Abstract
Electrical storm (ES) is a medical emergency characterized by repetitive episodes of sustained ventricular arrhythmias (VAs) in a limited amount of time (at least 3 within a 24-h period) leading to repeated appropriate implantable cardioverter defibrillator therapies. The occurrence of ES represents a major turning point in the natural history of patients with structural heart disease being associated with poor short- and long-term survival particularly in those with compromised left ventricular ejection fraction (LVEF) that can develop hemodynamic decompensation and multi-organ failure. Management of ES is challenging with limited available evidence coming from small retrospective series and a substantial lack of randomized-controlled trials. In general, a multidisciplinary approach including medical therapies such as anti-arrhythmic drugs, sedation, as well as interventional approaches like catheter ablation, may be required. Accurate patient risk stratification at admission for ES is pivotal and should take into account hemodynamic tolerability of VAs as well as comorbidities like low LVEF, advanced NYHA class and chronic pulmonary disease. In high risk patients, prophylactic mechanical circulatory support with left ventricular assistance devices or extracorporeal membrane oxygenation should be considered as bridge to ablation and recovery. In the present manuscript we review the available strategies for management of ES and the evidence supporting them.
Keywords: Electrical storm, Ventricular tachycardia, Catheter ablation, Mechanical hemodynamic support, Anti-arrhythmic drugs
Core tip: Electrical storm (ES) is a life-threatening condition characterized by ongoing ventricular arrhythmias leading to appropriate implantable cardioverter defibrillator therapies. It is associated with increased mortality and requires urgent medical care. In this review, we summarize the prognostic implications for ES as well as available treatment strategies to manage ES.
INTRODUCTION
Ventricular tachycardia (VT) electrical storm (ES) is a severe clinical condition characterized by clustering episodes of ventricular arrhythmia in a short amount of time. The current definition of ES implies at least 3 distinct episodes of sustained VT or ventricular fibrillation (VF) within the last 24-h or the occurrence of incessant VT for at least 12-h. In patients with ICD, ES is defined by ≥ 3 appropriate device interventions in the last 24-h (separated by at least 5-min one from the other) either with antitachycardia pacing (ATP) or direct-current shock[1]. Although ES mainly occurs in patients with structural heart disease and low left ventricular ejection fraction (LVEF), it may affect also patients with inherited arrhythmic syndromes and structurally normal heart (i.e., Brugada syndrome and catecholaminergic polymorphic VT) representing a life-threatening condition requiring urgent medical care[2]. Several strategies have been proposed to manage ES with most of the data coming from small retrospective series, lacking large randomized-controlled trials. There are several substantial differences in the approach and treatment of ES in the setting of structural heart disease compared to primitive arrhythmic syndromes. In this review, we will focus on the management of ES in the setting of structural heart disease by summarizing the current therapeutic strategies in a stepwise approach based on available evidence (Figure 1).
Figure 1.
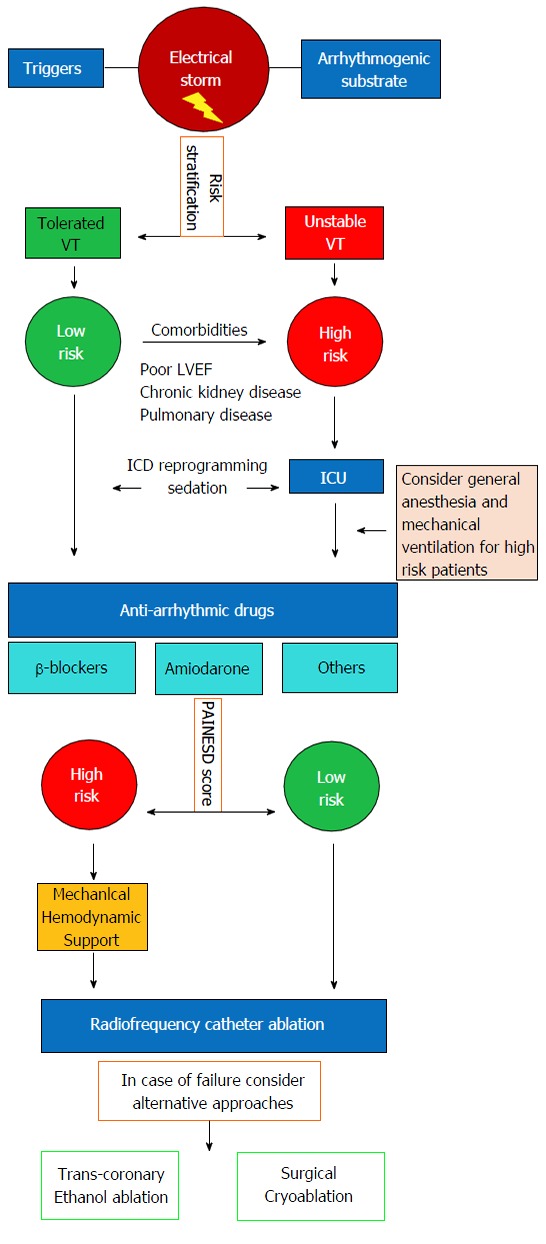
Proposed algorithm for acute management of patients presenting with electrical storm. VT: Ventricular tachycardia; LVEF: Left ventricular ejection fraction; ICU: Intensive care unit.
INITIAL CARE
Prolonged sustained VAs as well as multiple ICD shocks in the setting of ES, may contribute to worsening of systolic function and development of a low-output state leading to cardiogenic shock and multiple organ failure. In this setting, urgent ICD interrogation and reprogramming is mandatory. Documentation of appropriate ICD interventions triggered by VT/VF episodes is necessary to rule out all potentially reversible causes like electrolyte imbalances, acute ischemia, pro-arrhythmic drug effects, hyperthyroidism, infections and decompensated HF. However, reversible causes of ES account for less than 10%, and in the majority of cases no precipitating cause is identified (Table 1)[3]. Initial evaluation should include accurate patient risk stratification according to hemodynamic tolerability of the arrhythmia and presence of comorbidities (Figure 1)[4]. All patients with hemodynamic decompensation (persistent systolic blood pressure < 80-90 mmHg despite temporary resumption of sinus/paced rhythm and despite increasing doses of vasopressors) as well as patients with hemodynamically tolerated VT but with major comorbidities (i.e., LVEF ≤ 30%, moderate to severe chronic kidney disease and severe pulmonary obstructive disease) are considered at high risk and should be admitted to the intensive care unit in order to correct metabolic, respiratory and circulatory imbalances [mechanical ventilation and circulatory support with intra-aortic balloon pump (IABP), left ventricular assist device (LVAD), or extracorporeal membrane oxygenation (ECMO) may be required] and eventually undergo emergent CA. In both high and low-risk patients, every effort should be made to suppress VAs and avoid further ICD-shocks.
Table 1.
Reversible causes of electrical storm
| Acute myocardial ischemia |
| Electrolyte imbalances |
| Decompensated heart failure |
| Hyperthyroidism |
| Infections, fever |
| Pro-arrhythmic drug Effects |
| Early postoperative period |
ICD PROGRAMMING
Reprogramming of ICD settings is of great importance in the initial workup of patients presenting with ES. Repeated ICD-shocks are associated with increased mortality and low quality of life[5,6]. The end-point of ICD reprogramming should be the reduction of ICD-shocks favoring interruption of VAs with ATP. In large trials, increases in both detection duration and heart rate detection threshold have been shown to reduce ICD-shocks without increasing mortality or the incidence of syncope[5,7,8]. Moreover, ATP can effectively terminate most slow VTs with a low risk of acceleration[9,10].
ANTIARRHYTHMIC DRUG THERAPY
Antiarrhythmic drugs (AADs) are usually required for the acute management of ES and are often used as an adjunctive therapy to prevent long-term recurrences. In a recent meta-analysis of randomized-controlled trials, we found a 1.5-fold reduction of appropriate ICD interventions with AADs compared to standard medical therapy with also a significant reduction of inappropriate ICD interventions. However, pooled analysis did not show a significant impact of AADs on all-cause mortality compared to standard medical therapy[11]. The choice of a particular drug and its dose should take into account its efficacy in controlling VA but also potential pro-arrhythmic effects as well as other side effects. Pro-arrhythmic effects have been reported in up to 7% of the patients treated with AADs for VT/VF with the higher incidence in patients with severely reduced LVEF[12]. A list of the most common AADs used in the acute and long-term management of ES as well as indications on the proper use of them and their therapeutic drug monitoring is presented in Table 2.
Table 2.
Anti-arrhythmic medications for acute and long-term treatment of electrical storm
| Acute management | Long-term treatment | Desired plasma concentration | ||
| β-blockers | Propranolol | Bolus: 0.15 mg/kg IV over 10 min | 10-40 mg by mouth three-four times a day | NA |
| Metoprolol | Bolus: 2-5 mg IV every 5 min up to 3 doses in 15 min | 25 mg by mouth twice a day up to 200 mg a day | NA | |
| Esmolol | Bolus: 300 to 500 mg/kg IV for 1 min | Not recommended | NA | |
| Infusion: 25-50 mg/kg per minute up to a maximum dose of 250 mg/kg per minute (titration every 5-10 min) | ||||
| Class III agents | Amiodarone | Bolus: 150 mg IV over 10 min, up to total 2.2 g in 24 h | Oral load: 800 mg by mouth twice a day until 10 g total | 1.0-2.5 μg/mL |
| No efficacy proven for plasma concentrations < 0.5 μg/mL | ||||
| Infusion: 1 mg/min for 6 h, then 0.5 mg/min for 18 h | Maintenance dose: 200-400 mg by mouth daily | Serious toxicity risk for plasma concentrations > 2.5 μg/mL | ||
| Sotalol | Not recommended | 80 mg by mouth twice a day, up to 160 mg twice a day (serious side effects > 320 mg/d) | 1-3 µg/mL (not of great value, usually monitored by QT prolongation with indication to reduction/discontinuation if prolongation > 15%-20%) | |
| Class I agents | Procainamide | Bolus: 10 mg/kg IV over 20 min | 3-6 g by mouth daily fractionated in ≥ 3 administrations | 4-12 μg/mL |
| Infusion: up to 2-3 g/24 h | ||||
| Lidocaine | Bolus: 1.0 to 1.5 mg/kg IV, repeat dose of 0.5-0.75 mg/kg IV up to a total dose of 3 mg/kg | Not recommended | 2-6 μg/mL | |
| Infusion: 20 μcg/kg per minute IV | ||||
| Mexiletine | Not recommended | 200 mg by mouth three times a day, up to 400 mg by mouth three times a day | 0.6-1.7 μg/mL |
Beta-blockers
A significant increase in the sympathetic tone is inevitably observed in patients experiencing ES, being responsible for the occurrence and maintenance of VAs. In these patients a spiral of events may occur: ICD shocks may precipitate increased sympathetic tone, resulting in further VAs and shocks, and so forth. Therefore, suppression of adrenergic tone with β-blockers represents the cornerstone of AAD therapy of ES[13]. Although most of the benefits of β-blockers are related to a class effect, in this setting there are some important advantages of nonselective β1 and β2 blockade. Ventricular remodeling in patients with chronic HF leads to a downregulation of β-receptors mostly involving β1-receptors with relative spearing of β2-receptors. Moreover, the lipophilic nature of some unselective β-blockers like propranolol, enables their penetration into the central nervous system where they act by blocking presynaptic adrenergic receptors[14,15]. Propranolol has been demonstrated to be effective in suppressing VAs refractory to both metoprolol and amiodarone[16]. Short-acting intravenous drugs like esmolol can also be used, especially in patients at highest risk for hemodynamic compromise such as those with severely reduced LVEF[17].
Amiodarone
Amiodarone is widely used in the acute management of ES and can generally be safely administered unless hyperthyroidism or QT prolongation are present. Amiodarone has a mixed antiarrhythmic class action with a prevalent class III action (potassium channel blocker) prolonging the ventricular refractory period when administered orally and a prevalent class I (sodium channel), class IV (L-calcium channels) and class II (sympathetic blocker) action, not prolonging ventricular refractoriness, when is administered intravenously[18]. Amiodarone has demonstrated its efficacy in several trials being able to control VAs in up to 40% of patients within 24-h from intravenous administration as well as to reduce recurrent VT over follow-up[19-22]. The combined use of both amiodarone plus β-blockers significantly reduces the risk of recurrent ICD-shocks compared vs β-blockers alone[22]. In the specific setting of ES, amiodarone has been shown to reduce the risk of ES recurrence by 50% over 5-years follow-up[23]. Patients already under amiodarone treatment may benefit from a reloading dose or a dose adjustment based upon serum levels of amiodarone even if plasma concentration monitoring has been reported of very limited benefit because the drug and its active metabolite (desethylamiodarone) accumulates in tissues at higher concentrations that in plasma[24]. Importantly, amiodarone may increase defibrillation thresholds in patients with ICDs[25] and the risks and benefits of long-term administration of amiodarone should be carefully weighed because of its several side effects including liver dysfunction (elevated AST/ALT levels in up to 30% of patients but hepatitis requiring drug discontinuation in < 3% of the cases), thyroid disorders (hypothyroidism in up to 22%, hyperthyroidism in up to 12%), pulmonary fibrosis (2%), corneal deposits (> 90%, usually of no clinical importance), optic neuropathy (< 1%) and pro-arrhythmic effect (< 1%)[26]. A recent pooled analysis of randomized controlled trials comparing CA vs AADs demonstrated an association between amiodarone and increased mortality[11]. Furthermore, among patients undergoing CA for VT in the setting of structural heart disease, we have recently shown that higher amiodarone dose at discharge after CA was associated with increased mortality, suggesting that discontinuation or dose reduction of amiodarone should be considered in certain patients after successful CA[27].
Procainamide
Procainamide is a class IC agent no longer widely used (unavailable in most countries) that may be helpful to acutely terminate VAs and prevent recurrences. It acts as fast sodium channel blocker, while its active metabolite N-acetylprocainamide blocks potassium channels and accounts for much of the antiarrhythmic effect in vivo as well as side effects like QT interval prolongation. Up to date there are only two small randomized controlled trials analyzing its role in the acute treatment of tolerated VT. In the study by Gorgels et al[28], procainamide demonstrated its superiority to lidocaine in acute VT termination in 29 patients while in the more recent PROCAMIO trial, intravenous administration of procainamide was shown to be safe and more effective compared to amiodarone in the treatment of tolerated monomorphic VT[29,30]. The most important acute adverse reaction is hypotension (up to 30% patients) which requires drug discontinuation in 11% of cases[28-30]. Data regarding the long-term efficacy of procainamide in preventing VT are lacking, moreover chronic therapy is limited by a number of systemic side effects including lupus-like syndrome, gastrointestinal disturbances, and autoimmune blood impairments. Plasma procainamide concentrations can be useful in initial dose titration; however, monitoring of QRS and QT interval is a valid alternative to prevent drug toxicity.
Lidocaine and mexiletine
Lidocaine and mexiletine are both class IB AADs, acting as rapid sodium channel blockers binding to the receptor in a use-dependent fashion. The main difference between them is the bioavailability of mexiletine (80%) that allows its oral administration. The use of lidocaine in ES is more limited due to its lower efficacy in terminating scar-related VTs. During ischemic VT, the altered membrane potential as well as pH reduction increase the rate of drug binding, making lidocaine more effective in terminating VAs[31]. For this reason lidocaine is currently recommended mostly for the suppression of VAs in the setting of acute ischemia[32]. Mexiletine has shown to reduce the burden of VAs but with a trend toward increased mortality and is mostly used as a an adjunctive therapy to amiodarone being able to reduce appropriate therapies in patients with ICD in case of amiodarone inefficacy[33,34]. Side effects of lidocaine and mexiletine are dose dependent and predominantly related to central nervous system accumulation (particularly in patients with HF) including tremors, seizures and hallucinations. They are generally rapidly reversible with drug reduction or discontinuation.
Sotalol
The commercially available form of Sotalol is a racemic mix of d-isomer (acting as a class III potassium channel blocker) and l-isomer (acting as a non-selective β-blocker). Most of its antiarrhythmic (as well as pro-arrhythmic) effects result from its action on potassium channels resulting in prolongation of repolarization and the QT interval. While sotalol has shown to reduce the frequency of ICD-shocks among patients implanted for secondary prevention, it has failed to demonstrate his superiority to β-blocker therapy in preventing recurrent ICD-shocks in several randomized-controlled trials[22,35,36]. Moreover, an increased rate of arrhythmic deaths has been observed among patients with LV dysfunction and previous myocardial infarction treated with sotalol d-isomer alone for primary prevention of sudden death[37]. Basing upon this data it seems appropriate to consider sotalol only for VAs irresponsive to β-blockers. However, in patients with chronic kidney disease and severely depressed LVEF, it still should be avoided in favor of other medications like amiodarone[22].
GENERAL ANESTHESIA AND MECHANICAL HEMODYNAMIC SUPPORT
Sedation should be considered in all patients presenting with ES in order to minimize pain related to ICD-shocks and reduce the sympathetic surge triggered by repeated ICD therapies. Benzodiazepines such as midazolam in addition to short-acting analgesics such as remifentanil should be the first choice being able to suppress the sympathetic hyperactivity and provide analgesia without negative inotropic effects[38,39]. Propofol has been reported to suppress ES but must be used carefully since its negative inotropic effects can lead to cardiogenic shock[40]. Dexmedetomidine is an α2-presynaptic receptor agonist that reduces sympathetic activity by enhancing central vagal tone and inhibiting presynaptic catecholamine release. It should be used cautiously, however, since it may result in severe hypotension and bradycardia[41,42]. General anesthesia and mechanical ventilation should be preferred for patients with hemodynamic unstable VTs, because drugs used for anesthesia induction and maintenance can further depress cardiac function[43]. Patients with unstable VTs may also benefit from mechanical hemodynamic support like IABP, LVAD and ECMO. Hemodynamic support can reduce the arrhythmic burden by increasing coronary perfusion, reducing afterload and therefore myocardial wall stress and prevent multiple organ failure guarantying and adequate cardiac output[44-46].
NEURAXIAL MODULATION
Sympathetic hyperactivity plays a critical role in the onset and maintenance of VAs. Therefore, modulation of neuraxial efferents to the heart with epidural anesthesia or cardiac sympathetic denervation (CSD) may be a valuable option in selected patients refractory to standard medical treatment and CA[47,48]. Sympathetic denervation has been effectively used in the setting of inherited arrhythmic syndromes like long QT syndrome and catecholaminergic polymorphic VT[49,50]. However, it has been recently applied even to ES in patients with structural heart disease[47,48]. Surgical CSD is usually performed on the left side through a video-assisted thorascopic approach and entails removal of the lower third of the stellate ganglion (to avoid Horner syndrome) and T2-T4 thoracic ganglia. It has shown to suppress/significantly decrease the arrhythmic burden in 56% of patients refractory to AADs and CA[47]. Bilateral CSD may be considered in cases of failure of left CSD. In a small study involving 6 patients undergoing bilateral CSD after failed medical therapy, CA and epidural anesthesia, a complete response was observed in 4 (67%) of them and a partial response in another one (17%)[48]. In a recent series of 41 patients with refractory VT undergoing either left (14) or bilateral (27) CSD, a significant reduction of ICD-shocks during a mean follow-up of 367 ± 251 d was observed in 90% of the patients with a significantly higher ICD-shock free survival of 48% in the bilateral CSD group compared to 30% in the left CSD group[51].
CATHETER ABLATION
The last decade has seen a growing role for catheter ablation (CA) in the management of VT. Even if a mortality benefit has never been demonstrated in randomized-controlled trials, CA has repeatedly shown its superiority to medical therapy in reducing the arrhythmic burden[11,52,53]. Moreover, freedom from recurrent VT after CA ablation has been associated with improved survival[54,55]. For these reasons, CA should not be considered a bailout therapy but a valuable option in all patients presenting with ES related to structural heart disease. Radiofrequency CA is effective not only in the acute management of ES, leading to a control of VAs in up to 80%-90% of the patients but also over the long-term follow-up improving either VT- and ES-free survival (Table 3)[56,57]. In the recently published VANISH trial, a trend towards a 34% relative risk reduction of ES recurrences was observed in patients treated by CA compared to escalation of AADs[52]. In a pooled meta-analysis including 471 patients with ES treated invasively by different ablation strategies (i.e., CA, ethanol ablation and surgical ablation), acute elimination of all inducible VAs was reached in 72% of the cases with the clinical arrhythmia effectively suppressed in 91% of the patients and a complication rate of 2% with a procedure-related death < 1%. In terms of long-term outcomes, after a median follow-up of 1.2 years, 94% of the patients were free from ES and 72% were free from any VT. Overall mortality was 17% at 1.2-years follow-up with most of the deaths related to progressive HF (62%)[58]. Similar positive results have recently been found by our group in a large series of 267 patients undergoing CA for drug-refractory ES with an acute procedural success (non inducibility of any VT with cycle length < 250 ms at the end of the procedure) of 73%, a 54% VT-free survival and a 93% ES-free survival at 60-mo follow-up. We also observed a significant reduction of VT burden in patients experiencing VT recurrence after CA[59]. Regardless, patients with ES tend to have worse prognosis after CA compared vs patients without ES, as evidenced by the fact that those with ES have higher VT recurrence rates and are more likely to die or require heart transplantation or surgical LVAD over long-term follow-up after CA[60].
Table 3.
Principal studies analyzing the role of catheter ablation in controlling electrical storm
| Ref. | No. of patients | Left ventricular ejection fraction | Epicardial procedures | Acute success | VT recurrence | ES recurrence | Death | Follow-up duration, mo |
| Sra et al[64] | 19 | 27 ± 8 | 0% | 87% | 37% | - | 0% | 7 ± 2 |
| Silva et al[65] | 14 | 31 ± 13 | 20% | 80% | 13% | - | 27% | 12 ± 17 |
| Carbucicchio et al[56] | 95 | 36 ± 11 | 11% | 89% | 34% | 8% | 16% | Median 22 |
| Arya et al[66] | 13 | 33 ± 9 | 31% | 100% | 38% | - | 31% | Median 23 |
| Pluta et al[67] | 21 | - | 0% | 81% | 19% | 0% | 0% | 3 |
| Deneke et al[68] | 31 | 28 ± 15 | 9% | 94% | 25% | 12% | 9% | Median 15 |
| Kozeluhova et al[69] | 50 | 29 ± 11 | 0% | 85% | 52% | 26% | 29% | 18 ± 16 |
| Koźluk et al[70] | 24 | 27 ± 7 | 7% | - | 34% | 12% | 13% | 28 ± 16 |
| Di Biase et al[57] | 92 | 27 ± 5 | 47% | 100% | 34% | 0% | 2% | 25 ± 10 |
| Izquierdo et al[71] | 23 | 34 ± 10 | 0% | 56% | - | 35% | 30% | Median 18 |
| Jin et al[72] | 40 | 21 ± 7 | 0% | 80% | 53% | - | 25% | 17 ± 17 |
| Kumar et al[73] | 287 | 27 ± 10 in ICM and 33 ± 16 in NICM | 3.8% in ICM and 24% in NICM | 60% in ICM and 50% in NICM | 49% in ICM and 64% in NICM | 17% in ICM and 27% in NICM | 25% in ICM and 28% in NICM | Median 42 |
| Muser et al[59] | 267 | 29 ± 13 | 22% | 73% | 33% | 5% | 29% | Median 45 |
VT: Ventricular tachycardia; ES: Electrical storm.
As patients with chronic HF are living longer with their condition, technological advances to CA and better understanding of VT substrate has led to an increased number of procedures performed in high risk patients. Patients with advanced HF, several comorbidities as well as patients with unstable VTs are at highest risk of hemodynamic collapse during the ablation procedure and subsequent post-procedural mortality[43,61]. In a preliminary study of our group, a simple score (PAINESD score) accounting for baseline patient characteristics such as pulmonary chronic obstructive disease, age, Ischemic cardiomyopathy, NYHA class, LVEF, ES at presentation and diabetes has been demonstrated able to predict acute decompensation during VT ablation procedures and therefore has been proposed to select patients who may benefit from prophylactic mechanical support (Figure 2)[43]. Recently, the PAINESD score has been validated in a study assessing the outcomes of prophylactic vs rescue percutaneous LVAD in a cohort of 93 patients undergoing CA for VT related to structural heart disease[61]. The authors reported a higher 30-d mortality in patients who underwent rescue LVAD (58%) compared to patients who underwent prophylactic LVAD (4%) placement and patients who were ablated without LVAD (3%). Interestingly, patients who underwent rescue LVAD had similar PAINESD scores compared to those who underwent prophylactic LVAD insertion (mean 17.8 vs 16.5) while had a significantly higher score compared to the control group (mean 13.4), highlighting the importance of prophylactic mechanical support in high risk patients in order to improve post-procedural mortality[61]. Mechanical support is helpful in that it allows for prolonged mapping and ablation of inducible unstable arrhythmias. However, we have also found it to be useful when used prophylactically in high-risk patients with large areas of VT substrate undergoing a purely substrate-based ablation approach in which the long procedural times necessarily for complete substrate ablation and the consequent fluid overload related to irrigated CA may precipitate acute decompensation[43]. Importantly, some patients with advanced HF have significant biventricular dysfunction and LVAD support may be inadequate. In these cases, devices providing biventricular support like ECMO should be considered. In a recent study involving 64 patients undergoing CA of unstable VTs, the prophylactic use of ECMO has shown to allow to safely complete the procedure in 92% of the patients reaching the endpoint of VT non inducibility in 69% of them with a 88% overall survival after a median follow-up of 21 mo[46].
Figure 2.
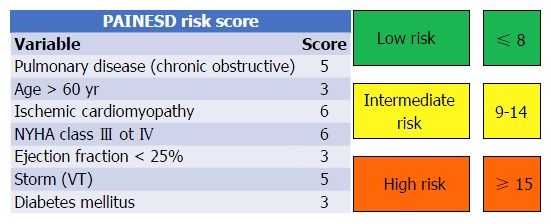
Proposed scoring system to identify patients at high risk of hemodynamic decompensation undergoing catheter ablation that may benefit from prophylactic mechanical circulatory support. Modified from Santangeli et al[43]. VT: Ventricular tachycardia.
ALTERNATIVE APPROACHES
In cases in whom radiofrequency CA has failed or is challenging (i.e., presence of mitral and aortic mechanical valves), alternative approaches like trans-coronary ethanol ablation and surgical cryoablation has been described[62]. Our group has recently reported a 73% VT-free survival at 1-year follow-up in a series of 20 consecutive patients with non-ischemic cardiomyopathy and VT refractory to conventional therapy who underwent surgical cryoablation[63]. Trans-coronary ethanol ablation performed through selective coronary angiography to identify the branches supplying the putative VT site of origin has been recently reported in a series of 46 patients with VT related to structural heart disease and refractory to CA[62]. At least partial procedural success was reached in 66% of the patients with a 74% and 82% VT recurrence rate at 6- and 12-mo follow-up, respectively and a complication rate of 32% (1 procedure related death).
CONCLUSION
Electrical storm is a life-threatening condition with an increasing incidence related to the wider use of ICD and the improved survival of patients with advanced HF. Management of ES requires a multimodality approach including optimal ICD-reprogramming, treatment of underlying conditions, anti-arrhythmic drug therapy, sedation and CA. Radiofrequency CA appears to be the most effective treatment option, being able to control arrhythmia burden in the acute phase and improve long-term arrhythmia free survival and therefore should be considered in all patients presenting with ES. A growing evidence supports the use of prophylactic mechanical hemodynamic support as a bridge to ablation and/or recovery in high risk patients.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors report no relevant conflicts of interest.
Peer-review started: February 17, 2017
First decision: April 14, 2017
Article in press: May 15, 2017
P- Reviewer: De Ponti R, den Uil CA, Dizon JM, Nam GB S- Editor: Song XX L- Editor: A E- Editor: Li D
References
- 1.Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm. 2014;11:e166–e196. doi: 10.1016/j.hrthm.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Conte G, Sieira J, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, La Meir M, Wellens F, Czapla J, et al. Implantable cardioverter-defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J Am Coll Cardiol. 2015;65:879–888. doi: 10.1016/j.jacc.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Hohnloser SH, Al-Khalidi HR, Pratt CM, Brum JM, Tatla DS, Tchou P, Dorian P; SHock Inhibition Evaluation with AzimiLiDe (SHIELD) Investigators. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006;27:3027–3032. doi: 10.1093/eurheartj/ehl276. [DOI] [PubMed] [Google Scholar]
- 4.Della Bella P, Baratto F, Tsiachris D, Trevisi N, Vergara P, Bisceglia C, Petracca F, Carbucicchio C, Benussi S, Maisano F, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. 2013;127:1359–1368. doi: 10.1161/CIRCULATIONAHA.112.000872. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, Greenberg H, Hall WJ, Huang DT, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 6.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, Birgersdotter-Green UM, Wathen MS, Van Gelder IC, Heubner BM, et al. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52:541–550. doi: 10.1016/j.jacc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Auricchio A, Schloss EJ, Kurita T, Meijer A, Gerritse B, Zweibel S, AlSmadi FM, Leng CT, Sterns LD; PainFree SST Investigators. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm. 2015;12:926–936. doi: 10.1016/j.hrthm.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney MO. Antitachycardia pacing for ventricular tachycardia using implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2004;27:1292–1305. doi: 10.1111/j.1540-8159.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 10.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 11.Santangeli P, Muser D, Maeda S, Filtz A, Zado ES, Frankel DS, Dixit S, Epstein AE, Callans DJ, Marchlinski FE. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–1559. doi: 10.1016/j.hrthm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Stanton MS, Prystowsky EN, Fineberg NS, Miles WM, Zipes DP, Heger JJ. Arrhythmogenic effects of antiarrhythmic drugs: a study of 506 patients treated for ventricular tachycardia or fibrillation. J Am Coll Cardiol. 1989;14:209–215; discussion 216-217. doi: 10.1016/0735-1097(89)90074-0. [DOI] [PubMed] [Google Scholar]
- 13.Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm: sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation. 2000;102:742–747. doi: 10.1161/01.cir.102.7.742. [DOI] [PubMed] [Google Scholar]
- 14.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 15.Billman GE, Castillo LC, Hensley J, Hohl CM, Altschuld RA. Beta2-adrenergic receptor antagonists protect against ventricular fibrillation: in vivo and in vitro evidence for enhanced sensitivity to beta2-adrenergic stimulation in animals susceptible to sudden death. Circulation. 1997;96:1914–1922. doi: 10.1161/01.cir.96.6.1914. [DOI] [PubMed] [Google Scholar]
- 16.Tsagalou EP, Kanakakis J, Rokas S, Anastasiou-Nana MI. Suppression by propranolol and amiodarone of an electrical storm refractory to metoprolol and amiodarone. Int J Cardiol. 2005;99:341–342. doi: 10.1016/j.ijcard.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Brodine WN, Tung RT, Lee JK, Hockstad ES, Moss AJ, Zareba W, Hall WJ, Andrews M, McNitt S, Daubert JP; MADIT-II Research Group. Effects of beta-blockers on implantable cardioverter defibrillator therapy and survival in the patients with ischemic cardiomyopathy (from the Multicenter Automatic Defibrillator Implantation Trial-II) Am J Cardiol. 2005;96:691–695. doi: 10.1016/j.amjcard.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Connolly SJ. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025–2034. doi: 10.1161/01.cir.100.19.2025. [DOI] [PubMed] [Google Scholar]
- 19.Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 20.Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 21.Kowey PR, Crijns HJ, Aliot EM, Capucci A, Kulakowski P, Radzik D, Roy D, Connolly SJ, Hohnloser SH; ALPHEE Study Investigators. Efficacy and safety of celivarone, with amiodarone as calibrator, in patients with an implantable cardioverter-defibrillator for prevention of implantable cardioverter-defibrillator interventions or death: the ALPHEE study. Circulation. 2011;124:2649–2660. doi: 10.1161/CIRCULATIONAHA.111.072561. [DOI] [PubMed] [Google Scholar]
- 22.Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, Thorpe K, Champagne J, Talajic M, Coutu B, Gronefeld GC, Hohnloser SH; Optimal Pharmacological Therapy in Cardioverter Defibrillator Patients (OPTIC) Investigators. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006;295:165–171. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 23.Greene M, Newman D, Geist M, Paquette M, Heng D, Dorian P. Is electrical storm in ICD patients the sign of a dying heart? Outcome of patients with clusters of ventricular tachyarrhythmias. Europace. 2000;2:263–269. doi: 10.1053/eupc.2000.0104. [DOI] [PubMed] [Google Scholar]
- 24.Holt DW, Tucker GT, Jackson PR, Storey GC. Amiodarone pharmacokinetics. Am Heart J. 1983;106:840–847. doi: 10.1016/0002-8703(83)90006-6. [DOI] [PubMed] [Google Scholar]
- 25.Jung W, Manz M, Pizzulli L, Pfeiffer D, Lüderitz B. Effects of chronic amiodarone therapy on defibrillation threshold. Am J Cardiol. 1992;70:1023–1027. doi: 10.1016/0002-9149(92)90354-2. [DOI] [PubMed] [Google Scholar]
- 26.Dixon DL, Dunn SP, Kelly MS, McLlarky TR, Brown RE. Effectiveness of Pharmacist-Led Amiodarone Monitoring Services on Improving Adherence to Amiodarone Monitoring Recommendations: A Systematic Review. Pharmacotherapy. 2016;36:230–236. doi: 10.1002/phar.1697. [DOI] [PubMed] [Google Scholar]
- 27.Liang JJ, Yang W, Santangeli P, Schaller RD, Supple GE, Hutchinson MD, Garcia F, Lin D, Dixit S, Epstein AE, et al. Amiodarone Discontinuation or Dose Reduction Following Catheter Ablation for Ventricular Tachycardia in Structural Heart Disease. JACC Clin Electrophysiol. 2017:309. doi: 10.1016/j.jacep.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Gorgels AP, van den Dool A, Hofs A, Mulleneers R, Smeets JL, Vos MA, Wellens HJ. Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am J Cardiol. 1996;78:43–46. doi: 10.1016/s0002-9149(96)00224-x. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J; PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marill KA, deSouza IS, Nishijima DK, Senecal EL, Setnik GS, Stair TO, Ruskin JN, Ellinor PT. Amiodarone or procainamide for the termination of sustained stable ventricular tachycardia: an historical multicenter comparison. Acad Emerg Med. 2010;17:297–306. doi: 10.1111/j.1553-2712.2010.00680.x. [DOI] [PubMed] [Google Scholar]
- 31.MacMahon S, Collins R, Peto R, Koster RW, Yusuf S. Effects of prophylactic lidocaine in suspected acute myocardial infarction. An overview of results from the randomized, controlled trials. JAMA. 1988;260:1910–1916. [PubMed] [Google Scholar]
- 32.European Heart Rhythm Association; Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain DA, Jewitt DE, Julian DG, Campbell RW, Boyle DM, Shanks RG. Oral mexiletine in high-risk patients after myocardial infarction. Lancet. 1980;2:1324–1327. doi: 10.1016/s0140-6736(80)92395-8. [DOI] [PubMed] [Google Scholar]
- 34.Gao D, Van Herendael H, Alshengeiti L, Dorian P, Mangat I, Korley V, Ahmad K, Golovchiner G, Aves T, Pinter A. Mexiletine as an adjunctive therapy to amiodarone reduces the frequency of ventricular tachyarrhythmia events in patients with an implantable defibrillator. J Cardiovasc Pharmacol. 2013;62:199–204. doi: 10.1097/FJC.0b013e31829651fe. [DOI] [PubMed] [Google Scholar]
- 35.Pacifico A, Hohnloser SH, Williams JH, Tao B, Saksena S, Henry PD, Prystowsky EN. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N Engl J Med. 1999;340:1855–1862. doi: 10.1056/NEJM199906173402402. [DOI] [PubMed] [Google Scholar]
- 36.Kettering K, Mewis C, Dörnberger V, Vonthein R, Bosch RF, Kühlkamp V. Efficacy of metoprolol and sotalol in the prevention of recurrences of sustained ventricular tachyarrhythmias in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2002;25:1571–1576. doi: 10.1046/j.1460-9592.2002.01571.x. [DOI] [PubMed] [Google Scholar]
- 37.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 38.Mandel JE, Hutchinson MD, Marchlinski FE. Remifentanil-midazolam sedation provides hemodynamic stability and comfort during epicardial ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22:464–466. doi: 10.1111/j.1540-8167.2010.01889.x. [DOI] [PubMed] [Google Scholar]
- 39.Ogletree ML, Sprung J, Moravec CS. Effects of remifentanil on the contractility of failing human heart muscle. J Cardiothorac Vasc Anesth. 2005;19:763–767. doi: 10.1053/j.jvca.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 40.Mulpuru SK, Patel DV, Wilbur SL, Vasavada BC, Furqan T. Electrical storm and termination with propofol therapy: a case report. Int J Cardiol. 2008;128:e6–e8. doi: 10.1016/j.ijcard.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 41.Tarvainen MP, Georgiadis S, Laitio T, Lipponen JA, Karjalainen PA, Kaskinoro K, Scheinin H. Heart rate variability dynamics during low-dose propofol and dexmedetomidine anesthesia. Ann Biomed Eng. 2012;40:1802–1813. doi: 10.1007/s10439-012-0544-1. [DOI] [PubMed] [Google Scholar]
- 42.Gerlach AT, Murphy CV. Dexmedetomidine-associated bradycardia progressing to pulseless electrical activity: case report and review of the literature. Pharmacotherapy. 2009;29:1492. doi: 10.1592/phco.29.12.1492. [DOI] [PubMed] [Google Scholar]
- 43.Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, Supple G, Frankel DS, Garcia FC, Bala R, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol. 2015;8:68–75. doi: 10.1161/CIRCEP.114.002155. [DOI] [PubMed] [Google Scholar]
- 44.Miller MA, Dukkipati SR, Mittnacht AJ, Chinitz JS, Belliveau L, Koruth JS, Gomes JA, d’Avila A, Reddy VY. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011;58:1363–1371. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Miller MA, Dukkipati SR, Chinitz JS, Koruth JS, Mittnacht AJ, Napolitano C, d’Avila A, Reddy VY. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1) Circ Arrhythm Electrophysiol. 2013;6:151–159. doi: 10.1161/CIRCEP.112.975888. [DOI] [PubMed] [Google Scholar]
- 46.Baratto F, Pappalardo F, Oloriz T, Bisceglia C, Vergara P, Silberbauer J, Albanese N, Cireddu M, D’Angelo G, Di Prima AL, et al. Extracorporeal Membrane Oxygenation for Hemodynamic Support of Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2016:9. doi: 10.1161/CIRCEP.116.004492. [DOI] [PubMed] [Google Scholar]
- 47.Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–2262. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ajijola OA, Lellouche N, Bourke T, Tung R, Ahn S, Mahajan A, Shivkumar K. Bilateral cardiac sympathetic denervation for the management of electrical storm. J Am Coll Cardiol. 2012;59:91–92. doi: 10.1016/j.jacc.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 50.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 51.Vaseghi M, Gima J, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–366. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong-Sit P, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016;375:111–121. doi: 10.1056/NEJMoa1513614. [DOI] [PubMed] [Google Scholar]
- 53.Liang JJ, Muser D, Santangeli P. Ventricular Tachycardia Ablation Clinical Trials. Card Electrophysiol Clin. 2017;9:153–165. doi: 10.1016/j.ccep.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12:1997–2007. doi: 10.1016/j.hrthm.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muser D, Santangeli P, Castro SA, Pathak RK, Liang JJ, Hayashi T, Magnani S, Garcia FC, Hutchinson MD, Supple GG, et al. Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol. 2016;9:pii: e004328. doi: 10.1161/CIRCEP.116.004328. [DOI] [PubMed] [Google Scholar]
- 56.Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 57.Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio C, Dello Russo A, Casella M, Mohanty S, Pump A, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–141. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 58.Nayyar S, Ganesan AN, Brooks AG, Sullivan T, Roberts-Thomson KC, Sanders P. Venturing into ventricular arrhythmia storm: a systematic review and meta-analysis. Eur Heart J. 2013;34:560–571. doi: 10.1093/eurheartj/ehs453. [DOI] [PubMed] [Google Scholar]
- 59.Muser D, Liang JJ, Pathak RK, Magnani S, Castro SA, Hayashi T, Garcia FC, Supple GE, Riley MP, Lin D, et al. Long-Term Outcomes of Catheter Ablation of Electrical Storm in Nonischemic Dilated Cardiomyopathy Compared With Ischemic Cardiomyopathy. JACC Clin Electrophysiol. 2017:371. doi: 10.1016/j.jacep.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 60.Frankel DS, Liang JJ, Supple G, Dixit S, Hutchinson MD, Elafros MA, Callans DJ, Marchlinski FE. Electrophysiological Predictors of Transplantation and Left Ventricular Assist Device-Free Survival in Patients With Nonischemic Cardiomyopathy Undergoing Ventricular Tachycardia Ablation. JACC Clin Electrophysiol. 2015;1:398–407. doi: 10.1016/j.jacep.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Mathuria N, Wu G, Rojas-Delgado F, Shuraih M, Razavi M, Civitello A, Simpson L, Silva G, Wang S, Elayda M, et al. Outcomes of pre-emptive and rescue use of percutaneous left ventricular assist device in patients with structural heart disease undergoing catheter ablation of ventricular tachycardia. J Interv Card Electrophysiol. 2017;48:27–34. doi: 10.1007/s10840-016-0168-8. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, Barbhaiya CR, Sobieszczyk P, Eisenhauer AC, Couper GS, Nagashima K, Mahida S, Baldinger SH, Choi EK, Epstein LM, et al. Role of alternative interventional procedures when endo- and epicardial catheter ablation attempts for ventricular arrhythmias fail. Circ Arrhythm Electrophysiol. 2015;8:606–615. doi: 10.1161/CIRCEP.114.002522. [DOI] [PubMed] [Google Scholar]
- 63.Liang JJ, Betensky BP, Muser D, Zado ES, Anter E, Desai ND, Callans DJ, Deo R, Frankel DS, Hutchinson MD, et al. Long-term outcome of surgical cryoablation for refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Europace. 2017 doi: 10.1093/europace/eux029. [DOI] [PubMed] [Google Scholar]
- 64.Sra J, Bhatia A, Dhala A, Blanck Z, Deshpande S, Cooley R, Akhtar M. Electroanatomically guided catheter ablation of ventricular tachycardias causing multiple defibrillator shocks. Pacing Clin Electrophysiol. 2001;24:1645–1652. doi: 10.1046/j.1460-9592.2001.01645.x. [DOI] [PubMed] [Google Scholar]
- 65.Silva RM, Mont L, Nava S, Rojel U, Matas M, Brugada J. Radiofrequency catheter ablation for arrhythmic storm in patients with an implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2004;27:971–975. doi: 10.1111/j.1540-8159.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 66.Arya A, Bode K, Piorkowski C, Bollmann A, Sommer P, Gaspar T, Wetzel U, Husser D, Kottkamp H, Hindricks G. Catheter ablation of electrical storm due to monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy: acute results and its effect on long-term survival. Pacing Clin Electrophysiol. 2010;33:1504–1509. doi: 10.1111/j.1540-8159.2010.02835.x. [DOI] [PubMed] [Google Scholar]
- 67.Pluta S, Lenarczyk R, Pruszkowska-Skrzep P, Kowalski O, Sokal A, Sredniawa B, Mazurek M, Kalarus Z. Transseptal versus transaortic approach for radiofrequency ablation in patients with cardioverter-defibrillator and electrical storm. J Interv Card Electrophysiol. 2010;28:45–50. doi: 10.1007/s10840-009-9464-x. [DOI] [PubMed] [Google Scholar]
- 68.Deneke T, Shin DI, Lawo T, Bösche L, Balta O, Anders H, Bünz K, Horlitz M, Grewe PH, Lemke B, et al. Catheter ablation of electrical storm in a collaborative hospital network. Am J Cardiol. 2011;108:233–239. doi: 10.1016/j.amjcard.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Kozeluhova M, Peichl P, Cihak R, Wichterle D, Vancura V, Bytesnik J, Kautzner J. Catheter ablation of electrical storm in patients with structural heart disease. Europace. 2011;13:109–113. doi: 10.1093/europace/euq364. [DOI] [PubMed] [Google Scholar]
- 70.Koźluk E, Gaj S, Kiliszek M, Lodziński P, Piątkowska A, Opolski G. Efficacy of catheter ablation in patients with an electrical storm. Kardiol Pol. 2011;69:665–670. [PubMed] [Google Scholar]
- 71.Izquierdo M, Ruiz-Granell R, Ferrero A, Martínez A, Sánchez-Gomez J, Bonanad C, Mascarell B, Morell S, García-Civera R. Ablation or conservative management of electrical storm due to monomorphic ventricular tachycardia: differences in outcome. Europace. 2012;14:1734–1739. doi: 10.1093/europace/eus186. [DOI] [PubMed] [Google Scholar]
- 72.Jin Q, Jacobsen PK, Pehrson S, Chen X. Acute and long term outcomes of catheter ablation using remote magnetic navigation for the treatment of electrical storm in patients with severe ischemic heart failure. Int J Cardiol. 2015;183:11–16. doi: 10.1016/j.ijcard.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 73.Kumar S, Fujii A, Kapur S, Romero J, Mehta NK, Tanigawa S, Epstein LM, Koplan BA, Michaud GF, John RM, et al. Beyond the Storm: Comparison of Clinical Factors, Arrhythmogenic Substrate, and Catheter Ablation Outcomes in Structural Heart Disease Patients With versus Those Without a History of Ventricular Tachycardia Storm. J Cardiovasc Electrophysiol. 2017;28:56–67. doi: 10.1111/jce.13117. [DOI] [PubMed] [Google Scholar]


