Abstract
AIM
To identify factors associated with depressive symptoms among inpatients with cardiovascular disease (CVD).
METHODS
This is a cross-sectional study performed in a subsample of a large cross-sectional research that investigated affective disorders and suicide behaviour among inpatients hospitalized in non-surgical wards of the University Hospital of the Federal University of Minas Gerais from November 2013 to October 2015. Sociodemographic and clinical data were obtained through a structured interview and medical record review. Depression was assessed by the depression subscale of the Hospital Anxiety and Depression Scale, with scores ≥ 8 considered as positive screening for depression. We used the Fageström Test for Nicotine Dependence to characterize nicotine dependence. For assessing resilience and early-life trauma, we used the raw scores of the Wagnild and Young Resilience Scale and Childhood Trauma Questionnaire, respectively.
RESULTS
At endpoint, we included 137 subjects. Thirty-eight (27.7%) subjects presented depressive symptoms and nine (23.7%) of those were receiving antidepressant treatment during hospitalization. The female sex; a lower mean educational level; a greater prevalence of previous suicide attempts; a higher level of pain; a higher prevalence of family antecedents of mental disorders; a lower resilience score; and higher childhood trauma score were the factors significantly associated with screening positive for major depression (P < 0.05). Multivariate analysis demonstrated that the factors independently associated with the depressive symptoms were a higher childhood trauma severity (OR = 1.06; P = 0.004); moderate to severe nicotine dependence (OR = 8.58; P = 0.008); and the number of previous hospital admissions (OR = 1.11; P = 0.034). The obtained logistic model was considered valid, indicating that the three factors together distinguished between having or not depressive symptoms, and correctly classified 74.6% of individuals in the sample.
CONCLUSION
Our results demonstrate that inpatients presenting both CVD and a positive screening for depression are more prone to have antecedents of childhood trauma, nicotine dependence and a higher number of previous hospitalizations.
Keywords: Inpatients, Depression, Cardiovascular disease, Depressive symptoms, General hospital
Core tip: The prevalence of depression is considerably higher among individuals with cardiovascular diseases (CVD) when compared to the general population. Both major depression and depressive symptoms are predictors of poor outcome in patients with CVD. Depressive disorder is frequently overlooked and untreated in individuals with CVD. Our results demonstrate that inpatients presenting both CVD and a positive screening for depression are more prone to have antecedents of childhood trauma, nicotine dependence and a higher number of previous hospitalizations. Clinicians may consider these factors in the assessment of CVD inpatients at risk for major depression. This measure can improve their treatment approach and patients’ prognosis.
INTRODUCTION
The prevalence of major depressive disorder (MD) is four times higher among individuals with cardiovascular diseases (CVD) when compared to the general population[1-4], and MD is a predictor for future CVD. MD increases the risk for coronary arterial disease (CAD) by 56%, independent of other traditional cardiovascular risk factors[1]. Moreover, MD and depressive symptoms are predictors of poor outcome in patients with CVD regarding morbidity and mortality[2,3].
Maltzberg[4] first reported the bidirectional relationship between MD and CVD in 1937. This author observed an increase in mortality from CVD in patients with severe depression. More recently, some authors reported that post-myocardial infarction depression increases the risk of all-cause mortality (RR = 2.25) and of cardiac events (RR = 1.59) within 24 mo after the event[5]. Even in the absence of depressive symptoms, a positive history of depression in first-degree relatives may influence the cardiovascular risk profile in adulthood, comparing to control group[6].
Various biological modifications, previously found in patients with depression, may explain these findings. An increased concentration of inflammatory biomarkers (C-reactive protein, interleukins 1 and 6), metabolic dysregulation, dysfunctions in the platelet clotting cascade, decreased variability in heart rate, hyperactivation of hypothalamus-pituitary-adrenal axis and reduction in circulating endothelial progenitor cells are some of the factors that may be at the pathophysiological origin of the association between depression and CVD[2,7-9]. Finally, both depression and CVD have notorious genetic determinants, which may underlie the development of one and another as shared risk factors[2,10]. Even acute and chronic life stressors may increase the risk for developing one of these diseases[2,10]. Adverse events in early life can directly affect genome through epigenetic mechanisms and contribute to the expression or exacerbation of a genetic susceptibility for depression, CVD or both in adulthood[2].
During hospitalization, depression decreases inpatients’ treatment adherence, increases functional disability and extends hospital length of stay[11,12]. Inpatients with CVD presenting positive screening for depression at discharge have a 2.5 fold increase in relative risk of experiencing a CVD-related hospitalization, even after adjustment for traditional cardiovascular risk factors and measures of disease severity[13].
Depression is still a frequently overlooked and untreated condition among individuals with CVD[14,15]. The same applies for those admitted to general hospitals, a population in which 28% present criteria for depressive disorders[16]. Less than one in four cases of major depression among inpatients are correctly diagnosed by assisting physicians[14]. In addition, only half the members of the American College of Cardiologists treat depression properly, according to a national survey[17].
Inpatients with CVD have increased vulnerability for depression. Improving the knowledge on how much risk factors influence the chances of becoming depressed may improve identification of MD among this population in the general hospital setting. We hypothesized that inpatients presenting both CVD and a positive screening for major depression are more prone to be of female sex; to present personal and familiar antecedents of psychiatric disorders (e.g., suicide attempts, addictions); to present worse indicators of CVD (e.g., increased number of previous hospital admissions, present pain and a worse score of functionality); and present lower resilience and antecedents of childhood trauma. Our primary goal was to assess the factors mentioned above in a population of inpatients with CVD in a university hospital.
MATERIALS AND METHODS
This cross-sectional design study encompassed a subsample of a larger study that investigated suicide behaviour among general hospital inpatients. We included all inpatients admitted to the wards of the University Hospital of the Federal University of Minas Gerais (UH-UFMG), hospitalized from November 2013 to October 2015. The UH-UFMG is a tertiary regional reference centre.
The Committee of Ethics in Research of UFMG approved the protocol, registered with the number CAAE 13605213.3.0000.5149. We obtained written informed consent from all participants, after providing a complete description of the study. All subjects screening positive for a psychiatric disorder received consultation-liaison psychiatric evaluations.
Subjects
For the initial study, we included inpatients aged 18-year-old or older, hospitalized in a clinical ward and excluded patients hospitalized with surgical indication. In the present study, we selected all patients with a diagnosis of CVD (e.g., CAD, congestive heart failure, Chagas cardiomyopathy, cardiac arrhythmia or any other cardiac disease that required hospitalization) and excluded subjects screening positive for delirium and mild to severe cognitive impairment or dementia as well as those unable to comprehend, illiterate or with major visual or hearing impairment. We summarized the inclusion procedure in the flowchart (Figure 1).
Figure 1.
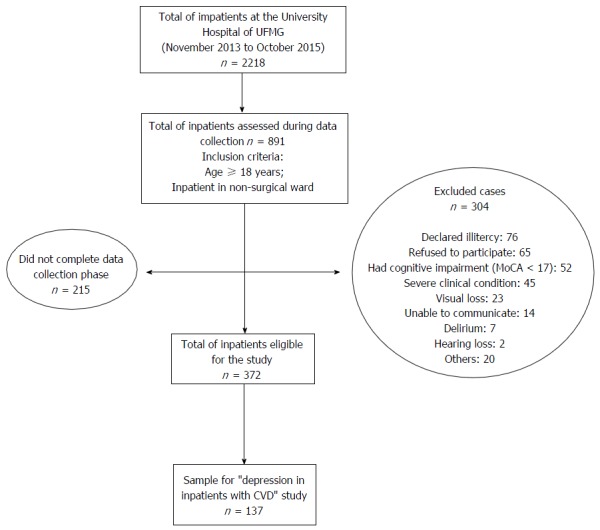
Flowchart of subjects included in the study. UFMG: The Federal University of Minas Gerais; CVD: Cardiovascular disease.
Instruments and procedures
After a clinical assessment and stabilization, we included all subjects at admission. Experienced psychiatrists, trained to perform the assessments foreseen in the study protocol, evaluated the subjects. All the scales used in this study had been previously translated and culturally adapted to Brazilian Portuguese.
We screened delirium using the Confusion Assessment Method[18] and cognitive impairment through the Montreal Cognitive Assessment[19] using 17 as a cut-off point[20].
For screening MD, we used the Hospital Anxiety and Depression Scale (HADS-d). This scale has acceptable properties for use in inpatients with CVD[21]. Scores of ≥ 8 indicate a positive screening for depression, according to the Brazilian validation study that issued a sensibility of 84.6% and specificity of 90.3%[22].
We used the visual analogue scale to assess pain intensity[23]. We determined the basic and instrumental activities of daily living (ADL) using the Katz Index and the Pfeffer’s Functional Activities Questionnaire (PFAQ), respectively[24]. We considered scores ≥ 5 in the PFAQ as characteristic of dependence on instrumental ADL[25].
We considered a score ≥ 8 on the Alcohol Use Disorders Identification Test to assess problematic alcohol use[26] and a score ≥ 4 on the Fageström Test for Nicotine Dependence to characterize moderate to severe nicotine dependence[27].
We considered the levels of positive psychosocial adjustment given major life events, like resilience, and used the Wagnild and Young Resilience Scale (WYRS) to evaluate resilience level. The WYRS is a paper-and-pencil scale, composed of 25 likert-type items[28] and, as the validation study did not establish a cut-off point, we used the raw results in our analysis. For assessing early-life trauma we used the Childhood Trauma Questionnaire (CTQ)[29]. The CTQ is a 28-item self-report inventory that provides a valid screening for early life abuse or negligence.
Statistical analysis
In the descriptive analysis, we calculated measures of central tendency and dispersion. The Shapiro-Wilk test assessed data normality. For univariate analysis, a χ2 test was considered for categorical variables and Mann-Whitney test for continuous variables. To determine which factors had a greater association with positive screening for depression in our sample, we conducted a multiple logistic regression with stepwise selection. Those variables with P value ≤ 0.2 in univariate analysis were apt to enter the model. The χ2 test model and Nagelkerke’s R2 used to evaluate the predictive ability of the logistic model obtained. Calculation of odds ratios (OR) considered a 95%CI and significance of P < 0.05. All analyses were performed using SPSS software version 20 (IBM Corporation© 2011).
RESULTS
Sample description
At endpoint, we included 137 subjects and found a higher prevalence of males [n = 92 (67.2%)], a mean age of 52.1 ± 12.5 years old and a mean educational level of 8.5 ± 4.4 years. The most prevalent CVD diagnoses were CAD (n = 76; 55.5%), congestive heart failure (n = 43; 31.4%) and cardiac arrhythmia (n = 15; 10.9%). CAD, the most frequent cardiovascular diagnosis, was not associated with positive screening for major depression. Seventy-five (54.7%) and 23 (16.8%) subjects presented hypertension and diabetes mellitus, respectively.
Factors associated with positive screening for depression
Thirty-eight (27.7%) subjects screened positive for depression and nine (23.7%) were taking antidepressants during hospitalization. Female sex, lower mean educational level, greater number of previous suicide attempts, higher level of pain, higher prevalence of family antecedents of mental disorders, lower resilience score and higher childhood trauma score were the factors significantly associated to screening positive for major depression (Table 1).
Table 1.
Factors associated with positive screening for depression (Hospital Anxiety and Depression scale - depression subscale score ≥ 8) in inpatients with cardiovascular diseases (n = 137) n (%)
|
Screening for depression |
Missing data (%) | P value | ||
| Yes | No | |||
| Sociodemographic variables | ||||
| Gender | ||||
| Male | 15 (39.5) | 77 (77.8) | - | < 0.001a |
| Female | 23 (60.5) | 22 (22.2) | - | |
| Age, mean (SD) | 53.7 ± 11.4 | 51.5 ± 12.8 | - | 0.444 |
| Elder (aged ≥ 60 yr) | 15 (39.5) | 24 (24.2) | - | 0.077 |
| Educational level in years (mean ± SD) | 7.4 ± 4.4 | 8.5 ± 4.3 | 1.5 | 0.035b |
| Married/lives with partner | 25 (65.8) | 70 (70.7) | 0.7 | 0.723 |
| Lives alone | 3 (8.1) | 9 (9.1) | 0.7 | 0.857 |
| Any son | 34 (91.9) | 83 (84.7) | 1.5 | 0.272 |
| Unemployed | 9 (24.3) | 23 (23.5) | 1.5 | 0.917 |
| Religion | 35 (94.6) | 91 (91.9) | 0.7 | 0.595 |
| Clinical variables | ||||
| No of previous hospital admissions, mean (SD) | 7.6 ± 9.1 | 5 ± 5.2 | 5.8 | 0.07b |
| CAD | 18 (47.4) | 58 (58.6) | - | 0.237 |
| Congestive heart failure | 12 (31.6) | 31 (31.3) | - | 0.976 |
| Cardiac arrhythymia | 5 (13.3) | 10 (10.1) | - | 0.76 |
| Hypertension | 19 (50) | 56 (56.6) | - | 0.489 |
| Diabetes mellitus | 5 (13.2) | 18 (18.2) | - | 0.481 |
| Pain level, mean (SD) | 2.2 ± 2.9 | 1.3 ± 2.4 | 0.7 | 0.136 |
| Propranolol | 8 (21.1) | 12 (13.5) | 8.0 | 0.255 |
| Dependence in basic ADL | 10 (26.3) | 14 (14.1) | 0.7 | 0.09 |
| Dependence in instrumental ADL | 2 (22.2) | 4 (17.4) | 76.6 | 0.753 |
| Psychosocial variables | ||||
| Family history of mental disorder | 18 (47.4) | 20 (20.2) | - | 0.001a |
| Previous suicide attempt | 11 (28.9) | 8 (8.2) | 0.7 | 0.002a |
| Moderate to severe nicotine dependence | 7 (18.4) | 10 (10.1) | - | 0.186 |
| Problematic alcohol use | 7 (30.4) | 13 (18.84) | 32.8 | 0.243 |
| Resilience score, WYS mean (SD) | 138.5 ± 14.5 | 144.9 ± 14.9 | 2.9 | 0.029b |
| Childhood trauma score, CTQ mean (SD) | 46.4 ± 20.1 | 37.5 ± 12.6 | 8.8 | 0.004b |
χ2 test significant if P < 0.05;
Mann-Whitney test significant if P < 0.05. CAD: Coronary arterial disease; ADL: Activities of daily living; WYS: Wagnild and Young Scale; CTQ: Childhood Trauma Questionnaire.
Three factors remained statistically associated with a positive screening for depression (Table 2) in multivariate analysis: (1) childhood trauma; (2) moderate or severe nicotine dependence; and (3) the number of hospital admissions.
Table 2.
Logistic regression analysis of factors associated with positive screening for depression (Hospital Anxiety and Depression scale - depression subscale score ≥ 8) in inpatients with coronary arterial disease
| Variable | B | EP | Wald | OR | 95%CI | P |
| Moderate to severe nicotine dependence | 2.15 | 6.91 | 7.135 | 8.58 | 1.77-41.57 | 0.008 |
| No of previous hospital admissions | 0.106 | 0.06 | 4.505 | 1.11 | 1.01-1.23 | 0.034 |
| High childhood trauma severity (CTQ) | 0.06 | 0.02 | 8.2 | 1.06 | 1.02-1.11 | 0.004 |
| Constant | -4.664 | 0.01 | 16.141 | 0.01 | 0.00-0.09 | < 0.001 |
χ2: 17.974, P < 0.001, D.f. = 1; Nagelkerke R2: 0.33. CTQ: Childhood Trauma Questionnaire.
Patients with moderate and severe nicotine dependence were 8.58 times more prone to screen positive for depression (P = 0.008).
The chance to screen positive for depression increased 11% for each hospital admission (P = 0.034) and 6% for each point of increase in the CTQ score for childhood trauma (P = 0.004).
The logistic model indicated that the three factors together distinguished between positive screening for depression or not, and correctly classified 74.6% of individuals in the sample (χ2: 17.974, P < 0.001, D.F. = 1; Nagelkerke R2: 0.33).
DISCUSSION
This study assessed the influence of the factors associated with positive screening for depression in a sample of patients with CVD hospitalized in a university hospital. After multiple comparisons, we found that positive screening for depression was significantly associated with childhood trauma, the severity of nicotine dependence, and the number of previous hospital admissions. These results partially agree with our initial hypothesis as no association was found with some of the factors previously related with major depression, such as: Personal and familiar antecedents of psychiatric disorders (e.g., suicide attempts, addictions), pain perception, a worse score of functionality, and a lower resilience level. To the best of our knowledge, only one study reported risk factors associated with depression in patients with CVD[30]. However, no study evaluated such a vast array of epidemiological, clinical and psychological factors associated with depression in patients hospitalized with CVD.
Our results should be regarded considering a few issues. First, our study has a cross-sectional design and, as such, hinders the evaluation of causality between the factors evaluated and major depression. Second, our sample included patients with different types of CVD. We have grouped the distinct disorders in a unique group as they share common risk factors and etiological mechanisms associated with inflammatory processes; previous studies have adopted this same strategy[30]. Finally, we have not been able to assess the severity of CVD with an objective measure within this study.
Our sample presented a rate of 27.7% of patients screening positive for depression. This result is comparable with previous studies, which reported a prevalence of 13.5% to 47% of major depression in inpatients with CVD[16,30-33]. Different from previous studies, that reported only 5% of patients with CVD and depression were being treated with antidepressants[33], in our sample, 23.7% of the subjects were taking an antidepressant.
Stressful experiences during the lifespan have been associated with CVD[34] and depression[35]. Childhood trauma is one of the most significant predictors of health problems, life expectancy, psychiatric disorders and the severity of clinical diseases’ courses[36]. The occurrence of childhood trauma can influence the development of CVD through changes in metabolic, cardiovascular risk factors like dyslipidemia, central obesity, and hyperglycemia. A Dutch cohort study found that among the childhood trauma subtypes and personality traits, sexual abuse was the primary factor that correlated negatively with serum cholesterol and abdominal circumference measurements[37]. Recurrent stressful events may induce a subtle chronic inflammatory response, enough to contribute to the progression of atherosclerosis and increased the risk of developing CAD[38]. Our results highlight the importance of the assessment of childhood trauma in patients with CVD, as the severity of trauma may predict depression in this population. Clinicians must consider that one possible mechanism of the association between childhood trauma, depression and CVD is the disruption of the key stress-response system, such as the catecholamine system, the hypothalamic-pituitary-adrenal axis, and neurotrophic factors, in early stages of child development. The impairment of the stress response system can influence arousal and emotional behaviour and contribute to increase the allostatic load, impairing brain development and increasing the risk for psychopathology[36].
As reported in the paper of Caro et al[30], our results point that nicotine dependence is associated with a positive screening for depression in CVD inpatients. Nicotine dependence is more prevalent in individuals with depression, possibly because these subjects are less prone to engage in smoking cessation programs[9] and tend to use nicotine to alleviate anxiety and dysphoria[39]. As other risk factors for CVD, cigarette use has been associated with damage of the arterial wall. Moreover, the intensification of cigarette use maintains inflammatory response, like chronic stress, and increases risk for depression and CVD[2]. Also, both depression and CVD increase the systemic pro-inflammatory state[2,7], aggravating the pathophysiological mechanisms related to CVD and closing a vicious cycle[38].
Presenting a greater number of previous hospital admissions was another factor associated with positive screening for depression in our study. These results agree with previous findings regarding inpatients with several medical illnesses[32]. Compared to those without depression, medical inpatients suffering from depression have longer hospital stays and higher readmission rates. Both factors underline the burden of this affective disorder among CVD patients, including the financial burden[12,40]. Moreover, each hospital admission can represent an acute stressor for those who experience it, raising negative feelings about an individual’s current health state and prognosis, augmenting depressive symptoms. In the same manner, a higher number of hospitalizations may represent a proxy for CVD severity. Other CVD severity measures were significantly associated with depression in inpatients, namely having an implantable cardioverter defibrillator or being in functional class III or IV[33].
How can we link these three factors to CVD and major depression? In our view, either childhood trauma, nicotine dependence and the number of previous hospitalizations have been associated with stress arousal and pro-inflammatory states. Both conditions are well-known risk factors for major depression and CVD[36].
Our results demonstrate that inpatients presenting both CVD and a positive screening for major depression are more prone to have antecedents of childhood trauma, nicotine dependence and a higher number of previous hospitalizations. Clinicians may consider these factors in the assessment of CVD inpatients at risk for major depression. This measure can improve their treatment approach and patients’ prognoses.
ACKNOWLEDGMENTS
We thank Mr. André A. C. de Freitas for performing the review and editing of this paper.
COMMENTS
Background
The prevalence of depression is considerably higher among individuals with cardiovascular diseases (CVD) when compared to the general population. Both major depression and depressive symptoms are predictors of poor outcome in patients with CVD. Depressive disorder is frequently overlooked and untreated in individuals with CVD.
Innovations and breakthroughs
This study assessed the influence of the factors associated with positive screening for depression in a sample of patients with CVD hospitalized in a university hospital. After multiple comparisons, the authors found that positive screening for depression was significantly associated with childhood trauma, the severity of nicotine dependence, and the number of previous hospital admissions.
Applications
The results demonstrate that inpatients presenting both CVD and a positive screening for major depression are more prone to have antecedents of childhood trauma, nicotine dependence and a higher number of previous hospitalizations. Clinicians may consider these factors in the assessment of CVD inpatients at risk for major depression. This measure can improve their treatment approach and patients’ prognoses.
Peer-review
This is a cross-sectional survey of risk factors associated with depression in patients hospitalized in non-surgical wards and suffering in cardiovascular disease. As both cardiovascular diseases and depression are frequent and possessing a great burden on the family and the society, the study is relevant and interesting.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The Committee of Ethics in Research of UFMG approved the protocol, registered with the number CAAE 13605213.3.0000.5149.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrolment.
Conflict-of-interest statement: Garcia FD received CME grants, received paid speakership, and participated in research from Janssen and Pfizer; Correa H received paid speakership, and participated in research from Janssen; Prado PHT and Neves MCL participated in research from Janssen. The other authors have no conflicts to declare.
Data sharing statement: We would like to inform that, related to the research described on our manuscript, no additional data is available.
Peer-review started: January 21, 2017
First decision: February 15, 2017
Article in press: April 24, 2017
P- Reviewer: Gazdag G, Hosak L, Shekhar A S- Editor: Song XX L- Editor: A E- Editor: Li D
References
- 1.Charlson FJ, Moran AE, Freedman G, Norman RE, Stapelberg NJ, Baxter AJ, Vos T, Whiteford HA. The contribution of major depression to the global burden of ischemic heart disease: a comparative risk assessment. BMC Med. 2013;11:250. doi: 10.1186/1741-7015-11-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak--the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012;9:526–539. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Vedanthan R. Cardiovascular disease and the UN Millennium Development Goals: time to move forward. Nat Clin Pract Cardiovasc Med. 2008;5:593. doi: 10.1038/ncpcardio1353. [DOI] [PubMed] [Google Scholar]
- 4.Maltzberg B. Mortality among patients with involutional melancholia. Am J Psychiatr. 1937:93. [Google Scholar]
- 5.Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203–216. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Mannie ZN, Williams C, Diesch J, Steptoe A, Leeson P, Cowen PJ. Cardiovascular and metabolic risk profile in young people at familial risk of depression. Br J Psychiatry. 2013;203:18–23. doi: 10.1192/bjp.bp.113.126987. [DOI] [PubMed] [Google Scholar]
- 7.Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:511–523. doi: 10.1016/j.pcad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Seligman F, Nemeroff CB. The interface of depression and cardiovascular disease: therapeutic implications. Ann N Y Acad Sci. 2015;1345:25–35. doi: 10.1111/nyas.12738. [DOI] [PubMed] [Google Scholar]
- 9.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case of depression. Prog Cardiovasc Dis. 2013;55:557–562. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botega NJ, Mitsuushi GN, Azevedo RC, Lima DD, Fanger PC, Mauro ML, Gaspar KC, Silva VF. Depression, alcohol use disorders and nicotine dependence among patients at a general hospital. Rev Bras Psiquiatr. 2010;32:250–256. doi: 10.1590/s1516-44462010005000016. [DOI] [PubMed] [Google Scholar]
- 12.Sibitz I, Berger P, Freidl M, Topitz A, Krautgartner M, Spiegel W, Katschnig H. ICD-10 or DSM-IV? Anhedonia, fatigue and depressed mood as screening symptoms for diagnosing a current depressive episode in physically ill patients in general hospital. J Affect Disord. 2010;126:245–251. doi: 10.1016/j.jad.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Meyer FA, Hugentobler E, Stauber S, Wilhelm M, Znoj H, von Känel R. Depressive symptoms at discharge from rehabilitation predict future cardiovascular-related hospitalizations. Cardiology. 2015;131:80–85. doi: 10.1159/000375231. [DOI] [PubMed] [Google Scholar]
- 14.Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 15.Rentsch D, Dumont P, Borgacci S, Carballeira Y, deTonnac N, Archinard M, Andreoli A. Prevalence and treatment of depression in a hospital department of internal medicine. Gen Hosp Psychiatry. 2007;29:25–31. doi: 10.1016/j.genhosppsych.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Yanzón de la Torre A, Oliva N, Echevarrieta PL, Pérez BG, Caporusso GB, Titaro AJ, Todaro Kicyla A, Cuatz M, Locatelli M, Nelson LM, et al. Major depression in hospitalized Argentine general medical patients: Prevalence and risk factors. J Affect Disord. 2016;197:36–42. doi: 10.1016/j.jad.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein RE, Blumenfield M, Orlowski B, Frishman WH, Ovanessian S. A national survey of cardiovascular physicians’ beliefs and clinical care practices when diagnosing and treating depression in patients with cardiovascular disease. Cardiol Rev. 2006;14:164–169. doi: 10.1097/01.crd.0000200977.41695.43. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri RM, Moreira MA, Garrido R, Almeida OP. Validity and reliability of the Portuguese version of the Confusion Assessment Method (CAM) for the detection of delirium in the elderly. Arq Neuropsiquiatr. 2001;59:175–179. doi: 10.1590/s0004-282x2001000200004. [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107. doi: 10.1186/s12877-015-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Botega NJ, Bio MR, Zomignani MA, Garcia C, Pereira WA. [Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD] Rev Saude Publica. 1995;29:355–363. doi: 10.1590/s0034-89101995000500004. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho DSK. P A: Avaliação da intensidade da dor. Migrâneas and Cefaleias. 2006;9:164. [Google Scholar]
- 24.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez MAS, Correa PCR, Lourenço RA. Cross-cultural adaptation of the “Functional Activities Questionnaire- FAQ” for use in Brazil. Dement Neuropsychol. 2011;5:322–327. doi: 10.1590/S1980-57642011DN05040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 27.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 28.Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. J Nurs Meas. 1993;1:165–178. [PubMed] [Google Scholar]
- 29.Grassi-Oliveira R, Stein LM, Pezzi JC. [Translation and content validation of the Childhood Trauma Questionnaire into Portuguese language] Rev Saude Publica. 2006;40:249–255. doi: 10.1590/s0034-89102006000200010. [DOI] [PubMed] [Google Scholar]
- 30.Caro MA, Sowden GL, Mastromauro CA, Mahnks S, Beach SR, Januzzi JL, Huffman JC. Risk factors for positive depression screens in hospitalized cardiac patients. J Cardiol. 2012;60:72–77. doi: 10.1016/j.jjcc.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Pakriev S, Kovalev J, Mozhaev M. Prevalence of depression in a general hospital in Izhevsk, Russia. Nord J Psychiatry. 2009;63:469–474. doi: 10.3109/08039480903062950. [DOI] [PubMed] [Google Scholar]
- 32.Yan ZY, Gu MJ, Zhong BL, Wang C, Tang HL, Ling YQ, Yu XW, Li MQ. Prevalence, risk factors and recognition rates of depressive disorders among inpatients of tertiary general hospitals in Shanghai, China. J Psychosom Res. 2013;75:65–71. doi: 10.1016/j.jpsychores.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Shiga T, Kuwahara K, Kobayashi S, Suzuki S, Nishimura K, Suzuki A, Omori H, Mori F, Ishigooka J, et al. Depression and outcomes in hospitalized Japanese patients with cardiovascular disease. - Prospective single-center observational study- Circ J. 2011;75:2465–2473. doi: 10.1253/circj.cj-11-0140. [DOI] [PubMed] [Google Scholar]
- 34.Bomhof-Roordink H, Seldenrijk A, van Hout HP, van Marwijk HW, Diamant M, Penninx BW. Associations between life stress and subclinical cardiovascular disease are partly mediated by depressive and anxiety symptoms. J Psychosom Res. 2015;78:332–339. doi: 10.1016/j.jpsychores.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte DG, Neves Mde C, Albuquerque MR, de Souza-Duran FL, Busatto G, Corrêa H. Gray matter brain volumes in childhood-maltreated patients with bipolar disorder type I: A voxel-based morphometric study. J Affect Disord. 2016;197:74–80. doi: 10.1016/j.jad.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 37.van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Personality traits and childhood trauma as correlates of metabolic risk factors: the Netherlands Study of Depression and Anxiety (NESDA) Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:85–91. doi: 10.1016/j.pnpbp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain Behav Immun. 2015;50:18–30. doi: 10.1016/j.bbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Minichino A, Bersani FS, Calò WK, Spagnoli F, Francesconi M, Vicinanza R, Delle Chiaie R, Biondi M. Smoking behaviour and mental health disorders--mutual influences and implications for therapy. Int J Environ Res Public Health. 2013;10:4790–4811. doi: 10.3390/ijerph10104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Ammon Cavanaugh S, Furlanetto LM, Creech SD, Powell LH. Medical illness, past depression, and present depression: a predictive triad for in-hospital mortality. Am J Psychiatry. 2001;158:43–48. doi: 10.1176/appi.ajp.158.1.43. [DOI] [PubMed] [Google Scholar]


