Abstract
Background
Antibiotics are frequently used in intensive care units (ICUs), and their use is associated with the emergence of bacterial resistance to antibiotics. The aim of this study was to investigate the association between the emergence of Pseudomonas aeruginosa resistance and the duration of antibiotic exposure or mode of administration in an ICU unit.
Methods
A 4-year cohort study of intensive care unit was performed in patients with P. aeruginosa isolates from clinical specimens, initially susceptible to the investigated antibiotics (piperacillin/tazobactam, ceftazidime, ciprofloxacin, meropenem and amikacin). Odds ratios (ORs) with 95% confidence interval (95% CI) of emergence of resistance were calculated using logistic regression analysis for various exposure periods to antibiotics (1–3, 4–7, 8–15 and >15 days) relative to no exposure with adjustment for age, sex, Simplified Acute Physiology Score 3 (SAPS 3) and length of stay. ORs on the emergence of P. aeruginosa resistance were also calculated for the various modes of administration.
Results
Included were 187 patients [mean age 61 years, 69% male, mean SAPS 3 score (SD): 59 (12.3)]. None of the antibiotics investigated showed the emergence of resistance within 1–3 days. Significant meropenem resistance emerged within 8–15 days [OR 79.1 (14.9–421.0)] after antibiotic exposure unlike other antibiotics (>15 days). No difference was observed between intermittent and extended administration of meropenem and between beta-lactam mono- or combined therapy.
Conclusions
Use of meropenem was associated with the emergence of resistance as soon as 8 days after exposure to the antibiotic.
Keywords: Pseudomonas aeruginosa, Antibiotic resistance, Extended infusion
Background
Pseudomonas aeruginosa is responsible for 9% of all healthcare-associated infections [1], and the resistance to P. aeruginosa is increasing [2]. Data from the European Centre for Disease Prevention and Control show that around 18% of P. aeruginosa strains are resistant to carbapenems, 15% resistant to at least three out of five antimicrobial classes with an antipseudomonal spectrum (piperacillin/tazobactam, ceftazidime, fluoroquinolones, carbapenems and aminoglycosides) and even 5% resistant to all five [3]. Antimicrobial resistance is of particular importance in the intensive care unit (ICU) because antibiotic use in this setting is extremely common [4]. Several studies have explored the risk factors for P. aeruginosa antimicrobial resistance in non-critically ill patients [5, 6]. These studies showed that among antipseudomonas antibiotics, meropenem was associated with the highest risk of resistance emergence [4, 7]. The risk factors for emerging antimicrobial-resistant Pseudomonas aeruginosa in the ICU are also worth investigating, especially to answer the question how quick the antibiotic resistance emerges after the exposure to antibiotics. Also, worthy of investigation is whether the use of extended infusion of meropenem and the combination of beta-lactam antibiotics with aminoglycosides are associated with less risk of the emergence of resistance. Extended infusion of meropenem (over 3–4 h) has been shown to improve microbiologic and clinical cure since it shows a time-dependent effect on bacterial eradication [8] but it is not clear whether extended infusion also reduces the emergence of resistance. Combination of beta-lactam antibiotics with aminoglycosides is often used in daily practice [9], for example in critically ill patients, because it increases the chance of treating the patients with one active antibiotic. Arguably, the risk of inappropriate antibiotic therapy (no active molecule) will increase in case of use of a beta-lactam alone, and inappropriate therapy is usually associated with a poor prognosis. Yet, the use of combination has not shown to have an added benefit, for example in neutropenic patients [10].
Therefore, the aim of this study was twofold: first to quantify the duration of antibiotic exposure and other risk factors for emergence of resistance and second to investigate whether the mode of administration of meropenem and combination beta-lactams and aminoglycosides were associated with lower emergence of resistance of previously susceptible P. aeruginosa.
Methods
Study setting and population
The cohort study was performed between June 2007 and June 2011 in adult patients admitted to the ICU of the Antwerp University Hospital (UZA) for at least 48 h. This ICU consists of 45 beds with approximately 2600 adult admissions yearly; it serves as tertiary care hospital in a city with the population of around 500,000 inhabitants. Included in the study were patients with >1 clinical (i.e., not screening) culture positive with P. aeruginosa from any site of the body (blood, upper (sputum) and lower respiratory tract (bronchoalveolar lavage), and wound, that were taken from a patient at least 2 days apart. Patients who were re-admitted after an initial stay in the ICU during the observed period as well as those on antibiotics prior to admission were excluded. On the included patients, we collected demographic data (age and sex), clinical characteristics (reason of ICU admission and Simplified Acute Physiology Score 3 ((SAPS 3), a validated marker of disease severity [11]), use of mechanical ventilation) and data on all antimicrobial drugs the patients received during the ICU stay (duration and mode of administration). The patients received standard dose of antibiotics or adjusted dose according to renal clearance. The standard intravenous doses were 4 g/2 g q.i.d. for piperacillin/tazobactam, 6 g o.d. for ceftazidime, 400 mg b.d. for ciprofloxacin, 1 g t.d. for meropenem and 15 mg/kg divided into three doses for amikacin. Amikacin was, apart from treatment of endocarditis with gentamicin, the only aminoglycoside used in our ICU.
Specimens
All cultures were analyzed according to standard hospital and laboratory protocol. Antibiotic susceptibility testing was performed using disk diffusion technique on Mueller–Hinton agar for piperacillin/tazobactam, ceftazidime, ciprofloxacin, meropenem and amikacin [12]. Zone diameters were interpreted according to CLSI (Clinical & Laboratory Standards Institute) guidelines (CLSI M100) as susceptible, intermediate or resistant. Intermediate isolates were considered in this present study as susceptible since the intermediate category also implies that a higher-than-normal dosage of a drug can be used [13].
Statistical analysis
All variables were assessed and described according to their distribution. Normally distributed variables were presented as mean with standard deviation (SD), and not normally distributed variables as median with range.
The outcome of the investigation was the emergence of resistance in P. aeruginosa, defined as phenotype switch of the relevant antibiotic from susceptible/intermediate at the initial culture to resistant at any follow-up culture. The determinant variable was the duration of the relevant antibiotic used by the patients. The patients were divided into groups according to the number of days of antibiotic exposure according to the literature [6, 14] as follows: not exposed, 1–3, 4–7, 8–15 and >15 days.
Goodman and Kruskal’s gamma tests were first used to investigate an association between higher categories of exposure and higher numbers of the emergence of resistance. Associations were considered significant if p-values were <0.05. To further quantify the effect of antibiotic exposure on emergence of resistance per antibiotic, logistic regression analysis was performed to calculate odds ratios (ORs) with 95% confidence interval (95% CI) of emerging resistance to an antibiotic for patients in one of the exposed groups (1–3, 4–7, 8–15 and >15 days) compared to the group without exposure with adjustment for possible confounders age, sex and SAPS 3 scores. Further adjustment was made for the length of stay. The analyses were repeated using the emergence of resistance antibiotic on the phenotype switch of any antipseudomonal antibiotic as the outcome. Re-analyses were also performed after excluding possible cross-transmission. Arbitrarily, we defined possible cross-transmission as cases with phenotype switch to identical antibiogram with a potential source patient with overlapping time periods of 30 days. Due to the small number of the included study participants, the effect of interaction between antipseudomonal antibiotics was not investigated.
To investigate the effect of meropenem administration through extended or intermittent infusion and the effect of combination therapy versus monotherapy, the ORs (95% CI) were calculated using logistic regression analysis comparing the risk of emerging resistance in one regimen versus the risk in the comparator regimen. All analyses were performed using IBM SPSS Statistics for Windows version 23.0 (IBM Corp., Armonk, NY).
Results
Demographic of study participants
We identified 405 patients with at least one positive culture with P. aeruginosa during the study period. After exclusion of patients with only one isolate (n = 168), the length of stay <48 h (n = 18) and patients re-admitted to the ICU (n = 32), 187 patients (mean age of 61 years, 69% male) were included. The flowchart of included patients is presented in Fig. 1. The included patients had mean SAPS 3 score of 59 (12.3). Most of the patients had mechanical ventilation (84.0%) and vasopressors (88.8%) (Table 1).
Fig. 1.
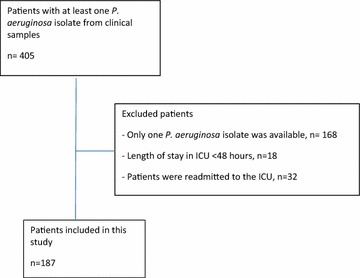
Flowchart of included patients
Table 1.
Demographic and clinical characteristics of the study cohort (n = 187)
| Characteristics | Values |
|---|---|
| Mean age (SD), years | 61 (14) |
| Male gender, n (%) | 129 (69.0) |
| Mean Simplified Acute Physiology Score 3 score (SD) | 59 (12) |
| Length of stay in intensive care unit, days, mean (range) | 29 (2–145) |
| Previous non-antipseudomonal antibiotics, n (%) | 79 (42.2) |
| Antibiotics during ICU stay | |
| Piperacillin/tazobactam, n (%) | 114 (61.0) |
| Meropenem, n (%) | 102 (54.8) |
| Ceftazidime, n (%) | 73 (39.0) |
| Amikacin, n (%) | 72 (38.5) |
| Ciprofloxacin, n (%) | 69 (36.9) |
| Types of antibiotics received | |
| None | 13 (7.4) |
| 1 | 35 (19.9) |
| 2–3 | 94 (53.5) |
| 4–5 | 34 (19.2) |
| Reason for ICU admission, n (%) | |
| Cardiovascular/vascular | 55 (29.4) |
| Respiratory | 46 (24.6) |
| Neurological | 32 (17.1) |
| Gastrointestinal | 25 (13.4) |
| Sepsis/septic shock | 15 (7.5) |
| Trauma | 10 (5.3) |
| Others | 5 (2.7) |
| Mechanical ventilation, n (%) | 160 (83.8) |
| Therapy with one or more vasopressors, n (%) | 169 (88.5) |
SD standard deviation
During the ICU admission, 96% of the patients received at least one of the antipseudomonal antibiotics. The most common antibiotics used were piperacillin/tazobactam (61.0%) and meropenem (54.8%). The median length of stay was 29 days (range 2–145 days).
From these patients, 1158 cultures were available (mean number cultures per patient 6, range 2–42). At baseline, the number of patients with an isolate susceptible to the antibiotic was 176 (94.1%) for piperacillin/tazobactam, 171 (91.4%) for ceftazidime, 133 for ciprofloxacin (71.1%), 124 (66.3%) for meropenem and 155 (82.9%) for amikacin. During the follow-up, phenotype switch from susceptible/intermediate to resistant for at least one antibiotic occurred in isolates from 38.1% of the patients during the ICU stay. During the ICU stay, 31.6% of Pseudomonas isolates became resistant to piperacillin/tazobactam, 27.5% to ceftazidime, 22.0% to meropenem, 13.3% to ciprofloxacin and 12.9% to amikacin.
Duration of antibiotics use and the emergence of resistance
Exposure to the relevant antibiotics was significantly associated with the emergence of resistance of P. aeruginosa for meropenem (p < 0.001), piperacillin/tazobactam (p = 0.006) and ceftazidime (p = 0.006). For ciprofloxacin, there was an association but did not reach statistic significant level (p = 0.06). There was no association with amikacin (p = 0.33). Other variables such as age, gender, SAPS 3 scores, previous antibiotic use, necessity use of mechanical ventilation or vasopressors and ICU length of stay were not associated with the emergence of P. aeruginosa resistance to any of the antipseudomonal antibiotics during ICU stay (data not shown).
Using antibiotics for less than 4 days was not significantly associated with emergence of resistance (Table 2). When the antibiotic was administered for longer than 15 days, we observed the emergence of resistance of P. aeruginosa to all antipseudomonal antibiotics, except to amikacin. The OR (95% CI) after adjusting for age, gender and SAPS 3 scores for the emergence of resistance for piperacillin–tazobactam was 4.7 (1.8–12.4) and for ciprofloxacin was 14.5 (2.8–75.0). Giving ceftazidime or meropenem between 8 and 15 days was associated with phenotypic switch of P. aeruginosa to these antibiotics. The ORs (95% CI) were 2.6 (1.1–6.0) and 10.0 (19.8–551.0), respectively. Further adjustment for length of stay changed the significant associations to not significant, except for meropenem (Table 2). The association between meropenem use and emergence of resistance remained even after adjustment for length of stay.
Table 2.
Odds ratios for the emergence of antipseudomonal antibiotic resistance by Pseudomonas aeruginosa in relation to antibiotic duration
| Antibiotic duration | Number of patients | Events (%) | Odds ratio (95% confidence interval) adjusted for age, gender and SAPS 3 scores | Odds ratio (95% confidence interval) adjusted for age, gender, SAPS 3 scores and length of stay |
|---|---|---|---|---|
| Piperacillin–tazobactam (n = 176) | ||||
| Reference (i.e., use of other antibiotic than piperacillin–tazobactam or no antibiotic) | 65 | 13 (21.2) | 1 | 1 |
| 1–3 days | 17 | 6 (35.3) | 2.5 (0.8–8.0) | 2.3 (0.7–8.2) |
| 4–7 days | 29 | 11 (37.9) | 2.3 (0.8–6.1) | 2.2 (0.7–6.4) |
| 8–15 days | 34 | 9 (26.5) | 1.5 (0.6–4.1) | 1.5 (0.5–4.3) |
| >15 days | 29 | 16 (55.2) | 4.7 (1.8–12.4)‡ | 1.7 (0.6–5.3) |
| Ceftazidime (n = 171) | ||||
| Reference (i.e., use of other antibiotic than ceftazidime or no antibiotic) | 99 | 22 (22.2) | 1 | 1 |
| 1–3 days | 13 | 0 | NA | NA |
| 4–7 days | 12 | 8 (66.7) | 1.7 (0.4–6.6) | 1.7 (0.4–7.4) |
| 8–15 days | 34 | 14 (41.2) | 2.6 (1.1–6.0)‡ | 1.7 (0.7–4.3) |
| >15 days | 13 | 7 (53.8) | 3.4 (1.0–12.4)‡ | 0.7 (0.2–3.4) |
| Ciprofloxacin (n = 128) | ||||
| Reference (i.e., use of other antibiotic than ciprofloxacin or no antibiotic) | 78 | 7 (9) | 1 | 1 |
| 1–3 days | 18 | 2 (5.6) | 1.5 (0.3–7.9) | 2.0 (0.3–11.7) |
| 4–7 days | 10 | 0 | 0 | 0 |
| 8–15 days | 13 | 3 (23.1) | 2.7 (0.5–13.3) | 1.4 (0.2–850) |
| >15 days | 9 | 5 (55.6) | 14.5 (2.8–75.0)‡ | 1.9 (0.2–15.6) |
| Meropenem (n = 123) | ||||
| Reference (i.e., use of other antibiotic than meropenem or no antibiotic) | 64 | 3 (4.7) | 1 | 1 |
| 1–3 days | 13 | 0 (0) | 0.0 | 0.0 |
| 4–7 days | 9 | 2 (5.6) | 4.9 (0.7–37.2) | 3.4 (0.4–30.2) |
| 8–15 days | 21 | 17 (81.0) | 104.5 (19.8–551.0)‡ | 79.1 (14.9–421.0)‡ |
| >15 days | 16 | 5 (31.2) | 10.0 (2.0–49.5)‡ | 4.6 (1.7–32.2)‡ |
| Amikacin (n = 148) | ||||
| Reference (i.e., use of other antibiotic than amikacin or no antibiotic) | 87 | 10 (11.5) | 1 | 1 |
| 1–3 days | 41 | 7 (17.1) | 1.3 (0.4–4.2) | 0.7 (0.2–2.6) |
| 4–7 days | 13 | 1 (7.7) | 0.3 (0.03–3.0) | 0.1 (0.01–2.2) |
| 8–15 days | 7 | 1 (14.3) | 0.5 (0.05–5.1) | 0.1 (0.01–2.9) |
| >15 days | 0 | 0 | 0.0 | 0.0 |
‡Statistically significant at p < 0.05
Among all antipseudomonal antibiotics, only meropenem use of 8–15 days and >15 days was associated with the emerging of resistance of any of antipseudomonal antibiotics. The ORs were 6.3 (2.4–16.8, p = 0.0001) and 2.6 (1.1–6.3, p = 0.04), respectively.
Eight patients (4.2%) were excluded due to possible cross-contamination. Re-analysis after excluding these patients did not significantly alter the results (data not shown).
Association between the mode of administration of meropenem and combination beta-lactams and aminoglycosides with emergence of resistance
Extended administration of meropenem was not associated with a lower risk of emerging P. aeruginosa resistance in comparison with intermittent administration (OR 1.1 (0.2–4.0). The proportion of patients who acquired resistance was 45.5% for extended administration (n = 46) and 43.5% for intermittent administration (n = 11).
The combination of piperacillin/tazobactam with amikacin was also not associated with a lower risk of acquiring P. aeruginosa resistance [OR 1.0 (0.4–2.5)]. The proportion of patients who acquired resistance was 37.5% for combination therapy (n = 24) and 37.2% for patients with monotherapy (n = 86).
Discussion
We observed in this study that exposure to meropenem, as short as 8 days, was already associated with emergence of resistance in Pseudomonas aeruginosa, even after adjustment for length of stay. We also found that extended infusion of meropenem and combination of beta-lactam antibiotics with amikacin did not reduce emergence of resistance. The present study used patient-based instead of surveillance-based approach. Instead of detecting the emergence of resistance in patients who endured regular screening cultures, the emergence of resistance was studied in clinical cultures. This approach is convenient in a clinical situation where surveillance cultures are not always routinely performed. The results of the present study are in line with those from surveillance-based approach studies that showed carbapenem as antibiotic class with the strongest potential for selection of resistance, for example from Carmeli et al. [15]. Unlike Carmeli and co-workers who used imipenem in their study, we used meropenem. Whether one type of carbapenem is more associated with emergence of resistance should be further explored. Earlier studies also showed that antipseudomonal antibiotics piperacillin/tazobactam, ceftazidime and ciprofloxacin were associated with the emergence of P. aeruginosa resistance in critically ill patients [7, 16, 17]. However, in our study, the significant effect disappeared after further adjustment with the length of stay, except for meropenem, which highlights meropenem as an antibiotic that selected for P. aeruginosa resistance.
Several studies have been published recently that investigated the molecular mechanisms of the resistance of P. aeruginosa to antipseudomonal antibiotics showing that mutated genes during the emergence of beta-lactam resistance differed for each antibiotic [18, 19]. Since we do not perform molecular analysis, our study does not allow the analysis of mechanisms involved in the emergence of resistance, either expression of intrinsic resistance mechanisms in response to antimicrobial exposure such as reduction in expression of the outer membrane proteins (porins) or increase production of an efflux pump to a variety of antibiotics.
To the best of our knowledge, the present study is the first to show that the emergence of resistance in initially susceptible P. aeruginosa isolates does not occur when the antibiotic was given for less than 4 days, even for meropenem. This is in line with results from Lodise et al. [6] that showed patients infected with a carbapenem-resistant P. aeruginosa were more likely to have been exposed to carbapenems for 3 days or more compared to patients infected with a carbapenem susceptible strain. The combination therapy beta-lactam and amikacin failed to decrease the emergence of resistance unlike in an in vitro study [20]. One of the possible explanations is that amikacin drug concentration may be suboptimal for P. aeruginosa in ICU patients. We did not always perform peak measurement of amikacin, and therefore, we do not know whether the pharmacokinetic/pharmacodynamic (PK/PD) target was achieved. In this study, the dose of 15 mg/kg divided into three doses was mostly used, yet some authors advised the use of 25 mg/kg/day [21].
Extended meropenem infusion was also not associated with less emergence of resistance compared with intermittent infusion. This effect may be true but alternative explanations such as optimal dosing are also possible and further study on this issue is needed.
This study has further limitations. Firstly, although using clinical specimens approach is convenient and mimic daily practice in many ICUs, this study cannot determine the exact date of phenotype switch because cultures were not regularly performed. Since the study was based on clinical specimens, the follow-up duration of the patients varied, i.e., the follow-up duration was the time frame between the first and the last positive culture for P. aeruginosa. A prospective study design with cultures taken at regular time frame will be a better study design despite in the routine practice P. aeruginosa could not always be re-isolated. Secondly, this study does not use genotyping to investigate the relatedness of the strains and to confirm the cross-transmission of resistant P. aeruginosa. However, cross-transmission estimated in this study (4.2%) is comparable with cross-transmission confirmed by genotyping (2.8%) as shown in a previous study [7], and the results of the analysis remain the same after excluding possible cross-transmission. It is also possible that several strains could be responsible for infection and this selection pressure cannot be differentiated from the emergence of resistance since no genotyping was performed. Thirdly, the results of this study might underestimate the effect since no minimum inhibitory concentration (MIC) values were available. Antipseudomonal antibiotics use might be associated with increasing MIC values that does not cause phenotypic switch on disk diffusion. Fourthly, the number of patients was relatively small and the study may be underpowered for several antibiotics that were used in low number, and lastly this study was performed in one center only.
Conclusions
The results of this study suggested that meropenem is associated with resistance of P. aeruginosa as soon after 8 days of use. This finding should be confirmed in other studies. All antipseudomonal antibiotics except amikacin are associated with the emergence of P. aeruginosa resistance. Physicians should weigh up the risk of resistance and the therapeutic benefit when meropenem or other antipseudomonal antibiotics are going to be used for a long period. Moreover, when physicians start empiric therapy with a carbapenem antibiotic, they should consider to de-escalate it within 48–72 h, once the culture results are known since the switch might be clinically beneficial for the patients.
Authors’ contributions
All authors contributed to the conception and design of the work. All authors performed the acquisition, analysis and interpretation of data. EY and BVH drafted the work. EY, BVH, WV, KW, PGJ and HG revised the work critically for important intellectual content. All authors gave final approval of the version to be published. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data can be requested from the corresponding author in SPSS format.
Ethics approval and consent to participate
The study was reviewed and approved by the hospital’s institutional review board (reference number EC110219), and informed consent was waived as the data were anonymized and gathered in a retrospective manner.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ICU
intensive care unit
- SAPS 3
Simplified Acute Physiology Score 3
- CLSI
Clinical & Laboratory Standards Institute
- SD
standard deviation
- OR
odds ratio
- 95% CI
95% confidence interval
- MIC
minimum inhibitory concentration
Contributor Information
Erlangga Yusuf, Phone: +32 (0)3 821 37 87, Email: angga.yusuf@gmail.com.
Bruno Van Herendael, Email: bruno.vanherendael@gza.be.
Walter Verbrugghe, Email: Walter.Verbrugghe@uza.be.
Margareta Ieven, Email: greet.ieven@uza.be.
Emiel Goovaerts, Email: Emiel.Goovaerts@uza.be.
Kristof Bergs, Email: Kristof.Bergs@uza.be.
Kristien Wouters, Email: kristien.wouters@uza.be.
Philippe G. Jorens, Email: Philippe.Jorens@uza.be
Herman Goossens, Email: Herman.Goossens@uza.be.
References
- 1.ECDC. European Centre for Disease Prevention and Control. Point prevalence survey of healthcareassociated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013.
- 2.ECDC. Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: European Centre for Disease Prevention and Control; 2015.
- 3.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2013. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). [Internet]. Ecdc; 2014. p. 1–211.
- 4.Cobos-Trigueros N, Solé M, Castro P, Torres JL, Hernández C, Rinaudo M, et al. Acquisition of Pseudomonas aeruginosa and its resistance phenotypes in critically ill medical patients: role of colonization pressure and antibiotic exposure. Crit Care. 2015;19:218. doi: 10.1186/s13054-015-0916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peña C, Suarez C, Tubau F, Dominguez A, Sora M, Pujol M, et al. Carbapenem-resistant Pseudomonas aeruginosa: factors influencing multidrug-resistant acquisition in non-critically ill patients. Eur J Clin Microbiol Infect Dis. 2009;28(5):519–522. doi: 10.1007/s10096-008-0645-9. [DOI] [PubMed] [Google Scholar]
- 6.Lodise TP, Miller C, Patel N, Graves J, McNutt LA. Identification of patients with Pseudomonas aeruginosa respiratory tract infections at greatest risk of infection with carbapenem-resistant isolates. Infect Control Hosp Epidemiol. 2007;28(8):959–965. doi: 10.1086/518972. [DOI] [PubMed] [Google Scholar]
- 7.Ong DS, Jongerden IP, Buiting AG, Leverstein-van Hall MA, Speelberg B, Kesecioglu J, et al. Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units. Crit Care Med. 2011;39(11):2458–2463. doi: 10.1097/CCM.0b013e318225756d. [DOI] [PubMed] [Google Scholar]
- 8.MacVane SH, Kuti JL, Nicolau DP. Prolonging β-lactam infusion: a review of the rationale and evidence, and guidance for implementation. Int J Antimicrob Agents. 2014;43(2):105–113. doi: 10.1016/j.ijantimicag.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents. 2013;41(4):301–310. doi: 10.1016/j.ijantimicag.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane database Syst Rev [Internet]. 2013;6(6):CD003038. [DOI] [PMC free article] [PubMed]
- 11.Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. CLSI document M100-S20. 2010; p. 1–153.
- 13.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev. 2007;20(3):391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. J Am Med Assoc. 2003;290(19):2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 15.Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43(6):1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georges B, Conil J-M, Dubouix A, Archambaud M, Bonnet E, Saivin S, et al. Risk of emergence of Pseudomonas aeruginosa resistance to beta-lactam antibiotics in intensive care units. Crit Care Med. 2006;34(6):1636–1641. doi: 10.1097/01.CCM.0000215517.51187.CA. [DOI] [PubMed] [Google Scholar]
- 17.Martínez JA, Delgado E, Martí S, Marco F, Vila J, Mensa J, et al. Influence of antipseudomonal agents on Pseudomonas aeruginosa colonization and acquisition of resistance in critically ill medical patients. Intensive Care Med. 2009;35(3):439–447. doi: 10.1007/s00134-008-1326-y. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Jonker MJ, Moustakas I, Brul S, ter Kuile BH. Dynamics of mutations during development of resistance by Pseudomonas aeruginosa against Five Antibiotics. Antimicrob Agents Chemother Internet. 2016;60(7):4229–4236. doi: 10.1128/AAC.00434-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solé M, Fàbrega A, Cobos-Trigueros N, Zamorano L, Ferrer-Navarro M, Ballesté-Delpierre C, et al. In vivo evolution of resistance of Pseudomonas aeruginosa strains isolated from patients admitted to an intensive care unit: mechanisms of resistance and antimicrobial exposure. J Antimicrob Chemother. 2015;70(11):3004–3013. doi: 10.1093/jac/dkv228. [DOI] [PubMed] [Google Scholar]
- 20.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(12):4920–4927. doi: 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med. 2014;40(7):998–1005. doi: 10.1007/s00134-014-3276-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be requested from the corresponding author in SPSS format.


