Abstract
AIM
To conduct a review of “interferon related pericarditis”.
METHODS
We searched MEDLINE, EMBASE, Cinahl, and the Cochrane Database from the earliest available date through September 2016. A search strategy using the Medical Subject Headings and text keywords “interferon” and ”pericarditis” were used.
RESULTS
Nine case reports were eligible for the present study. Six of 8 cases were women and the mean age was 43.8 ± 13.8 years with chronic hepatitis C in 6 cases, malignant melanoma in 2 cases and chronic myelogenous leukemia in 1 case. The patients complained of chest pain in 6 cases, dyspnea in 5 cases and edema in 2 cases. Pericardial friction rub was heard in 3 of 9 cases. Congestive heart failure occurred in 3 of 9 cases. Two mechanisms for pericarditis were demonstrated, one is autoimmune included lupus like syndrome in 2 cases and the other is cardio toxicity in 4 cases. Treatment of interferon related pericarditis is discontinuation of Interferon treatment. Four of 9 cases were treated with prednisone and 4 with nonsteroidal anti-inflammatory drugs.
CONCLUSION
Interferon related pericarditis still remains uncertain. Treatment of interferon related pericarditis rests mainly on early recognition and drug discontinuation. Interferon related pericarditis was treated with steroid and/or nonsteroidal anti-inflammatory drugs.
Keywords: Chronic hepatitis C, Chronic myelogeneous leukemia, Interferon, Malignant lymphoma, Pericarditis
Core tip: Interferon is considered to be one of the treatments for many diseases. However, interferon therapy is associated with side effects. Recently some reports demonstrated acute pericarditis complicating interferon therapy. Two mechanisms for pericarditis were demonstrated, one is autoimmune included lupus like syndrome and the other is cardio toxicity. However, these two mechanisms are controversial. The aim of this study is to review of “interferon related pericarditis”.
INTRODUCTION
Interferon is considered to be one of the treatments for many diseases. However, interferon therapy is associated with side effects, the most common being general symptoms such as fever, weight loss and headache. Some studies have demonstrated cardiac adverse effects of interferon for chronic hepatitis C (CHC)[1,2]. Most frequently reported are arrhythmia, congestive heart failure and sudden death. Reported with rarer frequency are polyneuropathy, paranoia and suicidal thoughts, diabetes mellitus, retinopathy, optical neuritis, diminution of hearing, seizures, loss of libido and cardio toxicity[3]. Recently some reports demonstrated acute pericarditis complicating interferon therapy[4-12]. We conducted a review of “interferon related pericarditis”.
MATERIALS AND METHODS
Selection of case reports
We searched MEDLINE, EMBASE, Cinahl, and the Cochrane Database from the earliest available date through September 2016. A search strategy using the Medical Subject Headings and text keywords “interferon” and “pericarditis” were used. The retrieved studies were manually screened to assess their appropriateness for this study. All references cited in the studies were also reviewed to identify additional published articles not indexed in the database. The search was not restricted by language.
RESULTS
Patients
Nine case reports were eligible for the present study; seven were in English[4,5,7,8,10-12] and two in French[6,9]. Clinical characteristics of patients are shown in Table 1. Six of 8 cases were women and the mean age was 43.8 ± 13.8 years with CHC in 6 cases, malignant melanoma in 2 cases and chronic myelogenous leukemia in 1 case. The patients complained of chest pain in 6 cases, dyspnea in 5 cases and edema in 2 cases. Pericardial friction rub was heard in 3 of 9 cases[5,8,9]. Congestive heart failure occurred in 3 of 9 cases[8-10]. Two mechanisms for pericarditis were demonstrated, one is autoimmune included lupus like syndrome (AI group) in 2 cases and the other is cardio toxicity (CT group) in 4 cases. Three of 9 articles didn’t mention the mechanisms for pericarditis.
Table 1.
Case reports of pericarditis associated with interferon
| Ref. | Age/gender | Disease | Administered | Duration of IFN therapy | Mechanism |
| Fava et al[4] | 28/NA | CML | IFNα | 13 mo | NA |
| Boonen et al[5] | 24/F | CHC | IFNα | 1 mo | Autoimmune |
| Wisniewski et al[6] | 42/F | CHC | IFNα | 6 h | Cardio toxicity |
| Gressens et al[7] | 40/F | CHC | IFNα2b | 3 mo | Cardio toxicity |
| Benjamini et al[8] | 63/M | MM | IFNα2b | 1 mo | Cardio toxicity |
| Hamdani et al[9] | 53/F | CHC | PEG IFN α2a | 6 mo | NA |
| Nishio et al[10] | 67/M | CHC | PEG IFN 2a | 15 d | Lupus like syndrome |
| Popescu et al[11] | 38/F | CHC | PEG IFN 2a | 7 mo | Cardio toxicity |
| Ashraf et al[12] | 39/F | MM | IFNα | 1 d | NA |
CHC: Chronic hepatitis C; CML: Chronic myelogenous leukemia; F: Female; IFN: Interferon; M: Male; MM: Malignant melanoma; NA: Data not available; PEG IFN: Pegylated interferon.
Clinical history
Three cases had clinical history. The 28-year-old patient had allergic asthma since infancy[4]. The 24-year-old woman following therapy with interferon α was diagnosed with systemic lupus erythematosus (SLE), and she was treated with prednisone (40 mg/d)[5]. When prednisone had been stopped completely for 3 mo, a pericarditis occurred. The patient had a recurrence of SLE. The 63-year-old man was diagnosed as diffuse large B cell non-Hodgkin’s lymphoma and was treated with a full course of chemotherapy consisting of cyclophosphamide, adriamycin, vincristine and dexamethasone[8].
Laboratory findings
The woman with SLE[5] experienced four episodes of fever and pain in the left shoulder while breathing. Antinuclear antibody and anti-ds DNA antibody tests were negative, whereas circulating immune complexes were positive at the second and the third episode. CH50 and C4 levels were decreased with slightly elevated C3d level. Laboratory results of the 63-year-old man with non-Hodgkin’s lymphoma presented antinuclear antibodies (titer 1/40)[8]. The blood sample examination of the 67-year-old man with CHC showed anti-DNA antibody and anti-ds DNA IgM were positive[10].
Interferon daily dose and duration of treatment with interferon
Figure 1 showed a relationship between the daily dose of interferon and the duration of treatment with interferon. Autoimmune due to interferon does not dependent on the daily dose but developed within one month with interferon treatment. Cardio toxicity due to interferon does not dependent on the daily dose or the duration of treatment with interferon.
Figure 1.
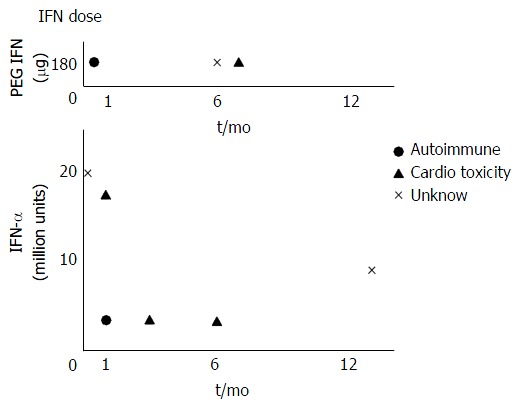
The relationship between interferon dose and duration of interferon therapy. PEG IFN: Pegylated interferon.
Chest radiography
Chest radiography demonstrated abnormalities in three cases. Chest X-ray of the 63-year-old man showed an enlarged heart silhouette and bilateral pleural effusion[8]. Chest radiograph of the 53-year-old woman showed cardiomegaly[9]. Portable chest radiograph of the 67-year-old man revealed pulmonary vascular congestion without pleural effusion[10].
Electrocardiogram
Electrocardiogram (ECG) demonstrated abnormality in one case. ECG showed gradual ST-segment elevation in leads V1 through V6 without elevated myocardial enzyme in the 67-year-old man[10]. Coronary angiography showed that there was no significant coronary arterial stenosis in this case.
Ultrasound cardiology
Ultrasound cardiology (UCG) demonstrated pericardial effusion in 7 of 9 cases; mild in 2 cases[6,12], moderate in 2 cases[4,10], severe in 1 case[9], and no presentation in 2 cases[7,11]. The 53-year-old female was diagnosed with constructive pericarditis with pre-tamponade[9].
Re-start and re-challenge test
The interferon treatment was restarted in three cases[4,6,11]. The 28-year-old patient suffered a pericarditis relapse at seven months after resumption of interferon α therapy[4]. The 42-year-old female felt chest pain, after 7 h from administration of 1 million interferon α[6]. After the first dose of interferon administration symptoms reappeared and UCG showed an increase of pericardial fluid in the 38-year-old female[11]. Re-challenge test was performed in one case[12]. Within ten hours of the re-initiation of interferon therapy, the 39-year-old woman developed chest pain identical to her previous pain.
Complications
Two cases developed other complications. An electromyography showed signs of polyneuropathy in the 40-year-old female[7]. The 67-year-old man developed chronic inflammatory demyelinating polyneuropathy during treatment with pegylated interferon-2a for chronic active hepatitis C viral infection[10].
Treatment
Treatment of drug-induced cardio toxicity rests mainly on early recognition and drug discontinuation. Interferon treatment was stopped in 7 of 9 cases. Four of 9 cases were treated with prednisone from 10 mg per day to 50 mg/d[4,5,8,10] and 4 with nonsteroidal anti-inflammatory drugs (NSAIDs) [5,7,11,12]. All of the AI group was treated with prednisone[5,10] and two of the CT group were treated with NSAIDs[7,11] and one of the CT group dexamethasone[8]. The 53-year-old female was treated with medications of anti-tuberculosis, but died because multiple organ failure[9]. Figure 2 showed an algorithm of the suspected interferon related pericarditis management.
Figure 2.
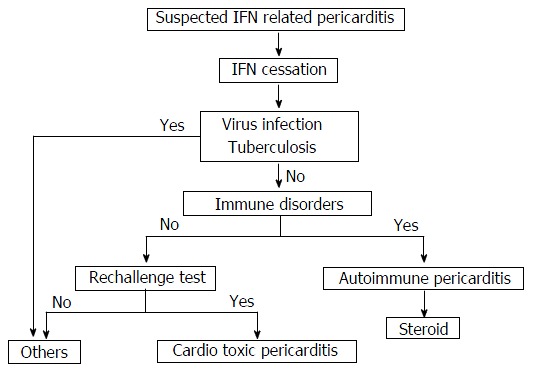
Algorithm of diagnoses and treatments for interferon related pericarditis. IFN: Interferon.
DISCUSSION
Cardiac adverse effects of interferon α for CHC have been demonstrated[1,2]. Most frequently reported are arrhythmias, congestive heart failure and sudden death. These side effects occurred during treatment with interferon α. However, pericarditis as a side effect of treatment with interferon is rare.
In 1988, the first report concerning interferon related pericarditis was presented by anonymity[13]. This report demonstrated two patients with continuous interferon therapy for chronic myelogenous leukemia had severe side effects consisting of pleural effusions and pericarditis. Montastruc et al[14] presented that there was an isolated pericarditis for which it was necessary to interrupt the interferon α treatment. However, these two articles didn’t describe in detail. Consequently, 9 articles were enrolled in the present study. Two mechanisms for pericarditis were demonstrated, one is autoimmune in 2 articles and the other is cardio toxicity in 4 articles. Three of 9 articles didn’t mention the mechanisms for pericarditis.
Prospective study reported on autoimmune phenomena in 987 patients treated with interferonα for CHC. Twelve patients developed hyperthyroidism, 6 hypothyroidism, 3 interstitial pneumonia, 1 SLE, 2 rheumatoid arthritis, 2 autoimmune hepatitis and 1 autoimmune thrombocytopenic purpura[15]. In the present study, the appearance of lupus-like syndrome by the interferon treatment has been reported in 1 article and autoimmune in 1 article. The mechanism by which interferon α induces autoimmune mediated complications is largely unknown. However, interferon alpha induses numerous target genes in antigen-presenting cells (APCs), such that APCs are stimulated and enhance humoral autoimmunity, promote isotype switching, and potently activate autoreactive T cells. Moreover, interferon alpha can synergistically amplify T cell autoreactivity by directly promoting T-cell activation and keeping activated T cells alive. Via the latter mechanisms, interferon can trigger autoimmune diseases[16]. There is a possibility that interferon may damage endothelial cells, cause the thickening of capillary walls, and induce deposition of immune complexes. Interferon evokes the release of several cytokines, including tumor necrosis factor alpha, and interleukins 2, 6 and 1, affecting autonomic sympathetic nerve activity and vasopressor responses[17]. Interferon induces an autoimmune reaction through various mechanisms including production of gamma globulins and interleukin-6 (IL-6)[18] and inhibition of Allo-specific suppressor T lymphocytes, as well as activation of natural killer cells[19]. IL-6 was significantly increased in pericardial effusion[20]. Interferon has been associated with exacerbation or induction of a wide variety of clinical and serological immune disorders, including systemic lupus erythematosus, rheumatoid arthritis, autoimmune hepatitis, thyroid disease and diabetes mellitus. On the other hand, Orságová et al[21] demonstrated that positivity of antinuclear antibodies and smooth muscle antibodies or increased rheumatoid factor and circulating immune complexes are often found in patients with chronic hepatitis B and CHC treated with interferon, but their presence does not correlate with the development of autoimmune diseases.
The cardiac toxicity of interferon alpha is also well known and uncommon. The mechanism of interferon cardio toxicity is unclear and probably multifactorial. There are no established predisposing factors for interferon cardio toxicity. The secondary effects of interferon described include arrhythmia (atrial fibrillation, sinus bradycardia, atrioventricular block), ischemic cardiomyopathy and cardiomyopathy with the dosage levels used in the treatment of hepatitis C[2]. Myocardial ischemia is mainly caused by cardio toxicity of interferon and antimetabolites[22]. Patients with previous heart disease are probably at higher risk for arrhythmia and ischemic manifestations[1]. Concerning drug toxicity, there have been reported cases of acute pericarditis after the administration of: Hydralazine, procainamide, izoniazid, phenylbutazone, dantrolene, doxorubicin, and penicillin. These situations are extremely rare. Sonnenblick et al[23] demonstrated that the cardiac effects of interferon were not related to the daily dose, cumulative total dose, or period of therapy and cardiac toxicity was reversible following the cessation of the drug therapy. Interferon inhibits cardiac cell function in vitro[24].
The Naranjo adverse drug reactions (ADR) Probability Scale[25] is a validated tool used to determine the likelihood that the adverse drug reaction is caused by the implicated medication. The Naranjo algorithm requires a series of questions to be answered and scored. The total calculated score indicates the likelihood of causing an adverse drug reaction. Popescu et al[11] used the Naranjo ADR Probability Scale to evaluate the correlation of pericarditis with interferon administration. This scale indicated a very probable association.
Treatment of interferon-induced cardio toxicity rests mainly on early recognition and drug discontinuation. There is a high degree of individual variation in toxicity, but most adverse events are reversible upon cessation of the drug[8]. In the present study, 4 of 9 patients were treated with prednisone and 4 with NSAIDs.
Chronic hepatitis C viral (HCV) infection and treatment with interferon are both associated with serological and clinical autoimmune manifestations[26,27]. The serological immune response to HCV infection may include the development of cryoglobulinemia, rheumatoid factor, anticardiolipin, antinuclear, anti-liver-kidney-microsome 1 and anti-smooth muscle antibodies. Serological autoimmune manifestations were explained by the lymphotropism of HCV and the polyclonal activation of B cells. Interferon-based treatment of HCV infection may precipitate or exacerbate the associated autoimmune disease. Classically, type II Cryoglobulinaemia, glomerulonephritis and thyroiditis are described.
Interferon related pericarditis still remains uncertain. There may be two mechanisms for pericarditis, one is autoimmune and the other is cardio toxicity. Treatment of interferon related pericarditis rests mainly on early recognition and drug discontinuation. Interferon related pericarditis was treated with steroid and/or NSAIDs.
COMMENTS
Background
When the authors examine a new unusual patient that the authors have never treated before, the authors need to research previous case reports. However, the case report is individual. The authors need to know what kind of examinations they need and what kind of treatments they need as soon as possible. This manuscript aimed to summarize those previous case reports concerning interferon related pericarditis.
Research frontiers
There may be two mechanisms for interferon related pericarditis, one is autoimmune and the other is cardio toxicity. Treatment of interferon related pericarditis rests mainly on early recognition and drug discontinuation. Interferon related pericarditis was treated with steroid and/or nonsteroidal anti-inflammatory drugs.
Innovations and breakthroughs
This is the first article that was summarized interferon related pericarditis.
Applications
Readers will understand the previous case reports concerning interferon related pericarditis in a short time.
Terminology
Interferon related pericarditis is one of the side effects of interferon treatment.
Peer-review
In the current manuscript, the authors reviewed the 9 published interferon-related pericarditis cases. This interferon regimen complication is rare and this review is helpful to understand this rare complication.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors of this manuscript having no conflicts of interest to disclose.
Data sharing statement: There is no additional data available.
Peer-review started: November 13, 2016
First decision: March 8, 2017
Article in press: May 5, 2017
P- Reviewer: Jin B, Kettering K S- Editor: Song XX L- Editor: A E- Editor: Li D
References
- 1.Mansat-Krzyzanowska E, Dréno B, Chiffoleau A, Litoux P. [Cardiovascular manifestations associated with interferon alfa-2A] Ann Med Interne (Paris) 1991;142:576–581. [PubMed] [Google Scholar]
- 2.Teragawa H, Hondo T, Amano H, Hino F, Ohbayashi M. Adverse effects of interferon on the cardiovascular system in patients with chronic hepatitis C. Jpn Heart J. 1996;37:905–915. doi: 10.1536/ihj.37.905. [DOI] [PubMed] [Google Scholar]
- 3.Georg M, Walker B. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Fava S, Luoni M, Stioui S. Pericarditis during interferon-alpha therapy in chronic myelogenous leukemia. Haematologica. 1996;81:484. [PubMed] [Google Scholar]
- 5.Boonen A, Stockbrügger RW, van der Linden S. Pericarditis after therapy with interferon-alpha for chronic hepatitis C. Clin Rheumatol. 1999;18:177–179. doi: 10.1007/s100670050081. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski B, Denis J, Fischer D, Labayle D. [Pericarditis secondary to interferon alpha in chronic hepatitis C] Gastroenterol Clin Biol. 2004;28:315–316. doi: 10.1016/s0399-8320(04)94929-9. [DOI] [PubMed] [Google Scholar]
- 7.Gressens B, Gohy P. Pericarditis due to interferon-alpha therapy during treatment for chronic hepatitis C. Acta Gastroenterol Belg. 2004;67:301–302. [PubMed] [Google Scholar]
- 8.Benjamini O, Kimhi O, Lishner M. Severe pleuropericarditis and cardiomyopathy induced by high dose interferon alpha-2b. Isr Med Assoc J. 2007;9:486–7. [PubMed] [Google Scholar]
- 9.Hamdani I, Kochlef A, Belhadj N, Kharrat J, Ghorbel A. [Acute pericardite which has occurred during the treatment of chronic hepatitis C by the interferon alpha2A] Tunis Med. 2008;86:404–405. [PubMed] [Google Scholar]
- 10.Nishio K, Konndo T, Okada S, Enchi M. Pericarditis and chronic inflammatory demyelinating polyneuropathy during therapy with pegylated interferon alfa-2a for chronic hepatitis C. World J Hepatol. 2010;2:358–361. doi: 10.4254/wjh.v2.i9.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu C, Arama V, Gliga S. Acute pericarditis due to pegylated interferon alpha therapy for chronic HCV hepatitis - case report. BMC Gastroenterol. 2011;11:30. doi: 10.1186/1471-230X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashraf F, Marmoush F, Shafi MI, Shah A. Recurrent Pericarditis, an Unexpected Effect of Adjuvant Interferon Chemotherapy for Malignant Melanoma. Case Rep Cardiol. 2016;2016:1342028. doi: 10.1155/2016/1342028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Interferon in CML. Br J Hospital Med. 1988;1:77. [PubMed] [Google Scholar]
- 14.Montastruc M, Reiffers J, Bilhou-Nabera C. Recombinant interferon alpha (IFN) as initial treatment for chronic myelogenous leukemia (CML). Journal Chronic Myeloid Leukemia, 2nd International Conference, Bologna; 1992. p. 103. [Google Scholar]
- 15.Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 16.Silva MO. Risk of autoimmune complications associated with interferon therapy. Gastroenterol Hepatol (N Y) 2012;8:540–542. [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens. 1992;5:224–229. doi: 10.1093/ajh/5.4.224. [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y, Okanoue T, Enjyo F, Morimoto M, Takeuchi T, Kagawa K, Kashima K. Elevated interleukin-6 and gamma-globulin during interferon therapy of hepatitis B. Am J Gastroenterol. 1992;87:1485–1487. [PubMed] [Google Scholar]
- 19.Edwards BS, Merritt JA, Fuhlbrigge RC, Borden EC. Low doses of interferon alpha result in more effective clinical natural killer cell activation. J Clin Invest. 1985;75:1908–1913. doi: 10.1172/JCI111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ristić AD, Pankuweit S, Maksimović R, Moosdorf R, Maisch B. Pericardial cytokines in neoplastic, autoreactive, and viral pericarditis. Heart Fail Rev. 2013;18:345–353. doi: 10.1007/s10741-012-9334-y. [DOI] [PubMed] [Google Scholar]
- 21.Orságová I, RoŽnovský L, Petroušová L, Konečná M, Kabieszová L, Martinek J, Kloudová A, Pavliska L. [Investigation of autoimmunity markers during interferon alpha therapy of chronic hepatitis B and C - twenty years of experience] Klin Mikrobiol Infekc Lek. 2016;22:61–67. [PubMed] [Google Scholar]
- 22.Castel M, Despas F, Modesto A, Gales C, Honton B, Galinier M, Senard JM, Pathak A. [Cardiotoxicity of chemotherapies] Presse Med. 2013;42:26–39. doi: 10.1016/j.lpm.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Sonnenblick M, Rosin A. Cardiotoxicity of interferon. A review of 44 cases. Chest. 1991;99:557–561. doi: 10.1378/chest.99.3.557. [DOI] [PubMed] [Google Scholar]
- 24.Lampidis TJ, Brouty-Boyé D. Interferon inhibits cardiac cell function in vitro. Proc Soc Exp Biol Med. 1981;166:181–185. doi: 10.3181/00379727-166-41043. [DOI] [PubMed] [Google Scholar]
- 25.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Pawlotsky JM, Ben Yahia M, Andre C, Voisin MC, Intrator L, Roudot-Thoraval F, Deforges L, Duvoux C, Zafrani ES, Duval J. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994;19:841–848. [PubMed] [Google Scholar]
- 27.Pawlotsky JM, Roudot-Thoraval F, Simmonds P, Mellor J, Ben Yahia MB, André C, Voisin MC, Intrator L, Zafrani ES, Duval J, et al. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med. 1995;122:169–173. doi: 10.7326/0003-4819-122-3-199502010-00002. [DOI] [PubMed] [Google Scholar]


