Abstract
The adult congenital heart disease (ACHD) population continues to grow and most cardiologists, emergency room physicians and family doctors will intermittently come into contact with these patients. Oftentimes this may be in the setting of a presentation with atrial tachyarrhythmia; one of the commonest late complications of ACHD and problem with potentially serious implications. Providing appropriate initial care and ongoing management of atrial tachyarrhythmia in ACHD patients requires a degree of specialist knowledge and an awareness of certain key issues. In ACHD, atrial tachyarrhythmia is usually related to the abnormal anatomy of the underlying heart defect and often occurs as a result of surgical scar or a consequence of residual hemodynamic or electrical disturbances. Arrhythmias significantly increase mortality and morbidity in ACHD and are the most frequent reason for ACHD hospitalization. Intra-atrial reentrant tachycardia and atrial fibrillation are the most prevalent type of arrhythmia in this patient group. In hemodynamically unstable patients, urgent cardioversion is required. Acute management of the stable patient includes anticoagulation, rate control, and electrical or pharmacological cardioversion. In ACHD, rhythm control is the preferred management strategy and can often be achieved. However, in the long-term, medication side-effects can prove problematic. Electrophysiology studies and catheter ablation are important treatments modalities and in certain cases, surgical or percutaneous treatment of the underlying cardiac defect has a role. ACHD patients, especially those with complex CHD, are at increased risk of thromboembolic events and anticoagulation is usually required. Female ACHD patients of child bearing age may wish to pursue pregnancies. The risk of atrial arrhythmias is increased during pregnancy and management of atrial tachyarrhythmia during pregnancy needs specific consideration.
Keywords: Congenital heart disease, Arrhythmia, Adult, Ablation
Core tip: This review highlights the importance of atrial tachyarrhythmia in adult congenital heart disease (ACHD) patients. It discusses causative mechanisms of arrhythmia, treatment of arrhythmia in the acute setting and on a long-term basis, including: Medications, catheter ablation, and anticoagulation. We include specific comments on the treatment of arrhythmias in ACHD patients who are pregnant.
INTRODUCTION
With an estimated incidence of 9 per 1000 live births, congenital heart disease (CHD) is the most frequent major birth defect[1]. Improved diagnosis and management have led to recent rapid growth in numbers of adult survivors, particularly in the subset of patients with complex heart lesions[2]. Individuals with adult congenital heart disease (ACHD) often have residual cardiac lesions that promote abnormal hemodynamics and electrical disturbance. Electrical abnormalities are exacerbated by surgical scar, prosthetic patches and diffuse myocardial fibrosis and increasingly as these patients age, by the additional burden of traditional cardiovascular risk factors[3,4]. It is perhaps not surprising that arrhythmia is one of the most important problems faced by ACHD patients and has become the leading cause of ACHD hospitalization[5]. In this population, atrial arrhythmia is far more common than ventricular and is associated with substantial morbidity and mortality.
Atrial arrhythmia in ACHD occurs with a prevalence 3 times greater than that seen in the general population[6]. The risk increases with age[7] and varies according to underlying congenital cardiac anomaly (Table 1[8-16]). The prevalence is greatest in those with complex defects where it is estimated at > 50% by the age of 65 years[6]. Atrial arrhythmias in ACHD can herald adverse or declining intra-cardiac hemodynamics and their occurrence is a risk factor for other significant clinical events[6,17]. Prompt diagnosis and appropriate management may help avoid important complications, the risks of which are higher in specific ACHD subgroups. In patients with simple lesions[18], atrial arrhythmia can be managed in a similar manner to arrhythmia in patients with structurally normal hearts. However, for patients with lesions of moderate or high complexity[18], involvement of an ACHD specialist and/or electrophysiologist with subspecialty expertise is recommended[17,18]. Most ACHD patients with arrhythmia will initially present locally, to general cardiologists, family physicians and emergency doctors and given population demographics, it is increasingly likely these care providers will encounter such patients. This review is intended to raise awareness of atrial arrhythmia as a complication of ACHD and of the necessary caveats to deliver care safely. It focuses on issues seen frequently in our day-to-day clinical practice and of importance to primary care providers. For a more comprehensive analysis and further reading we suggest the 2014 PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart disease[17].
Table 1.
Summary of studies describing the prevalence of arrhythmia in adult congenital heart disease
| Ref. | Diagnosis | No. of patients in study | Duration of follow-up (yr) | Prevalence of atrial arrhythmia (%) |
| [8] | Fontan | 94 | 11 | 41 |
| [9] | Fontan | 334 | 9 | 16 |
| [10] | TGA: Mustard or Senning | 86 Mustard | 8 | 48 |
| [11] | TGA: Mustard or Senning | 60 Mustard | Mustard 16 | Mustard 28.8 |
| 62 Senning | Senning 11 | Sennign 11.9 | ||
| [12] | TGA: Arterial switch | 374 | 19 | 2 |
| [13] | Ebstein anomaly | 285 | 20 | 36 |
| [14] | Tetralogy of Fallot | 242 | 16 | 12 |
| [15] | Repaired AVSD | 300 | 11 | 14 |
| [16] | Repaired ASD | 213 | 4 | 14 |
TGA: Transposition of great arteries; AVSD: Atrio-ventricular septal defect; ASD: Atrial septal defect.
CLINICAL IMPLICATIONS OF ATRIAL TACHYARRHYTHMIA IN ACHD
Given the retrospective and observational nature of most studies, teasing out “cause and effect” for the clinical implications of atrial arrhythmia in ACHD is challenging. However, it is certain that ACHD patients who develop atrial arrhythmia are at increased risk of other adverse clinical events. In their large (n > 38000) analysis of Quebec’s ACHD patients, Bouchardy et al[6] found patients with a history of atrial arrhythmia experienced a 50% increase in mortality, a 100% increase in stroke or heart failure and a 300% increase in the risk of cardiac interventions, as compared those with no history of atrial arrhythmia. These findings from a large and heterogeneous ACHD population are borne out by those from smaller studies of diagnosis-specific cohorts. Two large multi-center studies identified atrial arrhythmia as a powerful predictor of mortality and/or ventricular tachycardia in patients with tetralogy of Fallot (TOF)[19,20]. In a single-centre study of 321 Fontan patients “clinically relevant arrhythmia” was the strongest predictor of outcome, increasing the risk of death or transplant 6-fold[21]. In our own cohort of Mustard patients, patients who had experienced an atrial arrhythmia had worse subaortic right ventricular (RV) function and more tricuspid regurgitation than those who had not[22].
TYPES OF ATRIAL TACHYARRHYTHMIA IN ACHD AND THEIR MECHANISMS
Any type of atrial tachyarrhythmia can occur in ACHD patients. However, by nature of their underlying heart defects and previous surgeries, some subtypes are more frequently encountered than others. Intra-atrial reentry tachycardia (IART) is the sub-type encountered most frequently at the current time[23]. This may change as the ACHD patient population ages and increases in the incidence of atrial fibrillation have been already noted[24-26]. Atrioventricular nodal reentry, typical atrial flutter and focal atrial tachycardias[17,27] are also seen and atrial arrhythmia mediated by accessory pathway(s) is a particular idiosyncrasy of patients with Ebstein anomaly of the tricuspid valve[27].
Intra-atrial reentry tachycardia and atrial flutter
Macro reentry circuits within the atria of people without CHD usually produce typical isthmus-dependent atrial flutter, which can also occur in patients with ACHD. However, the scarred, dilated and thickened atria of ACHD patients produce additional barriers to conduction and promote macro reentry pathways independent of the tricuspid valve-inferior vena cava isthmus with low voltage electrocardiogram (ECG) signals and without the typical saw-toothed p-wave appearance of flutter waves. We refer to this type of arrhythmia as IART. With atrial rates ranging from 150-200 per minute, IART is usually slower than typical flutter and has a stable cycle length and p wave morphology[27]. Although both IART and atrial flutter can occur in ACHD and may coexist in individual patients[28,29] in our experience IART is the more frequently encountered and so we use this term preferentially when discussing atrial macro reentry in ACHD patients.
IART is the most common arrhythmia in adults with an atrial redirection procedure (Mustard or Senning operation) for transposition of the great arteries (TGA) and also in those with a Fontan circulation[23]. IART is also prevalent in patients with repaired TOF and reported to be an important cause of morbidity[25,30]. While the occurrence of atrial arrhythmia in ACHD increases with time[6] and subaortic ventricular dysfunction is a generalized risk factor[11], more specific risk factors for IART have been identified in some subgroups. In patients with TGA and an atrial redirection procedure identified IART risk factors include: A Mustard procedure[31], the occurrence of perioperative bradyarrhythmia, need for reoperation and loss of sinus rhythm during follow-up[26]. Older age at operation, history of an atrial septectomy, and an atriopulmonary connection have been identified as risk factors for IART in the Fontan population[10,32].
The propagation route for an IART circuit differs depending on the anatomic defect and type of surgical repair[33]. The pathway is often restricted to right atrial tissue, modified by regions of fibrosis from previous suture lines, patches, and baffles which act in combination with natural conduction barriers like crista terminalis, valve orifices, and the superior and inferior caval orifices to channel the wave front along a macroreentrant loop[34,35]. If a tricuspid valve is present, the isthmus between the valve ring and the inferior vena cava is often involved in such circuits, but in the absence of tricuspid valve or if there is a deformed valve, the circuit path is less predictable and can usually only be identified by electrophysiological mapping[36]. Multiple IART pathways can be present in the same patient[37].
In the setting of a healthy AV node A:V conduction may occur 1:1. If so, the resultant fast ventricular rhythm can produce hypotension, syncope, or possibly cardiac arrest in ACHD patients who often have abnormal baseline hemodynamics or impaired ventricular function[36]. Rapid conduction is of particular concern in patients with atrial baffles (Mustard and Senning) who are unable to augment sub-aortic RV filling rates and stroke volume during tachycardia[38,39] and also in patients with a single ventricle physiology. The clinical picture of instability combined with tachycardia sometimes give rise to erroneous interpretation of the rhythm as being of ventricular origin. We see this mistake made most frequently in patients with TOF, since they usually have a baseline broad RBBB which persists during atrial tachycardia.
Conversely, in patients who remain clinically stable, IART with 2:1 or 3:1 conduction can easily be misinterpreted as sinus rhythm on the surface ECG of a patient with ACHD. This is not infrequently encountered in patients with a Fontan circulation or atrial redirection procedure for TGA. These patients often experience IART with a ventricular rate only slightly above their baseline rate and may have fractionated, difficult to see p-waves. Reviewing previous ECGs is often key to establishing the correct diagnosis. Hidden p-waves may be uncovered by vagal maneuvers or intravenous adenosine infusion, which can be useful when used with caution if still uncertain[36]. A high degree of clinical suspicion is required when interpreting the ECG in an adult CHD patient who presents with palpitations, especially if that patient has a Fontan or Mustard/Senning circulation (Figures 1 and 2).
Figure 1.
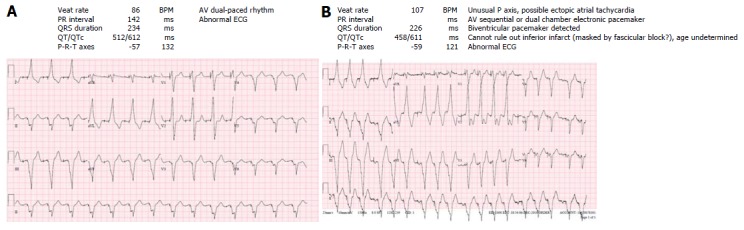
Electrocardiograms from a patient with an interatrial lateral tunnel Fontan for double inlet left ventricle. Patient has an epicardial DDI pacemaker for management of postoperative complete heart block. A: Atrio-ventricular sequentially paced rhythm. Patient feeling well; B: Grouped beating with V paced beats and intermittent p-waves. Underlying intra-atrial reentry tachycardia with AV Wenkebach demonstrated on device tracing (not shown). Patient complaining palpitations.
Figure 2.
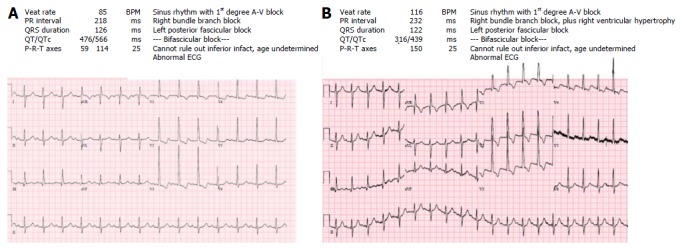
Electrocardiograms from a patient with a Mustard procedure. A: Sinus rhythm with 1st degree heart block. Patient feeling well; B: Intra-atrial reentry tachycardia with 2:1 ventricular conduction, alternate p-waves not seen as overlapping with QRS complexes. Patient complaining palpitations initially. Within 3 h unable to lie flat, requiring oxygen for desaturation and with pulmonary edema on chest X-ray. Note incorrect automated diagnosis of sinus tachycardia.
Atrial fibrillation
Atrial fibrillation is the result of multiple micro reentry circuits and in ACHD is less prevalent than IART. A single-centre study of 471 electrical cardioversions in 149 CHD patients found that 78% were for IART and 22% for AF[23]. However, the prevalence of AF is increasing as the ACHD population ages such that this diagnosis is becoming the most common atrial arrhythmia in older cohorts. A recent study of 4781 TOF patients across the age spectrum reported the average age of onset of IART as 27 years vs 44 years for AF[20]. In the same study, AF was the diagnosis in 10% of arrhythmias seen in patients born prior to 1961 vs < 1% patients born after 1981[20]. In another cohort of TOF patients AF was the most prevalent atrial arrhythmia after 55 years of age[25]. The increasing risk of AF with age relates to the underlying mechanism, which is usually chronic hemodynamic atrial stress and remodeling as well as increase in conventional risk factors such as hypertension, diabetes mellitus, obesity, and sleep apnea. Patients with residual AV valve regurgitation or left-sided obstructive lesions as well as those with unrepaired, palliated CHD are particularly vulnerable to AF[23,24]. Patients who have undergone ablation for IART may go on to experience late atrial fibrillation in follow-up. During follow-up approximately 30% of patients with surgically closed ASD who had previously undergone successful catheter ablation for IART develop AF[40].
Atrioventricular reentrant tachycardia
Wolff-Parkinson-White syndrome (WPW) occurs in 20% of cases with Ebstein anomaly of tricuspid valve[41]. The accessory pathway in Ebstein anomaly is usually located along the posterior and septal aspect of the tricuspid ring where the valve leaflets are most abnormal, and about half of these patients have multiple accessory pathways[42,43]. Ebstein-like malformation of a left-sided tricuspid valve is common in congenitally corrected TGA and often associated with accessory pathway(s)[36]. Tachycardia events for Ebstein patients are of concern in adult years when there is increased likelihood of coexisting atrial fibrillation and 1:1 anterograde conduction over the accessory pathway[36].
MANAGEMENT OF ACUTE ATRIAL ARRHYTHMIAS IN ACHD PATIENTS
Acute management of atrial tachyarrhythmia in hemodynamically stable patients includes anticoagulation to prevent thromboembolism, rate control, and cardioversion to restore sinus rhythm. In ACHD patients with simple anatomy and an atrial arrhythmia of ≥ 48 h or unknown duration, ≥ 3 wk of anticoagulation or transesophageal echocardiography (TEE) is recommended prior to cardioversion to rule out intra-cardiac thrombus[17]. In moderate and complex patients who are at higher risk of thrombosis formation, TEE or 3 wk of anticoagulation is recommended prior to cardioversion, even if the IART or atrial fibrillation is less than 48 h in duration[17,44]. In our own practice, we are reluctant to leave Fontan patients or those with a systemic right ventricle in an atrial tachyarrhythmia for a prolonged period of time, for fear of deteriorating hemodynamics and/or ventricular function. Therefore we rarely choose to anticoagulate and wait three weeks. In high-risk patients we prefer to perform TEE or low dose cardiac CT promptly followed by cardioversion within a day or two of diagnosis. Pharmacologic rate control can be useful for those who have a rapid ventricular response during their tachyarrhythmia while cardioversion is being planned. Options include use of beta-blockers, calcium channel blockers, or amiodarone[45].
Termination of atrial tachyarrhythmia can be achieved by electrical cardioversion, overdrive pacing, or drug therapy. Urgent electrical cardioversion is recommended in adults with ACHD who are hemodynamically unstable regardless of arrhythmia duration or anticoagulation status[45]. Anterior-posterior pad positioning increases cardioversion success in the face of marked atrial dilatation[17] and is important in the many ACHD patients who have a pacemaker. Patients with Fontan palliation, Mustard or Senning, and Glenn shunts, are at risk of sinus node dysfunction and may develop prolong sinus pause following rhythm conversion[17,9,46]. The team planning a cardioversion needs to be prepared for this possibility. Patients with an extra-cardiac or interatrial lateral tunnel Fontan circulation do not have ready venous access to enable ventricular pacing. The back-up plan for these patients should include percutaneous pacing and/or use of medications such as atropine or isopretrenol. Electrical cardioversion of complex CHD patients is best performed in the coronary care unit with continuous monitoring of heart rhythm post cardioversion till rhythm stability is established. Overdrive pacing of IART can be attempted in patients with either atrial or dual chamber pacemakers or defibrillators. Immediate anti-tachycardia pacing is effective in up to 50% of cases[47]. In pacemaker dependent patients, it is important to maintain back up ventricular pacing during attempted atrial overdrive pacing[17].
Pharmacologic cardioversion can be used to terminate atrial arrhythmias acutely, however, concerns include risk of torsades de pointes in Class III and ventricular tachycardia in Class IA and IC antiarrhythmic medications[17]. Amiodarone is the medication we use most often for acute termination of atrial arrhythmias in ACHD patients. Cardioversion can sometimes be achieved by following an intravenous bolus dose with a maintenance infusion for 24-48 h. Amiodarone may restore sinus rhythm, or assist in slowing the ventricular rate if cardioversion fails. There are currently no efficacy or safety data regarding the acute use of amiodarone in treatment of atrial tachyarrhythmia in ACHD patients[17]. Ibutilide and sotalol have also been shown as effective in acute treatment of atrial tachyarrhythmia[48,49]. When compared in a randomized study in non-ACHD population, ibutilide was superior to sotalol in conversion of atrial arrhythmia[50]. If using ibutilide, one must be cautious of torsades de pointes which is reported to occur in 2%-8% of non-ACHD patients[51]. In ACHD patients presenting with IART or atrial fibrillation, 1 to 2 mg of IV ibutilide over 10 min may be given if used with continuous heart monitoring where emergency defibrillation and resuscitation is available[17].
Patients with WPW, orthodromic atrioventricular reentrant tachycardia and AVNRT, who are hemodynamically stable can be treated with intravenous adenosine, which may terminate the tachycardia and restore sinus rhythm. This is because adenosine slows AV nodal conduction and these tachycardias include the AV node as an obligatory part of their circuit. In contrast, the effects of adenosine on atrial flutter, IART and atrial ectopic tachycardia are inconsistent[52]. Adenosine will not generally terminate these arrhythmias and is more likely to produce transient or increased AV block, which can unmask atrial activity on an ECG and aid diagnosis. It is important that adenosine be given rapidly and in a sufficient dose to reach the coronary circulation. Adenosine administration is generally safe but rare proarrhythmic and potentially life-threating effects have been reported[53]. It can precipitate atrial fibrillation, which as already stated, is a risk in patients with an accessory pathway where the atrial rate may be conducted 1:1 to the ventricles. Patients with pre-excited atrial fibrillation are usually treated with urgent electrical cardioversion because of the risk of cardiovascular collapse[54].
MANAGEMENT OF CHRONIC OR RECURRENT ATRIAL ARRHYTHMIAS IN ACHD PATIENTS
Medical management
Rhythm control is generally recommended as the initial strategy for patients with moderate or complex forms of CHD[17]. This is because loss of sinus rhythm, even with a controlled heart rate can adversely and importantly impact both hemodynamics and ventricular function in ACHD patients[17,27]. However, once an ACHD patient has experienced atrial arrhythmia, recurrences are often seen. In our experience, the initiation of antiarrhythmic drugs, before further cardioversion may be beneficial to the chances of reestablishing sinus rhythm and/or reducing the frequency of recurrence.
The pro-arrhythmic risk of Class I (fast sodium channel blockers) antiarrhythmic drugs in patients with CHD includes ventricular arrhythmias[55,56]. In addition, they are not recommended for maintenance of sinus rhythm in patients with coronary artery disease or moderately to severe systolic dysfunction of either ventricle[17,45].
In general cardiology practice, sotalol is used for maintenance of sinus rhythm in patients with AF who have normal baseline QT interval (< 460 ms) and minimal or no structural heart disease[45]. While some retrospective studies have suggested safety and efficacy of sotalol in adults with CHD[57-59], a meta-analysis of antiarrhythmic drugs for AF which included 12 clinical trials showed that sotalol doubles all-cause mortality[60]. Based on the current evidence, sotalol can be used with caution for maintenance of sinus rhythm in IART or AF in patients with ACHD[17].
In non-ACHD populations, amiodarone is the most effective antiarrhythmic medication in maintaining sinus rhythm in AF and is considered drug of choice in heart failure patients[61,62]. Expert consensus suggests amiodarone as first line for maintenance of sinus rhythm in ACHD patients with IART and those with AF in presence of ventricular hypertrophy or dysfunction, or coronary artery disease[17]. It is the drug we use most often across the spectrum in ACHD, including in patients with impaired ventricular function. Unfortunately, long-term treatment with amiodarone (which is often necessary in this population) can be complicated by pulmonary and liver toxicity, thyroid dysfunction, photosensitivity, and corneal microdeposits[63]. In our experience the most frequent problems in ACHD patients are thyroid related. Torsades de pointes is seen in fewer than 1% of non-ACHD patients[64].
Dofetilide has been shown to be safe in adult patients with recent myocardial infarction or heart failure[65]. The major concern is risk of torsade de pointes, which is seen in 0.9% to 3.3% of patients[66,67]. Dofetilide was studied in a multicenter series of 20 ACHD patients with refractory atrial arrhythmia and reasonable success was noted[68]. However, we rarely use this drug in our center and prefer the use of sotalol.
Catheter ablation
Catheter ablation is now used as an early treatment strategy for IART in many centers and is preferred over the long-term pharmacological management[17,69]. Advances in 3D mapping for improved circuit localization and irrigated-tip or large-tip ablation catheters which help with effective lesion creation has led to 81% acute success rate[69-72]. Newly developed software permits rapid automatic annotation of signals using multi-electrode catheters. This allows large chambers to be mapped rapidly and with a huge number of points reducing procedure time and potentially increasing success rates of ablation[73]. These needs to be interpreted in the context of programmed settings on the mapping system including the window of interest and electrogram annotation the discussion of which is beyond the scope of this review[74].
The acute success rate is lower in Ebstein anomaly with higher recurrence rate[75,76] due to challenges of distorted anatomic landmarks, difficulty identifying the true AV groove, extremely fractionated electrograms, and the high incidence of multiple pathways[31,75,76]. Recurrence rates following ablation are 34%-54%, mostly occurring within the first year and are higher in atriopulmonary anastomosis of Fontan palliation compared to other CHD patients[72,77,78]. Gaining access to the atrial tissue is difficult in patients with an interatrial lateral tunnel or extra-cardiac Fontan palliation and conduit puncture may be required[79]. When planning ablation, consideration should be given to the location of the AV node in the underlying CHD and the risk of AV block due to ablation. For example, the AV node is typically located anterior in the AV junction in congenitally corrected-TGA[80]. The AV node is displaced postero-inferiorly in inlet VSDs[81]. These should be kept in mind when planning ablations in that region as for typical slow fast AVNRT. The AV node does not have an intracardiac signal. The His bundle signal is used as its surrogate marker. Locating and identifying the His is often challenging in ACHD as the conduction system is often displaced in many conditions like AV canal defects and congenitally corrected transposition of great arteries. In other conditions like an extracardiac Fontan, the His bundle is not accessible unless a puncture is performed. If located, the His signal can be tagged by using 3D electroanatomic mapping systems. This is especially important in patients with single ventricle palliation where the complication of heart block would often require management with epicardial pacing[82].
Specific recommendations for AF ablation in CHD population have not yet been developed due to scarcity of published data on mechanism of arrhythmia, unclear ablation targets, and efficacy[27]. One study reported a success rate of 42% compared to 53% in controls without CHD. The value of repeat ablations and role of pulmonary vein isolation in complex CHD patients remains to be determined[40,83]. Limited experience is available on AV nodal ablation followed by permanent pacing in symptomatic patient with poor rate control[84].
Surgical treatment and percutaneous intervention
A disturbance of hemodynamics often underlies (or is a significant contributor to) atrial arrhythmia in ACHD. Sometimes the hemodynamic issues are amenable to treatment by surgery or a percutaneous intervention and if successful, such procedures may extinguish or ameliorate the patient’s arrhythmia burden. Examples would be replacement of a regurgitant left atrioventricular valve in a patient with an atrioventricular septal defect, pulmonary valve replacement in a patient with repaired tetralogy of Fallot and severe pulmonary regurgitation and replacement of a stenosed conduit for pulmonary blood flow. The decision to surgically revise or upgrade a Fontan circulation as an intervention for recurrent arrhythmia can have excellent results but is a specific example with unique considerations[85-87]. When patients with ACHD experience atrial arrhythmia consideration should not only be given to correction of residual hemodynamic lesions but also to the role of specific arrhythmia surgery, which can be performed either in conjunction with other procedures or in isolation. Mavroudis et al[88] have published an excellent and detailed review of arrhythmia surgery in ACHD based on their own experience in 248 patients. Most of the surgical procedures described are for the treatment of atrial arrhythmia in patients with repaired TOF or a single ventricle circulation. Operative techniques described include, methods for increasing the safety of repeat sternal reentry, direct ablation (cryoablation or radiofrequency), right atrial and biatrial Cox-maze procedures, as well as excision of automatic foci. The paper describes the differing anatomical and electrophysiological variations which need to be taken into account for each congenital cardiac diagnosis and arrhythmia[88].
Anticoagulation
Thromboembolic prevention in ACHD patients is an important aspect of pharmacological treatment of atrial arrhythmia. The prevalence of thromboembolic events in ACHD patients is estimated to be 10 to 100-fold higher than age-matched controls in the general population due to dilated chambers with sluggish flow, intra-cardiac prosthetic material, pacing/defibrillation leads, intracardiac shunts, and associated hypercoagulable states[89,90]. In particular, the risk of stroke is 10-fold higher in ACHD patients, with atrial arrhythmia being one of the strongest predictors[91,92]. In a small series of ACHD patients who underwent TEE prior to electrical cardioversion of an atrial arrhythmia, atrial thrombus was seen in 37%[93].
Risk scoring systems (CHA2DS2, CHA2DS2-VASc, and HAS-BLED) are widely used to guide anticoagulant prescription in non-ACHD patients, balancing the risks of thromboembolism against the risks of bleeding[94-96]. Initially, these scoring systems were not developed or tested in ACHD patients and did not include any component relating to the type or severity of congenital cardiac lesion[17]. For many years there has been controversy about whether or not they have any value in decision-making for ACHD patients. In 2015 a Dutch study of 229 ACHD patients with atrial arrhythmias attempted to address this issue[97]. The authors evaluated dichotomized CHA2-DS2-VASc and HAS-BLED scores in their ACHD cohort and reported that thrombotic and bleeding events can be predicted by using scoring systems[97]. In moderate/complex patients with IART or AF, long-term oral anticoagulation is recommended with vitamin K antagonist (VKA) however, in simple nonvalvular CHD, the decision of anticoagulation risk can be guided by CHA2DS2-VASc score[17,98]. Patients with CHA2DS2-VASc score of ≥ 2 need oral anticoagulation with VKA or novel oral anticoagulants[17].
The new oral anticoagulants (NOAC) have been introduced as an alternative to VKA in non-valvular atrial fibrillation. These drugs are either direct thrombin (Dabigatran) or factor Xa (Rivaroxaban, Apixaban) inhibitors. Limited data is available regarding the use of NOACs in ACHD population. One prospective study included data from 75 ACHD patients without prosthetic heart valves taking one of three NOACs[99]. Twenty-one percent of participants had complex CHD and the main indication was thrombosis prevention in atrial arrhythmia[99]. During a mean follow-up duration of 12 mo, there were no thrombotic or major bleeding events[99]. Results from further multi-centre studies are anticipated. However, it may be reasonable to consider NOAC in lieu of VKA in simple CHD and without prosthetic valves or hemodynamically significant valve disease[17,99].
ATRIAL ARRHYTHMIAS DURING PREGNANCY IN ACHD PATIENTS
The risk of atrial arrhythmia is known to increase during pregnancy due to anticipated adaptive hemodynamic, hormonal, and autonomic changes[100,101]. The incidence of AF/IART in patients with structural heart disease was reported at 1.3% by the Registry on Pregnancy and Cardiac Disease with the majority of arrhythmias occurring during second trimester[102]. The frequency of arrhythmia during pregnancy is higher than this in other studies and certain ACHD subgroups. In a systematic review by Drenthen et al[103], the highest risk of arrhythmia was reported in patients with Fontan palliation, atrial redirection for TGA (Mustard or Senning) and repaired atrio-ventricular septal defect. In a multi-centre study of 157 pregnancies in 74 women with repaired TOF the incidence of arrhythmia was 6.5% vs < 1% in controls[104]. In a study of women with valvular heart disease the rate of arrhythmia during pregnancy was 15% vs 0% in controls (P = 0.001)[105]. The following have been identified as risk factors for the occurrence of atrial arrhythmias during pregnancy: Arrhythmia before pregnancy, mitral valve disease, beta-blocker use before pregnancy, and left-sided cardiac lesions[101,102]. Atrial arrhythmias during pregnancy area associated with pregnancy-related mortality and morbidity. This may be due to severity of underlying heart disease and also to an increased risk of thromboembolic events[102].
Electrical cardioversion can be used in all trimesters of pregnancy and must not be delayed in hemodynamically unstable patients[106]. The risk of inducing fetal arrhythmias is minimal but unless cardioversion is emergent, fetal monitoring should be performed[101,106,107]. Most antiarrhythmic drugs are United States Food and Drug Administration class C medications; i.e., animal studies have shown risk to the fetus, there are no controlled studies in women or studies in women and animals are not available. This is a confusing classification, meaning that there is insufficient data to make a statement regarding safety. Use of these drugs must be carefully discussed with pregnant women to allow them the ability to weigh potential pros and cons. Beta-blockers (often metoprolol) are commonly used as first line rate control therapy. Atenolol is an FDA class D medication (positive evidence of fetal risk) and should be avoided due to association with low birthweight. Oral verapamil and digoxin (FDA Class C) are used for atrial arrhythmia in pregnancy but should not be used when an accessory pathway is suspected[106]. Sotalol (FDA class B - animal studies show no risk, no studies in humans) can be used to maintain sinus rhythm although, its side effects include fetal bradycardia and long QT in the mother and therefore, close monitoring is required. Due to its side effects on fetal thyroid function, amiodarone should not be used during pregnancy unless other antiarrhythmic agents are contraindicated or ineffective. Flecainide (FDA class C) is a drug which crosses the placenta and is often used for treatment of fetal tachycardia, however there are proarrhythmic risks in patients with structural heart disease[108,109]. Pregnancy is a prothrombotic state and sustained atrial arrhythmia will further increase risk of thromboembolic events in pregnancy, therefore anticoagulation should be considered. Specific guidelines and recommendations exist[100,110] and any decision about anticoagulation during pregnancy should be made with the input of specialist advice and in full consultation with the patient. Different regimens can be recommended depending on patient preference and the individualized balance of risks: Obstetric risks (miscarriage, retroplacental hematoma, bleeding during delivery) vs off-spring risks (warfarin associated embryopathy, premature birth, inter-cranial bleeding) vs maternal risks (thrombosis and bleeding). The risk of maternal thrombosis and thromboembolism are highest in women who in addition to their atrial arrhythmia also have either mechanical valves or Fontan circulations.
CONCLUSION
The ACHD population continues to expand and is also aging. Atrial arrhythmias are common in these patients and the cause of significant morbidity. They also associated with increased risk of subsequent mortality. A comprehensive understanding of the underlying anatomy, previous surgeries and mechanism of the arrhythmia is essential to optimal management of arrhythmias in this population and clear, open communication between ACHD specialists, electrophysiologists and primary care providers necessary.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good):B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None.
Peer-review started: January 16, 2017
First decision: March 8, 2017
Article in press: April 10, 2017
P- Reviewer: Maeda S, Soliman EZ, Trohman RG S- Editor: Song XX L- Editor: A E- Editor: Li D
References
- 1.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 3.Moons P, Van Deyk K, Dedroog D, Troost E, Budts W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612–616. doi: 10.1097/01.hjr.0000197472.81694.2b. [DOI] [PubMed] [Google Scholar]
- 4.Roche SL, Silversides CK. Hypertension, obesity, and coronary artery disease in the survivors of congenital heart disease. Can J Cardiol. 2013;29:841–848. doi: 10.1016/j.cjca.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. doi: 10.1016/j.jacc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 7.Kaemmerer H, Bauer U, Pensl U, Oechslin E, Gravenhorst V, Franke A, Hager A, Balling G, Hauser M, Eicken A, et al. Management of emergencies in adults with congenital cardiac disease. Am J Cardiol. 2008;101:521–525. doi: 10.1016/j.amjcard.2007.09.110. [DOI] [PubMed] [Google Scholar]
- 8.Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37:585–592. doi: 10.1016/s0735-1097(00)01141-4. [DOI] [PubMed] [Google Scholar]
- 9.Fishberger SB, Wernovsky G, Gentles TL, Gauvreau K, Burnett J, Mayer JE, Walsh EP. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86. doi: 10.1016/s0022-5223(97)70402-1. [DOI] [PubMed] [Google Scholar]
- 10.Puley G, Siu S, Connelly M, Harrison D, Webb G, Williams WG, Harris L. Arrhythmia and survival in patients & gt; 18 years of age after the mustard procedure for complete transposition of the great arteries. Am J Cardiol. 1999;83:1080–1084. doi: 10.1016/s0002-9149(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 11.Helbing WA, Hansen B, Ottenkamp J, Rohmer J, Chin JG, Brom AG, Quaegebeur JM. Long-term results of atrial correction for transposition of the great arteries. Comparison of Mustard and Senning operations. J Thorac Cardiovasc Surg. 1994;108:363–372. [PubMed] [Google Scholar]
- 12.Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127:331–339. doi: 10.1161/CIRCULATIONAHA.112.135046. [DOI] [PubMed] [Google Scholar]
- 13.Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol. 2008;52:460–466. doi: 10.1016/j.jacc.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DA, Siu SC, Hussain F, MacLoghlin CJ, Webb GD, Harris L. Sustained atrial arrhythmias in adults late after repair of tetralogy of fallot. Am J Cardiol. 2001;87:584–588. doi: 10.1016/s0002-9149(00)01435-1. [DOI] [PubMed] [Google Scholar]
- 15.El-Najdawi EK, Driscoll DJ, Puga FJ, Dearani JA, Spotts BE, Mahoney DW, Danielson GK. Operation for partial atrioventricular septal defect: a forty-year review. J Thorac Cardiovasc Surg. 2000;119:880–889; discussion 889-890. doi: 10.1016/S0022-5223(00)70082-1. [DOI] [PubMed] [Google Scholar]
- 16.Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. New Engl J Med. 1999;340:839–846. doi: 10.1056/NEJM199903183401103. [DOI] [PubMed] [Google Scholar]
- 17.Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot Nd, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD) Can J Cardiol. 2014;30:e1–e63. doi: 10.1016/j.cjca.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JIE, Somerville J, Williams RG, Webb GD. Task Force 1: The Changing Profile of Congenital Heart Disease in Adult Life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 19.Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu MH, Lu CW, Chen HC, Chiu SN, Kao FY, Huang SK. Arrhythmic burdens in patients with tetralogy of Fallot: a national database study. Heart Rhythm. 2015;12:604–609. doi: 10.1016/j.hrthm.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Diller GP, Giardini A, Dimopoulos K, Gargiulo G, Müller J, Derrick G, Giannakoulas G, Khambadkone S, Lammers AE, Picchio FM, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–3083. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 22.Gatzoulis MA, Walters J, McLaughlin PR, Merchant N, Webb GD, Liu P. Late arrhythmia in adults with the mustard procedure for transposition of great arteries: a surrogate marker for right ventricular dysfunction? Heart. 2000;84:409–415. doi: 10.1136/heart.84.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsh JA, Walsh EP, Triedman JK. Prevalence of and risk factors for atrial fibrillation and intra-atrial reentrant tachycardia among patients with congenital heart disease. Am J Cardiol. 2002;90:338–340. doi: 10.1016/s0002-9149(02)02480-3. [DOI] [PubMed] [Google Scholar]
- 24.Philip F, Muhammad KI, Agarwal S, Natale A, Krasuski RA. Pulmonary vein isolation for the treatment of drug-refractory atrial fibrillation in adults with congenital heart disease. Congenit Heart Dis. 2012;7:392–399. doi: 10.1111/j.1747-0803.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–875. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 26.Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, Gow RM, Williams WG, Trusler GA, Freedom RM. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194–201. doi: 10.1016/s0735-1097(96)00424-x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Tedrow UB, Triedman JK. Arrhythmias in Adult Congenital Heart Disease: Diagnosis and Management. Cardiol Clin. 2015;33:571–888, viii. doi: 10.1016/j.ccl.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Akar JG, Kok LC, Haines DE, DiMarco JP, Mounsey JP. Coexistence of type I atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol. 2001;38:377–384. doi: 10.1016/s0735-1097(01)01392-4. [DOI] [PubMed] [Google Scholar]
- 29.Love BA, Collins KK, Walsh EP, Triedman JK. Electroanatomic characterization of conduction barriers in sinus/atrially paced rhythm and association with intra-atrial reentrant tachycardia circuits following congenital heart disease surgery. J Cardiovasc Electrophysiol. 2001;12:17–25. doi: 10.1046/j.1540-8167.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 30.Roos-Hesselink J, Perlroth MG, McGhie J, Spitaels S. Atrial arrhythmias in adults after repair of tetralogy of Fallot. Correlations with clinical, exercise, and echocardiographic findings. Circulation. 1995;91:2214–2219. doi: 10.1161/01.cir.91.8.2214. [DOI] [PubMed] [Google Scholar]
- 31.Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, Suys B, Budts W, Pasquet A, De Wolf D, et al. Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart. 2004;90:307–313. doi: 10.1136/hrt.2002.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, et al. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56:890–896. doi: 10.1016/j.jacc.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86:969–974. doi: 10.1016/s0002-9149(00)01132-2. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa H, Shah N, Matsudaira K, Overholt E, Chandrasekaran K, Beckman KJ, Spector P, Calame JD, Rao A, Hasdemir C, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow "focal" ablation. Circulation. 2001;103:699–709. doi: 10.1161/01.cir.103.5.699. [DOI] [PubMed] [Google Scholar]
- 35.Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72–76. doi: 10.1046/j.1540-8167.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 36.Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 37.Delacretaz E, Ganz LI, Soejima K, Friedman PL, Walsh EP, Triedman JK, Sloss LJ, Landzberg MJ, Stevenson WG. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol. 2001;37:1665–1676. doi: 10.1016/s0735-1097(01)01192-5. [DOI] [PubMed] [Google Scholar]
- 38.Derrick GP, Narang I, White PA, Kelleher A, Bush A, Penny DJ, Redington AN. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load-independent indexes of right ventricular performance after the Mustard operation. Circulation. 2000;102:III154–III159. doi: 10.1161/01.cir.102.suppl_3.iii-154. [DOI] [PubMed] [Google Scholar]
- 39.Fratz S, Hager A, Busch R, Kaemmerer H, Schwaiger M, Lange R, Hess J, Stern HC. Patients after atrial switch operation for transposition of the great arteries can not increase stroke volume under dobutamine stress as opposed to patients with congenitally corrected transposition. Circ J. 2008;72:1130–1135. doi: 10.1253/circj.72.1130. [DOI] [PubMed] [Google Scholar]
- 40.Teh AW, Medi C, Lee G, Rosso R, Sparks PB, Morton JB, Kistler PM, Halloran K, Vohra JK, Kalman JM. Long-term outcome following ablation of atrial flutter occurring late after atrial septal defect repair. Pacing Clin Electrophysiol. 2011;34:431–435. doi: 10.1111/j.1540-8159.2010.03005.x. [DOI] [PubMed] [Google Scholar]
- 41.Attenhofer Jost CH, Connolly HM, Edwards WD, Hayes D, Warnes CA, Danielson GK. Ebstein's anomaly - review of a multifaceted congenital cardiac condition. Swiss Med Wkly. 2005;135:269–281. doi: 10.4414/smw.2005.10985. [DOI] [PubMed] [Google Scholar]
- 42.Levine JC, Walsh EP, Saul JP. Radiofrequency ablation of accessory pathways associated with congenital heart disease including heterotaxy syndrome. Am J Cardiol. 1993;72:689–693. doi: 10.1016/0002-9149(93)90886-h. [DOI] [PubMed] [Google Scholar]
- 43.Smith WM, Gallagher JJ, Kerr CR, Sealy WC, Kasell JH, Benson DW, Reiter MJ, Sterba R, Grant AO. The electrophysiologic basis and management of symptomatic recurrent tachycardia in patients with Ebstein's anomaly of the tricuspid valve. Am J Cardiol. 1982;49:1223–1234. doi: 10.1016/0002-9149(82)90048-0. [DOI] [PubMed] [Google Scholar]
- 44.Idorn L, Jensen AS, Juul K, Reimers JI, Johansson PI, Sørensen KE, Ostrowski SR, Søndergaard L. Thromboembolic complications in Fontan patients: population-based prevalence and exploration of the etiology. Pediatr Cardiol. 2013;34:262–272. doi: 10.1007/s00246-012-0431-4. [DOI] [PubMed] [Google Scholar]
- 45.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Flinn CJ, Wolff GS, Dick M, Campbell RM, Borkat G, Casta A, Hordof A, Hougen TJ, Kavey RE, Kugler J. Cardiac rhythm after the Mustard operation for complete transposition of the great arteries. N Engl J Med. 1984;310:1635–1638. doi: 10.1056/NEJM198406213102504. [DOI] [PubMed] [Google Scholar]
- 47.Stephenson EA, Casavant D, Tuzi J, Alexander ME, Law I, Serwer G, Strieper M, Walsh EP, Berul CI; ATTEST Investigators. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92:871–876. doi: 10.1016/s0002-9149(03)00905-6. [DOI] [PubMed] [Google Scholar]
- 48.Hoyer AW, Balaji S. The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol. 2007;30:1003–1008. doi: 10.1111/j.1540-8159.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 49.Rao SO, Boramanand NK, Burton DA, Perry JC. Atrial tachycardias in young adults and adolescents with congenital heart disease: conversion using single dose oral sotalol. Int J Cardiol. 2009;136:253–257. doi: 10.1016/j.ijcard.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 50.Vos MA, Golitsyn SR, Stangl K, Ruda MY, Van Wijk LV, Harry JD, Perry KT, Touboul P, Steinbeck G, Wellens HJ. Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. The Ibutilide/Sotalol Comparator Study Group. Heart. 1998;79:568–575. doi: 10.1136/hrt.79.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowey PR, VanderLugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/flutter. Am J Cardiol. 1996;78:46–52. doi: 10.1016/s0002-9149(96)00566-8. [DOI] [PubMed] [Google Scholar]
- 52.Trohman RG. Adenosine for diagnosis of wide QRS tachycardia: rapid infusion for an easier conclusion. Crit Care Med. 2009;37:2651–2652. doi: 10.1097/CCM.0b013e3181abfb9f. [DOI] [PubMed] [Google Scholar]
- 53.Mallet ML. Proarrhythmic effects of adenosine: a review of the literature. Emerg Med J. 2004;21:408–410. doi: 10.1136/emj.2004.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NA, Field ME, Goldberger ZD, Hammill SC, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67:e27–e115. doi: 10.1016/j.jacc.2015.08.856. [DOI] [PubMed] [Google Scholar]
- 55.Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, Fernandes SM, Beauchesne L, Therrien J, Chetaille P, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363–370. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- 56.Fish FA, Gillette PC, Benson DW. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18:356–365. doi: 10.1016/0735-1097(91)90586-x. [DOI] [PubMed] [Google Scholar]
- 57.Koyak Z, Kroon B, de Groot JR, Wagenaar LJ, van Dijk AP, Mulder BA, Van Gelder IC, Post MC, Mulder BJ, Bouma BJ. Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol. 2013;112:1461–1467. doi: 10.1016/j.amjcard.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 58.Miyazaki A, Ohuchi H, Kurosaki K, Kamakura S, Yagihara T, Yamada O. Efficacy and safety of sotalol for refractory tachyarrhythmias in congenital heart disease. Circ J. 2008;72:1998–2003. doi: 10.1253/circj.cj-08-0194. [DOI] [PubMed] [Google Scholar]
- 59.Beaufort-Krol GC, Bink-Boelkens MT. Sotalol for atrial tachycardias after surgery for congenital heart disease. Pacing Clin Electrophysiol. 1997;20:2125–2129. doi: 10.1111/j.1540-8159.1997.tb03642.x. [DOI] [PubMed] [Google Scholar]
- 60.Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012;5:CD005049. doi: 10.1002/14651858.CD005049.pub3. [DOI] [PubMed] [Google Scholar]
- 61.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagné P, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 62.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 63.Goldschlager N, Epstein AE, Naccarelli GV, Olshansky B, Singh B, Collard HR, Murphy E. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 64.Hohnloser SH, Singh BN. Proarrhythmia with class III antiarrhythmic drugs: definition, electrophysiologic mechanisms, incidence, predisposing factors, and clinical implications. J Cardiovasc Electrophysiol. 1995;6:920–936. doi: 10.1111/j.1540-8167.1995.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 65.Køber L, Bloch Thomsen PE, Møller M, Torp-Pedersen C, Carlsen J, Sandøe E, Egstrup K, Agner E, Videbaek J, Marchant B, Camm AJ; Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–296. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 67.Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, Billing CB. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 68.Wells R, Khairy P, Harris L, Anderson CC, Balaji S. Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study. Pacing Clin Electrophysiol. 2009;32:1313–1318. doi: 10.1111/j.1540-8159.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- 69.Triedman JK, Bergau DM, Saul JP, Epstein MR, Walsh EP. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032–1038. doi: 10.1016/s0735-1097(97)00252-0. [DOI] [PubMed] [Google Scholar]
- 70.Triedman JK, Alexander ME, Love BA, Collins KK, Berul CI, Bevilacqua LM, Walsh EP. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827–1835. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- 71.Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010;56:1589–1596. doi: 10.1016/j.jacc.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 72.Hebe J, Hansen P, Ouyang F, Volkmer M, Kuck KH. Radiofrequency catheter ablation of tachycardia in patients with congenital heart disease. Pediatr Cardiol. 2000;21:557–575. doi: 10.1007/s002460010134. [DOI] [PubMed] [Google Scholar]
- 73.Haldar S, Porta-Sanchez A, Oechslin E, Downar E, Benson L, Nair K. Transbaffle Multielectrode Mapping of Atrial Flutter Post-Double Switch Operation. J Cardiovasc Electrophysiol. 2016;27:1240–1241. doi: 10.1111/jce.12988. [DOI] [PubMed] [Google Scholar]
- 74.Selvaraj RJ, Shankar B, Subramanian A, Nair K. Chasing red herrings: making sense of the colors while mapping. Circ Arrhythm Electrophysiol. 2014;7:553–556. doi: 10.1161/CIRCEP.113.001391. [DOI] [PubMed] [Google Scholar]
- 75.Reich JD, Auld D, Hulse E, Sullivan K, Campbell R. The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998;9:1370–1377. doi: 10.1111/j.1540-8167.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 76.Roten L, Lukac P, DE Groot N, Nielsen JC, Szili-Torok T, Jensen HK, Zimmermann M, Delacrétaz E. Catheter ablation of arrhythmias in ebstein's anomaly: a multicenter study. J Cardiovasc Electrophysiol. 2011;22:1391–1396. doi: 10.1111/j.1540-8167.2011.02161.x. [DOI] [PubMed] [Google Scholar]
- 77.Kannankeril PJ, Anderson ME, Rottman JN, Wathen MS, Fish FA. Frequency of late recurrence of intra-atrial reentry tachycardia after radiofrequency catheter ablation in patients with congenital heart disease. Am J Cardiol. 2003;92:879–881. doi: 10.1016/s0002-9149(03)00908-1. [DOI] [PubMed] [Google Scholar]
- 78.de Groot NM, Atary JZ, Blom NA, Schalij MJ. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol. 2010;3:148–154. doi: 10.1161/CIRCEP.109.909838. [DOI] [PubMed] [Google Scholar]
- 79.Lobo RG, Griffith M, De Bono J. Ablation of Arrhythmias in Patients with Adult Congenital Heart Disease. Arrhythm Electrophysiol Rev. 2014;3:36–39. doi: 10.15420/aer.2011.3.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911–23. doi: 10.1161/01.cir.50.5.911. [DOI] [PubMed] [Google Scholar]
- 81.Milo S, Ho SY, Wilkinson JL, Anderson RH. Surgical anatomy and atrioventricular conduction tissues of hearts with isolated ventricular septal defects. J Thorac Cardiovasc Surg. 1980;79:244–255. [PubMed] [Google Scholar]
- 82.A Pilcher Md T, V Saarel Md E. Anatomic Challenges In Pediatric Catheter Ablation. J Atr Fibrillation. 2014;7:1054. doi: 10.4022/jafib.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mondésert B, Abadir S, Khairy P. Arrhythmias in adult congenital heart disease: the year in review. Curr Opin Cardiol. 2013;28:354–359. doi: 10.1097/HCO.0b013e32835fb7c2. [DOI] [PubMed] [Google Scholar]
- 84.Friedman RA, Will JC, Fenrich AL, Kertesz NJ. Atrioventricular junction ablation and pacemaker therapy in patients with drug-resistant atrial tachyarrhythmias after the Fontan operation. J Cardiovasc Electrophysiol. 2005;16:24–29. doi: 10.1046/j.1540-8167.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 85.Mavroudis C, Deal BJ, Backer CL, Stewart RD, Franklin WH, Tsao S, Ward KM, DeFreitas RA. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg. 2007;84:1457–1465; discussion 1465-1466. doi: 10.1016/j.athoracsur.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 86.Agnoletti G, Borghi A, Vignati G, Crupi GC. Fontan conversion to total cavopulmonary connection and arrhythmia ablation: clinical and functional results. Heart. 2003;89:193–198. doi: 10.1136/heart.89.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh M, Yagihara T, Uemura H, Kagisaki K, Hagino I, Ishizaka T, Kitamura S. Optimal timing of the Fontan conversion: change in the P-wave characteristics precedes the onset of atrial tachyarrhythmias in patients with atriopulmonary connection. J Thorac Cardiovasc Surg. 2007;133:1295–1302. doi: 10.1016/j.jtcvs.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Mavroudis C, Deal B, Backer CL, Stewart RD. Operative techniques in association with arrhythmia surgery in patients with congenital heart disease. World J Pediatr Congenit Heart Surg. 2013;4:85–97. doi: 10.1177/2150135112449842. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann A, Chockalingam P, Balint OH, Dadashev A, Dimopoulos K, Engel R, Schmid M, Schwerzmann M, Gatzoulis MA, Mulder B, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223–1226. doi: 10.1136/hrt.2010.196147. [DOI] [PubMed] [Google Scholar]
- 90.Khairy P. Thrombosis in congenital heart disease. Expert Rev Cardiovasc Ther. 2013;11:1579–1582. doi: 10.1586/14779072.2013.854703. [DOI] [PubMed] [Google Scholar]
- 91.Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson PO, Dellborg M. Ischemic Stroke in Children and Young Adults With Congenital Heart Disease. J Am Heart Assoc. 2016;5:pii: e003071. doi: 10.1161/JAHA.115.003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Opotowsky AR, Webb GD. Population-Based Data on Congenital Heart Disease and Stroke. J Am Heart Assoc. 2016;5:pii: e003257. doi: 10.1161/JAHA.116.003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feltes TF, Friedman RA. Transesophageal echocardiographic detection of atrial thrombi in patients with nonfibrillation atrial tachyarrhythmias and congenital heart disease. J Am Coll Cardiol. 1994;24:1365–1370. doi: 10.1016/0735-1097(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 94.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 95.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 96.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 97.Heidendael JF, Bokma JP, de Groot JR, Koolbergen DR, Mulder BJ, Bouma BJ. Weighing the risks: Thrombotic and bleeding events in adults with atrial arrhythmias and congenital heart disease. Int J Cardiol. 2015;186:315–320. doi: 10.1016/j.ijcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 98.Jensen AS, Idorn L, Nørager B, Vejlstrup N, Sondergaard L. Anticoagulation in adults with congenital heart disease: The who, the when and the how? Heart. 2015;101:424–429. doi: 10.1136/heartjnl-2014-305576. [DOI] [PubMed] [Google Scholar]
- 99.Pujol C, Niesert AC, Engelhardt A, Schoen P, Kusmenkov E, Pittrow D, Ewert P, Kaemmerer H. Usefulness of Direct Oral Anticoagulants in Adult Congenital Heart Disease. Am J Cardiol. 2016;117:450–455. doi: 10.1016/j.amjcard.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 100.Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961–967. doi: 10.1161/CIRCEP.114.001517. [DOI] [PubMed] [Google Scholar]
- 101.Silversides CK, Harris L, Haberer K, Sermer M, Colman JM, Siu SC. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206–1212. doi: 10.1016/j.amjcard.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Salam AM, Ertekin E, van Hagen IM, Al Suwaidi J, Ruys TP, Johnson MR, Gumbiene L, Frogoudaki AA, Sorour KA, Iserin L, et al. Atrial fibrillation or flutter during pregnancy in patients with structural heart disease: data from the ROPAC (Registry on Pregnancy and Cardiac Disease) JACC Clin Electrophysiol. 2015;1:284–92. doi: 10.1016/j.jacep.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 103.Drenthen W, Pieper PG, Roos-Hesselink JW, van Lottum WA, Voors AA, Mulder BJ, van Dijk AP, Vliegen HW, Yap SC, Moons P, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–2311. doi: 10.1016/j.jacc.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 104.Balci A, Drenthen W, Mulder BJ, Roos-Hesselink JW, Voors AA, Vliegen HW, Moons P, Sollie KM, van Dijk AP, van Veldhuisen DJ, et al. Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. Am Heart J. 2011;161:307–313. doi: 10.1016/j.ahj.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 105.Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, Goodwin I, Zapadinsky N, Elkayam U. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37:893–899. doi: 10.1016/s0735-1097(00)01198-0. [DOI] [PubMed] [Google Scholar]
- 106.Burkart TA, Conti JB. Cardiac arrhythmias during pregnancy. Curr Treat Options Cardiovasc Med. 2010;12:457–471. doi: 10.1007/s11936-010-0084-7. [DOI] [PubMed] [Google Scholar]
- 107.Barnes EJ, Eben F, Patterson D. Direct current cardioversion during pregnancy should be performed with facilities available for fetal monitoring and emergency caesarean section. BJOG. 2002;109:1406–1407. doi: 10.1046/j.1471-0528.2002.02113.x. [DOI] [PubMed] [Google Scholar]
- 108.Silversides C, Spears D. Atrial Fibrillation and atrial flutter in pregnant women with heart disease. Contributions from the ROPAC Investigators. JACCEP. 2015;1:284–292. doi: 10.1016/j.jacep.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285–288. doi: 10.1053/j.semperi.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]


