly higher. By ROC analysis, the optimum ACR to predict preeclampsia was 9.85 mg/g of creatinine. The relative risk of developing preeclampsia in women with urinary albumin-to-creatinine ratio more than 9.85 mg/g of creatinine was higher than in the women who had urinary albumin-to-creatinine ratio less than 9.85 mg/g of creatinine.
Conclusion
A spot urinary albumin-to-creatinine ratio of more than 9.8 mg/g of creatinine can predict the development of preeclampsia in later pregnancy with the sensitivity and specificity of 67 and 76%, respectively. However, additional studies and cost–benefit analysis are required to confirm these finding before recommending this test for screening purposes.
Keywords: Preeclampsia, Proteinuria, Microalbuminuria, Pregnancy
Introduction
Preeclampsia is a major cause of perinatal and maternal morbidity and mortality. [1]. Till date, it can neither be predicted nor is any established preventive therapy available. If it is possible to predict the development of preeclampsia, appropriate monitoring can ensue as well as some evidence to support the prophylactic benefit of early introduction of aspirin, calcium and possibly heparin in high-risk prothrombotic conditions can be obtained [2–4].
The pathophysiological event resulting in preeclampsia begins early in pregnancy and precedes the onset of clinical features [5]. Most important pathophysiological hallmark is endothelial cell damage [5, 6]. Microalbuminuria is a marker of endothelial dysfunction and can be used as an early marker of endothelial dysfunction of preeclampsia, before the onset of overt syndrome as it is likely that overt proteinuria is preceded by a microalbuminuric phase. Although 24-h collection of urine is the gold standard for quantifying urine albumin excretion, it is cumbersome and results in delay of at least 24 h in diagnosis [7, 8]. Therefore, the spot urinary albumin-to-creatinine ratio has been advocated as an alternative [7, 8].
In this study, we aim to establish whether spot urinary albumin-to-creatinine ratio can predict the development of preeclampsia later on in pregnancy.
Material and Methods
This was a prospective study of 250 women with singleton pregnancy attending antenatal clinic at ESI hospital, Basaidarapur, New Delhi. All women attending antenatal clinic were asked to participate in study at the time of booking (between 12 and 20 weeks of gestation) and were recruited if they agreed. The study was approved by the regional ethics committee, and written informed consent was obtained from all participating women.
Inclusion Criteria
Singleton pregnancy.
Gestation age between 12 and 20 weeks by last menstrual period verified by ultrasound.
Urine sample provided at gestational age 12–20 weeks.
Normal renal function and no evident proteinuria upon measurement with a dipstick.
Exclusion Criteria
-
5.
Multi-fetal pregnancy.
-
6.
Women with hematuria, dipstick-positive proteinuria, ongoing urinary tract infection, acute renal failure or chronic kidney disease (CKD).
-
7.
Mental retardation or other mental disorders that raise doubts regarding the subject’s true willingness to participate in the study.
-
8.
Gestation age below 12 or above 20 weeks by last menstrual period verified by ultrasound.
-
9.
Lack of urine sample at the specified enrollment period.
-
10.
Known major fetal anomaly or fetal demise.
-
11.
Lack of demographic data.
Between 17 and 20 weeks of gestation, all women were given a sterile urine container without preservative and after instruction a midstream urinary sample was collected. Routine microscopic and spot urinary albumin-to-creatinine ratio was calculated. Participants were then followed until delivery. Primary outcome measure was preeclampsia, secondary outcome measures included were gestational hypertension, gestational diabetes mellitus (GDM), IUGR, and normal range estimate of urinary albumin-to-creatinine ratio was established.
The definition of gestational hypertension (GH) was a blood pressure of ≥140/90 mmHg occurring after 20 weeks of gestation in a previously normotensive woman [9, 10]. GDM was defined as a positive 50-g oral glucose challenge test [with a venous plasma glucose level 1 h after glucose challenge of at least 7.8 mmol/l (140 mg/dl)], and a positive 75-g oral glucose-tolerance test at 24–28 weeks of gestation [with venous plasma glucose levels of <7.8 mmol/l after an overnight fast and 7.8–11.0 mmol/l (198 mg/dl) at 2 h]. IUGR was diagnosed when the birth weight was below the tenth centile for the gestational age.
Urine albumin was measured by immunoturbidimetric method using commercially available kit (Beckman Coulter) through Beckman AU 480 fully automated biochemistry analyzer. Urine creatinine was measured by modified kinetic Jaffe reaction without deproteinization.
Statistical Method
Comparison of spot urinary albumin creatinine ratio and birthweight between unaffected and affected groups was performed by Kruskal–Wallis test. ANOVA and Chi-square test were used for comparison of continuous and categorical data between the groups. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at different values of urinary albumin creatinine ratio were calculated using receiver operating curve (ROC).
Result
After obtaining informed consent, 250 women were enrolled between 12 and 20 weeks of gestation, 10 women were excluded subsequently because of missing outcome data; therefore, 240 women were included in final analysis (Tables 1, 2). The mean maternal age was 25 years, and mean weight of the mother was 49 kg. One hundred and three (42.9%) were primigravidae. Out of 240, 6 (2.5%) developed preeclampsia and 5 developed gestational hypertension. Three out of 6 women who developed preeclampsia were nulliparous, and the other three had no history of preeclampsia. Out of 240, 6(2.5%) developed IUGR and 3 (1.5%) developed GDM.
Table 1.
Baseline clinical parameter
| Unaffected (n = 220) | Gestational hypertension (n = 5) | Preeclampsia (n = 6) | GDM (n = 3) | IUGR/OLIGOHYDROAMNIOS (n = 6) | P value | |
|---|---|---|---|---|---|---|
| Age (years) | 25 (22–29) | 25 (21–27) | 30 (20.75–32.75) | 31 (22–32) | 26.5 (23.00–28.75) | .715 |
| Weight of mother | 49 (46–56) | 58 (48–75) | 53 (48.25–58.50) | 58 (55–72) | 48.00 (41.25–51.50) | .091 |
| Gravida, n (%) | ||||||
| Primi | 94 (42.7) | 2 (40.0) | 3 (50.0) | 2 (66.7) | 2 (33.3) | .785 |
| G2 | 85 (38.6) | 3 (60.0) | 0 | 0 | 3 (50.0) | |
| G3 | 25 (11.4) | 0 | 2 (30.0) | 1 (33.3) | 1 (16.7) | |
| G4 | 13 (5.9) | 0 | 1 (20.0) | 0 | 0 | |
| G5 | 3 (1.4) | 0 | 0 | 0 | 0 | |
| SBP (mmHg) | 116 (110–122) | 126 (100–131) | 123 (113.5–127.5) | 120 (110–126) | 105 (99–118.5) | .213 |
| DBP (mmHg) | 72 (68–78) | 76 (60–82) | 75 (67.5–80.5) | 74 (58–82) | 64 (55.5–73.5) | .362 |
Table 2.
Spot urinary ACR and outcome
| Unaffected (n = 220) | Gestational hypertension (n = 5) | Preeclampsia (n = 6) | GDM (n = 3) | IUGR/OLIGOHYDROAMNIOS (n = 6) | P | |
|---|---|---|---|---|---|---|
| Child’s weight | 2.9 (2.8–3.3) | 3 (2.8–3.05) | 1.95 (1.6–2.65) | 3.1 (2.4–3.5) | 2 (1.75–2.3750) | .000 |
| Mode of delivery, n (%) | ||||||
| ND | 173 (78.6) | 4 (80.0) | 2 (33.3) | 2 (66.7) | 1 (16.7) | .001 |
| ISCS | 47 (21.4) | 1 (20.0) | 4 (66.7) | 1 (33.3) | 5 (83.3) | |
| Result of UACR | 5.25 (2.525–9.6) | 5.2 (0.65–7.23) | 30.795 (9.7250–92.850) | 0.5 (0.08–1.00) | 102.6 (5–650) | .000 |
No significant correlation was found between child’s weight and result of UACR (UA or preeclampsia or gestational hypertension) (r = −0.028; P = .673)
Significant correlation was found between child’s weight and result of UACR (all groups) (r = −0.205; P = .001)
At the time of booking, the mean systolic BP and mean diastolic BP were slightly lower in unaffected group, but the difference was not statistically significant.
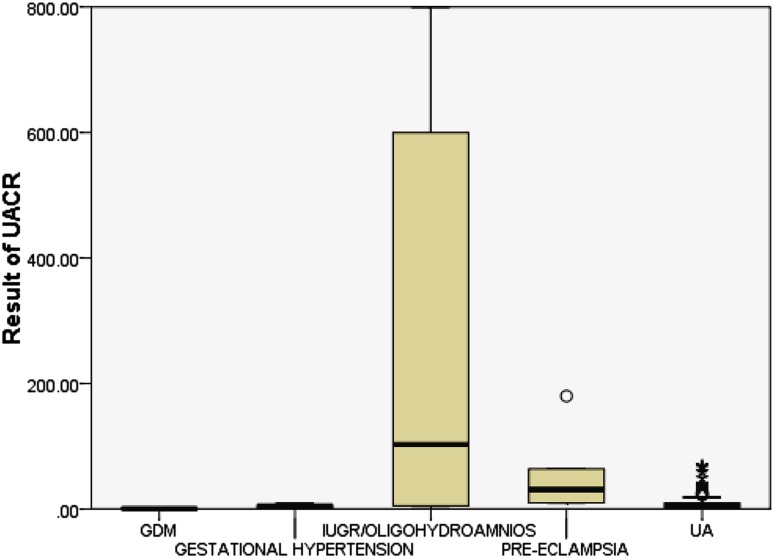
Overall, median spot urinary albumin-to-creatinine ratio measured between 17 and 20 weeks of gestation was 5.2 mg/g of creatinine (2.5–9.6). Women who subsequently developed preeclampsia had higher spot urinary albumin-to-creatinine ratio (median 30.795 [9.7–92.8]) in comparison with women who developed gestational hypertension (median 5.2 [0.7–7.2]) and unaffected women (median 5.2 [2.5–9.6]). The difference was statistically significant (Fig. 1).
Fig. 1.
Graph detailing median and IQRs of preeclampsia, unaffected (UA), GDM, gestational hypertension and IUGR groups. End of the box represents quartiles, lower end represents first quartile, and upper end represents third quartile, and the central line represents median. Lower bar joining the lower end of the box represents minimum value, and upper bar joining the upper end of the box represents the maximum value
The median urinary albumin-to-creatinine ratio of mothers who developed IUGR was 102.6 [5–650] which was statistically significant.
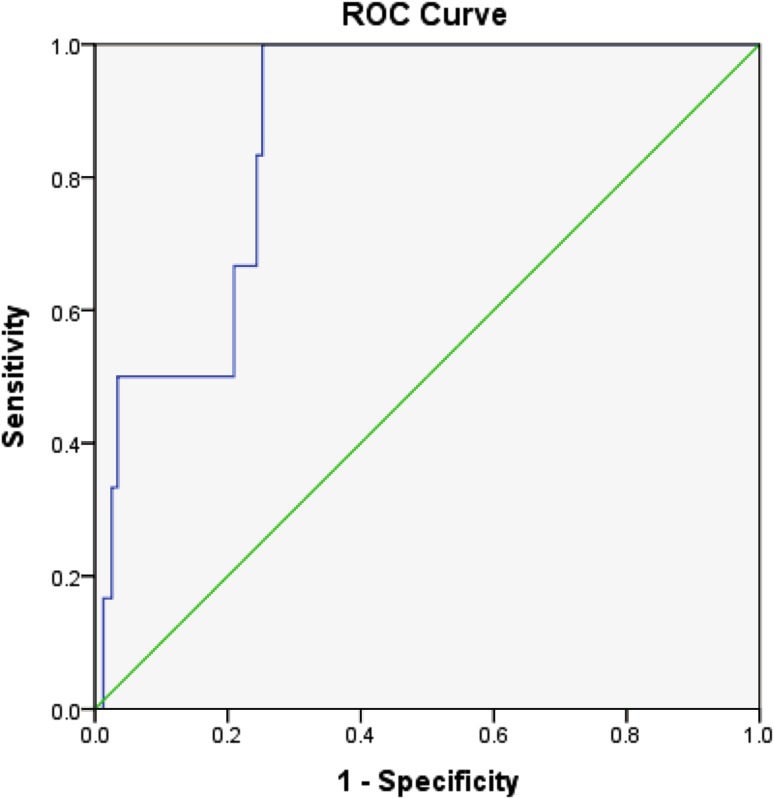
Median birth weight among the unaffected group was 2.9 kg (2.8–3.3), while in preeclampsia it was 1.9 kg (1.6–2.6 kg) and in IUGR it was 2 kg (1.75–2.3 kg) which was found to be statistically significant. The sensitivity and specificity of spot urinary albumin-to-creatinine ratio were obtained from the ROC curve. The optimum spot urinary albumin-to-creatinine ratio to predict preeclampsia was 9.85 which had a sensitivity of 67% and specificity of 76%. The area under curve was 0.879 with SE = 0.048 and 95% CI from 0.785 to 0.972. (Fig. 2).
Fig. 2.
ROC curve to predict preeclampsia (all groups). The ROC curve obtained by plot at different cutoffs is shown in above figure. A statistical software found that the area under the curve is C = 0.870 with SE = 0.046 and 95% CI from 0.779 to 0.961. The best cutoff that maximizes (sensitivity + specificity) 9.85. At this duration, the sensitivity is 0.67 and specificity is 0.76 (1—specificity = 0.24)
The relative risk of developing preeclampsia in women with urinary albumin-to-creatinine ratio more than 9.85 mg/g of creatinine was higher than the women who had urinary albumin-to-creatinine ratio less than 9.85 mg/g of creatinine.
Discussion
Preeclampsia remains a leading cause of maternal and fetal morbidity and mortality [1]. Studies have shown that alteration in the regulation and signaling of angiogenic pathway contributes to the inadequate cytotrophoblast invasion, resulting in preeclampsia. Endothelial dysfunction has been demonstrated as early as 22 weeks of gestation, and levels of antiangiogenic factors starts rising as early as 17 weeks of gestation. It could be expected that microalbuminuria, a marker of endothelial dysfunction, might also be apparent by this time.
In our study, we found that spot urinary albumin-to-creatinine ratio at 17–20 weeks of gestation was significantly higher in women who developed preeclampsia and IUGR than in those who did not.
Figure 1 shows although urinary albumin-to-creatinine ratio from preeclampsia and IUGR was significantly higher than average, unfortunately some values still fell within the normal range and this may limit the usefulness of this test in clinical practice.
Other factors that might have been relevant but were not assessed included women’s level of activity before the urine was collected. Although 24-h collection of urine is the gold standard for quantifying urinary albumin, a spot urinary albumin-to-creatinine ratio was used in this study because it was more likely to reflect clinical practice for a screening test, and because an attempted 24-h collection would very likely had been futile and erroneous [11]. Some of the past studies have shown excellent correlation between spot urinary albumin-to-creatinine ratio and albumin excretion in 24-h urine sample in normal pregnancy and preeclampsia [8, 12, 13].
Many previous studies has measured microalbuminuria in an attempt to predict preeclampsia in early pregnancy, postulating that the state of gross proteinuria is preceded by the stage of microalbuminuria. In one study, it was found that the logarithmic conversion of urinary albumin-to-creatinine ratio at 11–13 weeks of gestation helped to predict preeclampsia.
Singh et al. [14] identified the presence of microalbuminuria at 28–30 weeks of gestation to be predictive of subsequent preeclampsia with an odds ratio of 2.1 (95% CI 1.26–3.53). Another study showed that microalbuminuria at 10–12 weeks of gestation had 50% of sensitivity, 58% specificity, 50% PPV and 91% NPV for the later development of preeclampsia.
It has been shown the presence of microalbuminuria at 28–30 weeks of gestation has higher specificity and PPV when predicting preeclampsia; however, in one meta- analysis it has been documented that when spot urinary albumin- to-creatinine ratio to predict 24-h urine protein was used, no definite inference can be made. This is because only one study suggested that the most predictive value for significant proteinuria was with the DCA 2000 quantitative analyzer ([2 mg/mmol) with a sensitivity of 0.94 (95% CI 0.86–0.98) and a specificity of 0.94 (0.87–0.98) [12]. The major limitation of our study was the number of affected cases was small and the finding therefore should be interpreted with caution.
A study from Australia showed that women who developed preeclampsia had a higher albumin creatinine ratio (ACR) (median 50 mg/mmol) as compared to women who were unaffected (median 28 mg/mmol). A value of ACR > 35.5 mg/mmol in midstream sample of urine between 17 and 20 weeks predicted preeclampsia well before the onset of clinical symptoms. The urinary albumin was measured by high-performance liquid chromatography (HPLC). HPLC may not be available to clinicians in resource poor countries [13].
Conclusion
A spot urinary albumin-to-creatinine ratio of more than 9.8 mg/g of creatinine between 17 and 20 weeks of gestation can predict the development of preeclampsia in later pregnancy with the sensitivity and specificity of 67 and 76%, respectively; however additional studies and cost–benefit analysis are required to confirm these finding before recommending this test for screening purposes.
Dr. Nupur Gupta
is working as Assistant Professor and senior specialist at ESI PGIMSR, Basaidarapur, New Delhi. She has a keen interest in academics and is actively involved in CME presentations. Her area of interest is high-risk obstetrics and infertility. She is a competent doctor and engages herself in taking care of patients.
Compliance with Ethical Requirements
Conflict of interest
Dr. Nupur gupta, Dr. Taru Gupta and Dr. Deepti Asthana declare that they have no conflict of interest.
Ethical Standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Animal Rights
This article does not contain any studies with animal subjects.
Footnotes
Dr. Nupur Gupta is an Assistant Professor at ESI PGIMSR New Delhi, Dr. Taru Gupta is a Professor at ESI PGIMSR New Delhi, Dr. Deepti Asthana is a Senior Resident at ESI PGIMSR New Delhi.
References
- 1.Townsend R, O’Brien P, Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integr Blood Press Control. 2016;9:79–94. doi: 10.2147/IBPC.S77344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014;2014:297397. doi: 10.1155/2014/297397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villa PM, Kajantie E, Räikkönen K, et al. Aspirin in the prevention of pre-eclampsia in high-risk women. BJOG. 2013;120(6):773. doi: 10.1111/1471-0528.12135. [DOI] [PubMed] [Google Scholar]
- 4.Thangaratinam S, Langenveld J, Mol BW, et al. Prediction and primary prevention of pre-eclampsia. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):419–433. doi: 10.1016/j.bpobgyn.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Torrado J, Farro I, Zócalo Y, et al. Preeclampsia is associated with increased central aortic pressure, elastic arteries stiffness and wave reflections, and resting and recruitable endothelial dysfunction. Int J Hypertens. 2015;2015:720683. doi: 10.1155/2015/720683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkuchi A, Hirashima C, Takahashi K, et al. Prediction and prevention of hypertensive disorders of pregnancy. Hypertens Res. 2016. doi:10.1038/hr.2016.107. [DOI] [PubMed]
- 7.Fagerstrom P, Sallsten G, Akerstrom M, et al. Urinary albumin excretion in healthy adults: a cross sectional study of 24-hour versus timed overnight samples and impact of GFR and other personal characteristics. BMC Nephrol. 2015;16:8. doi: 10.1186/1471-2369-16-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Q, Gao Y, Yu Y, et al. Urinary spot albumin:creatinine ratio for documenting proteinuria in women with preeclampsia. Rev Obstet Gynecol. 2012;5(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- 9.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Magee LA, Pels A, Helewa M, et al. SOGC hypertension guideline committee. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(7):575–576. doi: 10.1016/S1701-2163(15)30533-8. [DOI] [PubMed] [Google Scholar]
- 11.Demirci O, Kumru P, Arınkan A, et al. Spot protein/creatinine ratio in preeclampsia as an alternative for 24-hour urine protein. Balkan Med J. 2015;32(1):51–55. doi: 10.5152/balkanmedj.2015.15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris RK, Riley RD, Doug M, Deeks JJ, Kilby MD. Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre-eclampsia: systematic review and meta-analysis. BMJ. 2012;345:e4342. doi: 10.1136/bmj.e4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baweja S, Kent A, Masterson R, et al. Prediction of pre-eclampsia in early pregnancy by estimating the spot urinary albumin: creatinine ratio using high-performance liquid chromatography. BJOG. 2011;118(9):1126–1132. doi: 10.1111/j.1471-0528.2011.02960.x. [DOI] [PubMed] [Google Scholar]
- 14.Singh H, Samal S, Mahapatro A, et al. Comparison of obstetric outcome in pregnant women with and without microalbuminuria. J Nat Sci Biol Med. 2015;6(1):120–124. doi: 10.4103/0976-9668.149112. [DOI] [PMC free article] [PubMed] [Google Scholar]