Abstract
Objective
To systematically review the procedure, applications, and outcomes of autologous fat grafting, a promising technique with various clinical applications.
Patients and methods
Literature review of publications concerning autologous fat grafting.
Results
Since its introduction, lipofilling has become increasingly popular; however, its results are variable and unpredictable. Several modifications have been made to the procedures of fat harvesting, processing, and injecting. Surgical excision and low negative-pressure aspiration with large-bore cannulas minimize adipocyte damage during fat harvesting. The “wet” method of fat harvesting involves fluid injection at the donor site and facilitates lipoaspiration while minimizing pain and ecchymosis. For fat processing, centrifugation at a low speed is preferable to high-speed centrifugation, gravity separation or filtration. Fat injection at the recipient site should be performed using small-gauge cannulas in a fanning out pattern over multiple sessions, rather than a single session. Fat grafts exhibit not only dermal filler properties but also regenerative potential owing to the presence of stem cells in fat tissue. Thus, the clinical applications of autologous fat grafting include correction of secondary contour defects after breast reconstruction, release of painful scar contractures, and treatment of burn scars and radiodermatitis. Lipofilling is also used in aesthetic surgery, such as facial and hand rejuvenation, augmentation rhinoplasty, and breast and gluteal augmentation. The complications of lipofilling are minimal and include bruising, swelling, pain, infection, necrosis, and calcification.
Conclusions
Lipofilling is a low-risk procedure that can be used to correct soft-tissue defects in the face, trunk, and extremities, with minimal discomfort for patients.
Keywords: Autologous fat grafting, Procedure, Applications, Outcomes
Highlights
-
•
Fat grafts are used to correct post-surgery defects, release of scars contractures, radiodermatitis, and cosmetic surgery.
-
•
Different fat harvesting, processing, and injecting procedures have been proposed by various authors.
-
•
Fat grafts exhibit regenerative potential owing to the presence of adipose stem cells.
-
•
Autologous fat grafting is a low-risk procedure with minimal discomfort for patients.
1. Introduction
Historically, the use of fat grafts to correct congenital deformities and complex traumatic wounds with soft-tissue loss after radical oncological surgery was proposed in 1893 by Neuber, by Hollander in 1912, by Neuhof in 1921, and by Josef in 1931 [1]. The liposuction technique, introduced by Fisher in 1974, followed by the tumescent technique, introduced by Klein in 1985, accelerated the development of the lipofilling technique. The tumescent technique allowed patients to undergo liposuction under local anaesthesia administered using small cannulas [2]. In 1987, Coleman introduced a new technique to decrease traumatic handling of fat during liposuction. His technique consisted of three steps: manual lipoaspiration under low pressure, centrifugation for 3 min at 3400 rpm, and reinjection in 3D. This technique remains the gold standard for liposuction and lipofilling, but has undergone some technical modifications [2], [3]. Since the 1980s, autologous fat transplantation has been one of the most popular procedures performed by plastic surgeons [4]. In 2009, fat grafting represented 5.9% of all non-surgical aesthetic procedures [5]. However, because the results of lipofilling are variable, optimization of the procedure is required. The long-term results of fat grafting are often disappointing because of unpredictable partial absorption of up to 70% of the volume of the fat graft. A number of studies have reported resorption rates of 30%–70% within a year [6]. Thus, autologous fat grafting has unpredictable success rates, and there is no agreement among physicians as to the ideal method for the harvesting and handling of fat grafts [5], [6], [7]. The Coleman technique should be considered as the standard and preferred method for harvesting and processing. However, one of the problems observed is a decrease in the number of fat cells because of damage caused during the aspiration and centrifugation steps [8]. Another limitation is the requirement to infiltrate cells in direct contact with well-vascularized tissues [8]. Furthermore, the Coleman technique can be operator dependent and time-consuming if performed by less-experienced surgeons. Numerous modifications of the Coleman have been attempted in order to improve the survival of the injected fat, including atraumatic fat-harvesting, fat washing to eliminate inflammatory mediators, centrifugation, and incubation of fat grafts with different bioactive agents. Fat is a filler with ideal properties: it naturally integrates into tissues, is autologous, and is 100% biocompatible. However, this is not the only function of lipofilling; fat is an active and dynamic tissue composed of several different cell types, including adipocytes, fibroblasts, smooth muscle cells, endothelial cells, and adipogenic progenitor cells called “preadipocytes” [9], [10], [11]. Adipose-derived stem cells (ASCs) have a differentiation potential similar to that of other mesenchymal stem cells as well as a higher yield upon isolation and a greater proliferative rate in culture when compared to bone marrow–derived stem cells [12], [13], [14]. Because of these properties and because these cells can be easily harvested in great amounts with minimal donor-site morbidity, ASCs have proved to be particularly promising for regenerative therapies [12], [15].
2. Fat harvesting
It is widely accepted that less-traumatic methods of fat harvesting result in increased adipocyte viability and graft survival [16], [17]. Several techniques have been proposed for fat harvesting, and there is an ongoing debate in the literature as to which method produces more viable and functional adipocytes. The main techniques are vacuum aspiration, syringe aspiration, and surgical excision. Recent experimental as well as some clinical studies support direct fat excision over aspiration. Fagrell et al. [16], [18] introduced a technique called “fat cylinder graft,” in which fat is drilled out in cores by a punching device, whereas Qin et al. [16], [19] recommended the core graft for block grafting because it maintains the structure and viability of harvested fat tissue by avoiding damage to the adipocytes. Pu et al. [16], [20] found significantly impaired adipocyte function in conventional liposuction aspirates compared with fresh fatty tissue samples and syringe-aspirated fat. Low negative-pressure lipoaspiration may yield fat faster than syringe aspiration and can be used when a large volume of fat is required, as in breast surgery. The high vacuum pressures of conventional liposuction may cause structural disruption in up to 90% of adipocytes [16], [17]. Cannula size may also affect the viability of harvested fat [21]. The use of the excisional method and fat harvesting with large-bore cannulas reduce the occurrence of cellular rupture and preserve the native tissue architecture. Campbell et al. found an inverse relationship between cellular damage and the diameter of the instrument used to extract fat [22]. Erdim et al. [23] reported higher graft viability with lipoaspirates that were obtained using a 6-mm cannula rather than a 4-mm or 2-mm cannula.
Coleman et al. [24] described a technique for fat harvesting that minimized trauma to the adipocytes. With a 3-mm, blunt-edged, 2-hole cannula connected to a 10-mL syringe, fat is suctioned manually by withdrawing the plunger. The cannula is pushed through the harvest site, as the surgeon uses digital manipulation to pull back on the plunger of the syringe and create a gentle negative pressure [24]. A combination of slight negative pressure and the curetting action of the cannula through the tissues allows parcels of fat to move through the cannula and Luer-Lok aperture into the barrel of the syringe [24]. When filled, the syringe is disconnected from the cannula, which is replaced with a plug that seals the Luer-Lok end of the syringe [24]. The plunger is removed from the syringe before it is placed into a centrifuge [24].
There are different natural fat deposits in the body; surgeons should identify the most suitable area after an accurate examination of the patient. The abdomen is the most common site of fat harvesting; the second is the trochanteric region (saddlebags) and the inside of the thighs and knees [25], [26]. The harvesting of fat grafts can be performed via a “wet” method or a “dry” method. In 1993, Klein et al. [16], [27] described the “wet” method, which involves the injection of the donor site with a fluid solution (Klein's solution) containing 0.9% NaCl, epinephrine, and a local anaesthetic. Illouz and de Villers [16], [28] highlighted the fact that the wet technique causes hydrodissection and enlarges the target fat layer, thus facilitating the subsequent aspiration, with decreased pain and ecchymosis. It has been observed that low shear stress leading improves graft survival; in fact, the shear stress exerted on harvested fat has been determined to be a factor affecting adipocyte viability [29]. Alternatively, a “dry” method without the tumescent fluid could be used. However, the “dry” technique may lead to a greater requirement for analgesics [27].
Micro- and nanofat grafts, typically harvested with cannulas as small as 0.7 mm in diameter, can be used to treat delicate areas of the face such as the eyelids and lips [30], [31]. Tonnard et al. highlighted the clinical application of micro- and nanofat grafts compared with macrofat [32]. Microfat particles were harvested from the abdomen using a cannula with a 1-mm diameter. An amount of the microfat was sheared into finer particles using a Leur-to-Leur connector with two 10-mL syringes [32], [33]. The nanoparticles were then filtered and collected. Macrofat particles were also harvested using a standard 3-mm cannula to serve as controls [32], [33]. The study provided micrographs, which showed normal cellular architecture and sparse nonviable cells for both the macro- and microfat particles [32], [33]. The nanofat grafts were devoid of adipocytes, and the native architecture was disrupted [32], [33]. However, the nanografts retained a rich supply of ASCs, which were similar to the ASCs in the macro- and microfat samples in terms of proliferation and differentiation [32], [33]. In several clinical cases, the use of nanofat grafts has resulted in improved skin quality by 6 months postoperatively [32], [33]. Therefore, the author suggests that while nanografts do not contain viable adipocytes, the high content of stem cells in these grafts may be clinically useful for skin rejuvenation [32], [33].
3. Fat processing
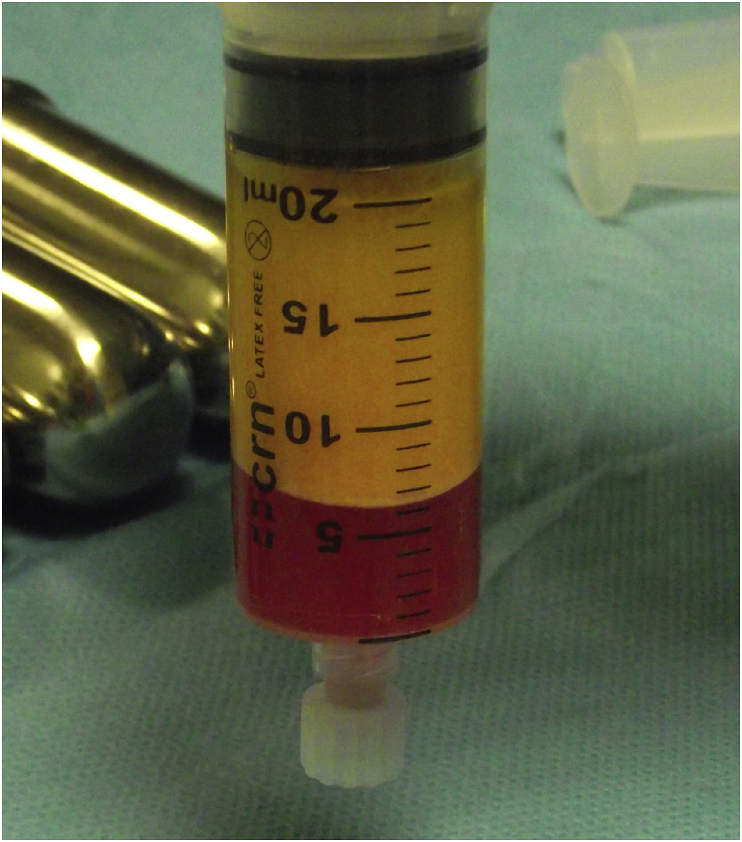
The most commonly used methods to prepare fat grafts are sedimentation, filtering, washing, and centrifugation. Fat processing is necessary because the lipoaspirate contains not only adipocytes but also collagen fibres, blood, and debris. These elements can cause inflammation at the recipient site, which can be detrimental for the fat graft [34]. Blood must be extracted because blood accelerates the degradation of the transplanted fat [35]. Moreover, the injection of debris gives an erroneous impression of the volume of correction because the debris will be absorbed after a few hours [34]. In animal experiments, no significant differences have been observed in the weight or architecture of fat grafts obtained using the centrifugation, filtration, or sedimentation methods [36], [37], [38], [39], [40]. In contrast, studies conducted in patients have demonstrated more favourable outcomes with centrifugation rather than gravity separation [36]. Comparative studies investigating the effects of fat processing with centrifugation, washing, and filtration have showed no significant differences in fat retention; however, filtration resulted in nodule formation, whereas centrifugation did not [41], [42]. Ferraro et al. [8] demonstrated that centrifugation with a force greater than 50 g resulted in damage to the structural integrity of adipose tissue, increased necrosis and apoptosis of cells, and decreased adipogenic differentiation capacity and tubule formation. Tubule formation during angiogenesis provides blood supply and nutrients to adipose tissue and ultimately sustains the fat graft for long-term retention [36], [43], [44]. Higher centrifugation speeds have also been correlated with increased fluid portion, reduced injectable tissue volume, and increased oil portion, which are associated with damage to adipocytes [36], [45]. Coleman suggested a processing method that has gained popularity and has been since integrated in many fat-transfer clinical protocols. Aspirated fat in syringes is spun at 3000 rpm for 3 min to isolate the fat [46]. After the centrifugation, three layers are observed: the first layer includes lipids, which can be poured off using absorbent material; the second layer consists of fatty tissue; and the third layer contains blood, tissue fluid, and local anaesthetic and is ejected from the base of syringe. The middle layer is routinely used for adipose tissue grafting [5], [47], [48], [49] (Fig. 1). The identification of an optimal processing method will increase the number of viable cells and ultimately increase fat engraftment and retention over time.
Fig. 1.
Lipoaspirate after centrifugation. From top to bottom: First layer of lipids, second layer of fatty tissue, and third layer of blood and local anaesthetics.
4. Fat injection
Despite a long history of clinical use and the evolution of fat-transfer techniques, no consensus exists on the best technique and the longevity of results, yet the principles of fat reimplantation are based on optimal recipient-site vascularity for increased fat survival [16]. Through a skin incision of a size corresponding to the diameter of the cannula, the fat graft is inserted at the level of the anatomical region affected. Small-gauge cannulas are thought to reduce trauma to the recipient site, thus reducing the risks of bleeding, haematoma formation, and poor graft oxygen diffusion [16]. Because revascularization starts at the periphery, ischaemic time is longer in the centre of the graft [34]. Therefore, fat reinjection in multiple small-volume sessions is preferred over one single injection [34]. Usually, through multiple access sites, multiple tunnels are created on insertion, but fat is injected only during withdrawal of the cannula in a “fanning-out” pattern [16]. Fat grafts are distributed in small aliquots and fanned out to varying depths in the soft tissue to avoid excessive interstitial pressure at the recipient site and overcrowding of the transplanted adipocytes [16]. Studies on fat-graft maintenance have demonstrated that mobile areas of the face, such as the glabella and lips, are less amenable to correction than are less-mobile areas, such as the malar and lateral cheek areas [16]. Regarding the cannula size, several authors use different calibre cannulas for fat injection, and the nature of the recipient site is the major determinant in the choice of cannula size. Ozsoy et al. [21] observed a greater vitality of adipose tissue if infiltrated with cannulas of at least 2.5 mm in diameter. However, Erdim et al. [23] found no significant differences in cell viability with differing needle gauge.
5. Role of ASCs
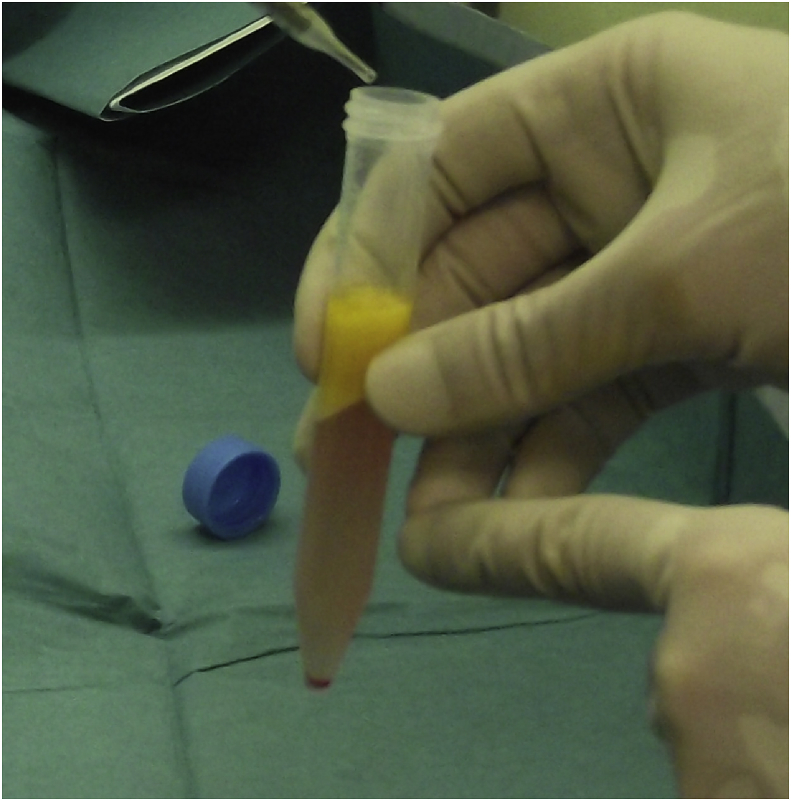
Fat transplantation techniques have dramatically changed over the last two decades, from simple free transfers of intact adipose tissue, which had limited success in the consistent replacement of volume defects, to free composite fat-cell transplantation strategies that, if properly executed, could have a high regenerative potential for both simple volume replacement as well as functional enhancement of recipient tissues. ASCs are similar to bone marrow–derived stem cells in that they are capable of differentiating into multiple mesodermal tissue types and show similar surface protein marker expression [15], [50]. The cytometric analysis of ASCs has shown that they do not express CD31 and CD45, but do express CD34, CD73, CD105, and the mesenchymal stem cell marker CD90 [51], [52]. Human ASCs are distinct from other mesenchymal progenitors in terms of surface-marker expression profile; notably, only ASCs express the stem-cell–associated marker CD34 in higher percentages than bone marrow–derived mesenchymal stem cells and dermal fibroblasts [53]. ASCs are different from bone marrow–derived mesenchymal stem cells because they can be easily obtained using a standard wet liposuction procedure under local anaesthesia, without the need for expansion in culture [15], [54]. For these reasons, ASCs are appealing for cell-based therapies involving tissue repair and regeneration. Stem cells isolated from lipoaspirates have demonstrated in vitro differentiation into adipogenic, osteogenic, chondrogenic, myogenic, cardiomyogenic, and neurogenic lineages [55], [56], [57]. We have previously reported two methods of ASC isolation (Fig. 2): one based on a mechanical + enzymatic procedure [51], and the other exclusively mechanical [52].
Fig. 2.
Adipose-derived stem cells (ASCs). Pellet of ASCs at the bottom of the tube.
ASCs are part of the stromal vascular fraction (SVF) of adipose tissue, together with a heterogeneous population of many other cell types, including preadipocytes, endothelial cells, pericytes, haematopoietic-lineage cells, and fibroblasts [58]. The regenerative features of the SVF are attributable to its paracrine effects: SVF cells secrete vascular endothelial growth factor, hepatocyte growth factor, and transforming growth factor-β in the presence of stimuli such as hypoxia and other growth factors [15], [59] and strongly influence the differentiation of stem cells, promoting angiogenesis and wound healing, and potentially aiding new tissue growth and development [60]. Other studies have emphasized the plasticity of preadipocytes and macrophages, and suggested that some or all of the healing effect maybe secondary to enhanced immune response [61], [62] or removal of dying or defective cells, leading to permanent tissue remodelling [63]. In 2006, Matsumoto et al. [64] provided evidence to support a novel method of autologous tissue transfer, which they named cell-assisted lipotransfer (CAL). CAL is the concurrent transplantation of aspirated fat and ASCs. In CAL, ASCs were supportively used to boost the efficacy of autologous lipoinjection (resulting in a higher survival rate and the persistence of transplanted fat) and to decrease the known adverse effects of lipoinjection, such as fibrosis, pseudocyst formation, and calcification [64].
6. Applications
6.1. Breast reconstruction
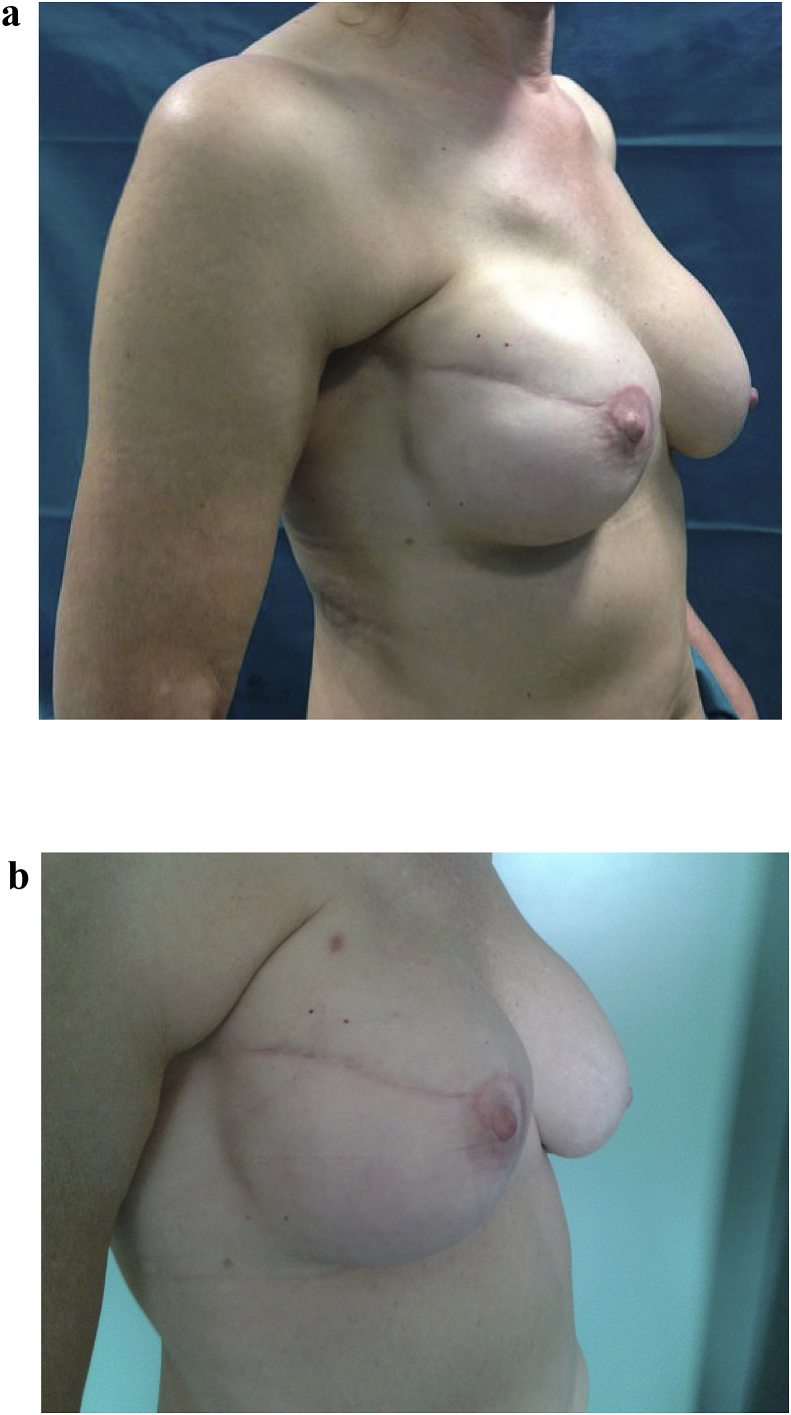
Autologous fat transplantation is widely used in reconstructive breast surgery. Plastic surgeons and patients seeking breast reconstruction may have drastically different images in mind of what constitutes an attractive, natural, and ideal breast shape [65], [66]. Lipofilling represents a simple solution to restore the correct profile of the breast after reconstruction. In fact, in the immediate or late postoperative period, secondary contour defects of the reconstructed breast can develop [67], [68]. Indeed, there are important landmarks in the female breast, for example, the creation of a well-defined inframammary fold is a fundamental element in obtaining a good aesthetic result after breast reconstruction [69]. Lipofilling can be used after reconstruction with implants or muscle flaps with or without tissue expansion (Fig. 3a–b). Appropriate tissue expansion allows the use of autologous flaps or the insertion of definitive prosthetic implants for breast reconstruction. This could be carried out with the aid of a computer program to help the surgeon select the proper tissue expander while planning breast reconstruction [70], [71]. In Poland syndrome of the chest wall and breast, autologous fat injection seems to be a particularly useful technique to fill the subclavicular and anterior axillary fold defects. Fat injection is rarely used as the sole treatment, rather it is often performed in combination with other routine techniques of breast surgery [72]. When fat tissue is not perfused, it can die and result in necrotic cysts and even calcification, but this complication can occur in any surgical breast procedure [73]. Fat grafting to the breast could potentially interfere with breast cancer detection; however, no evidence has been found that strongly supports such an interference [74]. Early studies noted that graft re-absorption was the main drawback of fat grafting, with 50%–90% graft loss [75], [76], [77], [78]. Large grafts exhibit higher rates of liquefaction, necrosis, and cyst formation, while too small a graft volume is associated with resorption [75], [79]. To ensure maximal take, many surgeons practice repeated transfers [3], [79], [80], [81].
Fig. 3.
a. Autologous fat grafting in secondary breast reconstruction. 39 years old patient subjected to mastectomy and recostruction with implants. Before treatment with lipofilling. b. 39 years old patient subjected to mastectomy and recostruction with implants. After treatment with lipofilling at the level of the scar of right breast.
6.2. Scars
Patients with retractile and painful scars compromising the normal daily activity/mobility of the joint involved can take advantage of lipofilling treatment. In fact, fat transplantation can be used not only to fill atrophic scars but also to reduce scar contracture as a regenerative alternative to other surgical techniques [82]. This is made possible by the presence of ASCs in the fat tissue. From a histological point of view, autologous fat grafts show the ability to regenerate the dermis and subcutaneous tissue and improve the dermal and dermohypodermic quality in scar areas, with increasing amount of fat layer - largely destroyed in cases of thermal insults and poorly regenerated during tissue repair after any type of trauma - new collagen deposition, and local neoangiogenesis [83], [84], [85]. Mojallal et al. [86] showed that fat tissue grafting stimulates the neosynthesis of collagen fibres at the recipient site and makes the dermis thicker, thereby improving skin quality. The regenerative role of fat in scarred areas is thought to be attributable to the release of multiple nerve entrapments, so that neuropathic pain is improved. In addition, the improvement in neurogenic pain may be maintained by placing fat grafts around the nerve to avoid the recurrence of scar contracture [86]. Klinger et al. [85] described the use of lipofilling to treat scars in 694 patients. They observed quality improvement in all treated scars, from both an aesthetic and a functional point of view. In particular, the relief from pain and increase in scar elasticity were clinically assessable in all patients. The first results were observed soon after the procedure (at 14 days); relief from pain and improvement in scar elasticity were noted at 3 months and were sustained in all patients until the 1-year follow-up. Autologous fat grafts allow skin to become softer and more flexible and extensible, and very often the colour seems similar to that of the surrounding unaffected skin. Another important quality of scars release, both superficial and deep, is the improvement in the mobility of the body part involved, in particular, the affected joints, eyelids, nasal valve, and mouth, as well as the possibility for the patient to have a partial restoration of facial expression. In patients with marked skin depression, scar release by autologous fat grafting often fills these volume deficits, leading to excellent cosmetic results and positively affecting the patient's body image [85].
6.3. Burns
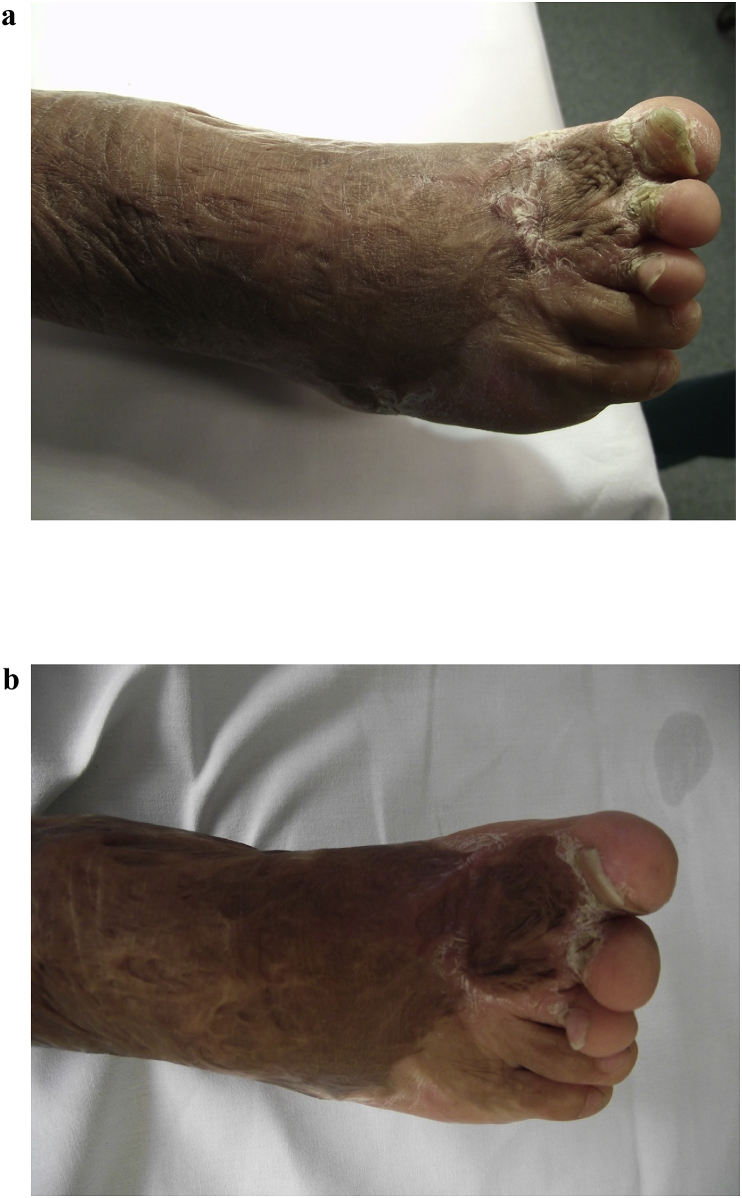
Burn injury is a devastating trauma with systemic consequences. Although survival rates are increasing, burn injury remains a great challenge in the field of cutaneous wound healing. Major burns patients lack enough skin to cover their burns, and the currently used cutaneous substitutes and cultured epithelial autografts are still neither efficient nor effective solutions [86], [87]. Transplanted skin from donors is currently not an option due to graft rejection; however, augmenting immunotolerance via stem cell therapy may overcome this problem. Regenerative medicine using stem cells is an efficient, low-morbidity, and high-quality therapy for skin coverage in burns, mainly due to the regeneration of skin appendages [88] and the minimal risk of hypertrophic scarring [89], [90]. Hypertrophic burn scars occur in approximately 75% of white patients with third-degree burns [91], [92]. Burn outcomes still represent a problem because of aesthetic and functional concerns as well as concerns regarding the patient's social and psychological life [93]. Subscar and intrascar fat grafting are relatively recent techniques that improve scar quality (Fig. 4a–b). Bruno et al. [93], in their immunohistochemical study, reported that burn scars, even old ones, cannot be considered as quiescent scars because they are characterized by maturation block and a proinflammatory and hypervascularized status. Lipofilling allows a dramatic change in this status, making the tissue much more similar to healthy tissues from a histological point of view [93]. In another study, Brongo et al. [94] reported their experience with the use of lipofilling to treat burn scars. They evaluated the evolution of the scars at 1 year after the treatment by means of a questionnaire and physical and histopathological examinations. At the 1-year follow-up, all patients reported an improvement in their clinical condition. The histological findings showed new collagen deposition, neoangiogenesis, and dermal hyperplasia in the context of new tissue, demonstrating tissue regeneration. Clinically, improved texture, softness, thickness, colour, and elasticity of the treated skin were observed, as was a reduction in scar retraction [94]. Conversely Gal et al. [95] treated eight burned pediatric patients with a single autologous fat transplantation and did not observed any scar improvement when compared to a control group treated with saline injections. The authors theorized that their findings may be explained by the fact that they performed only one single session of fat grafting. Indeed Strong et al. [36] theorized that serial fat transplant sessions may be required to improve scarred recipient sites.
Fig. 4.
a. Autologous fat grafting of burn scars. 25 years old patient with a burn scar with a retracted and hypertrophic burn scar. Before treatment with lipofilling. b. 25 years old patient with a burn scar with a retracted and hypertrophic burn scar. After treatment with lipofilling.
To enhance the therapeutic response after stem cell treatment in burns patients, intense tissue engineering with the development of 3D scaffolds or matrices is of vital importance as well as improved preconditioning cell treatments and optimized culture conditions [96].
6.4. Radiodermatitis
Radiation dermatitis is caused by prolonged exposure of the skin to ionizing radiation [97]. It can be seen in patients receiving radiation therapy, with or without adjuvant chemotherapy [98]. Inflammation of the skin after exposure to radiotherapy (radiodermatitis) can be classified into three specific types: acute radiodermatitis, chronic radiodermatitis, and eosinophilic, polymorphic, and pruritic eruption associated with radiotherapy [98]. Rigotti et al. [99] reported that the transplantation of lipoaspirates containing adult ASCs is a highly effective therapeutic approach for the treatment of degenerative, chronic lesions induced as late effects of oncologic radiation treatments. In fact, ultrastructural analysis of the radio-damaged tissue revealed a significant reduction of the capillary bed [99]. Owing to the angiogenic factors released from ASCs, lipofilling interrupted a vicious circle of vascular lesion, ischaemia, hyperpermeability, and fibrosis leading to increased ischaemia, and favoured the growth of a microvascular bed with the correct ratio of adipocytes to capillaries [99]. These changes lead to the production of new microvessels, which ultimately increases circulation. They advanced the idea that the chain of events leading to mesemchymalization of the tissue would be the following: targeting of damaged areas by stem cells, release of angiogenic factors, formation of new vessels, and oxygenation [99].
This process would favour the development of stem cells into mature adipocytes and in a newly formed microcirculation replacing the replacing the existing, seriously damaged one [99]. Because some damaged vessels could still be found in persisting areas of fibrosis late after the treatment, they stressed the importance of performing repeated injections to obtain homogeneous improvement throughout the radio-damaged area [99]. In fact, they reported a linear relationship between clinical improvement and the number of transplants. The reason for this is probably that the healing of tissue microangiopathy increased as a function of the total number of stem cells [99].
6.5. HIV-associated lipodystrophy
The redistribution of fat deposits in HIV-associated lipodystrophy includes visceral fat accumulation in the abdomen and subcutaneous fat accumulation in the breasts and the cervical and dorsal areas (buffalo hump) with fat wasting in the legs, arms, buttocks, and face [100]. Many hypotheses have been proposed for its aetiology: most of them focus on the mitochondrial toxic effects and altered adipocyte differentiation induced by protease inhibitors and nucleoside reverse transcriptase inhibitors [100], [101], [102], but lipodystrophy has also been represented as a selective neuropathy [100], [103]. Following the consistent experience of Coleman in facial lipofilling for aesthetic purposes [2], [100], [104], autologous fat injection has been considered as one of the most reliable treatments for facial subcutaneous augmentation. However, not all patients are candidates for this treatment because some patients do not possess enough subcutaneous tissue in the lower abdominal area, which is the fattiest area of the body in these patients. In many cases, the treating physician informs the patients of the possibility of a plastic surgery treatment for their facial lipoatrophy. This strategy makes the patients adhere to their therapy more readily [101].
6.6. Aesthetic surgery
6.6.1. Facial rejuvenation
Autologous fat grafting has an important role in facial rejuvenation. In fact, the unique regenerative potential of lipofilling leads to excellent results due to its filling properties and the role of ASCs. For this reason, lipofilling has unique features, and plastic surgeons can use it not only to correct soft-tissue deficiencies but also to rejuvenate the skin of the face [87]. The loss of facial volume, especially in the periorbital region, is an important component of aging and is due to the redistribution and atrophy of facial fat [105], [106], [107], [108]. Traditional approaches to facial rejuvenation have relied on subtractive surgical techniques, focusing on the excision of skin, muscle, and/or fat [109]. Modern approaches concentrate instead on filling the “empty” facial compartments, mainly through fat grafting [110]. Traditional fat grafting involves Coleman's harvesting technique with 2-mm side-port cannulas, followed by the distribution of a structural fat implant throughout the various dermal layers of the face, from deep to superficial [104]. Disadvantages of traditional fat grafting include the risks of irregular fat accumulation, fat necrosis, and visible lumpiness. Because the eyelid skin is usually thin, the periocular area is most susceptible to contour problems, and thus, deep implantation of fat is recommended [110]. In response to concerns such as those outlined above, many authors have recently focused on microfat grafting techniques [104], [111]. A major effect of microfat injection is improvement in the viability of adipocytes via the disruption of fat lobules [112], which is contrary to Coleman's thesis that preservation of the lobular structure is essential for fat survival [2]. Moreover, Moscatello et al. [111] demonstrated that the greater surface area of the disrupted fat lobules on the recipient bed significantly improved fat survival after injection. More recently, various authors have proposed “ultra-micro” fat as a very superficial implant in the periocular and perioral areas [113], [114]. These newer techniques are based on fat harvesting with Coleman's traditional cannulas, followed by various modalities of fat processing to disrupt the large fat lobules harvested [111], [112], [113], [114]. Tonnard et al. [113] reported that manual fat emulsification provides a nanofat solution rich in the SVF and consequently ASCs, but devoid of viable adipocytes. Consequently, the indications of nanofat are reportedly limited to skin regeneration and do not include volume restoration [113], [115]. In fact, it may be questioned whether a nanofat transfer actually is a “fat grafting” procedure, as adipocytes did not survive the emulsification process.113 The major effect of nanofat injection is probably a stem cell activity so nanofat injection might rather be considered as an in vivo tissue-engineering process [113]. It might be logical to discard the dead adipocyte fraction from the nanofat and to inject the purified stromal vascular fraction only. Moreover, it is known that apoptotic cells release cytokines and attract macrophages that induce growth factors and play an important role in regeneration of the damaged tissue 113 Thus, coinjection of fragmented adipocytes might have a stimulating effect on stem cell differentiation and tissue regeneration [113]. Aesthetically, the main surgical indications of lipofilling for facial rejuvenation are the correction of dark circles [116], as an adjuvant to blepharoplasty, or as an alternative treatment for hollow eyes and malar bags [113], [117]. Fat reinjection is an important step in the overall success of the graft. Fat is injected in longitudinal tunnels that form a 3D mesh to promote revascularization and graft survival, per Coleman's technique [110], [117]. In the upper eyelids, fat grafting is generally used to fill hollow eyelids. The injections are sometimes carried out in conjunction with blepharoplasty [117], [118]. The injection sites are located at the medial 2/3 of the upper eyelid, the inferomedial 1/3 of the eyebrow, and the lateral part of the eyebrow [113], [117]. In the lower eyelids, fat grafting helps restore volume, including that of the periorbital region. The injection points are located external to the zygomatic bone [113], [117], [119] and in “the valley of tears” [117], [119]. In these areas, injections should not be administered between the skin and muscle, as the skin here is thin and such injections may lead to palpable irregularities [117], [110]. A greater understanding of facial aging mechanisms, consisting of fat atrophy and ptosis of the different facial compartments, has allowed fat grafting to be considered as a possible technique for facial rejuvenation, particularly of the eyelids [117].
6.6.2. Hand rejuvenation
The appearance of the hands is a tell-tale sign of a person's true age [120], [121]. Studies have shown that people are able to roughly estimate a person's age solely by viewing their hands [120], [122]. Extrinsic effects on the hand include dermatoheliosis and photoaging, which lead to wrinkles and irregular pigmentation in the form of solar lentigines, solar purpura, punctuate hypopigmentation, actinic keratosis, seborrhoeic keratosis, and telangiectasia [120], [123], [124]. Aging also leads to intrinsic effects such as the gradual disappearance of subcutaneous fullness and tissue atrophy due to collagen depletion and dehydration [125], [126], [127]. This leads to dorsal skin wrinkling and greater visibility of the extensor tendons, and makes subcutaneous veins appear more blue and tortuous [120], [121], [128], [129]. Because fat not only serves as a filler but also has the regenerative potential to improve the quality of soft tissue and skin on the dorsal side of the hands, fat grafting is an attractive procedure for hand rejuvenation [24]. Under local anaesthesia, the fat graft is injected using blunt cannulas to reduce the risk of dorsal vein perforation. Between 10 and 30 mL of fat should be injected to give the hand a puffy, slightly overfilled look. A small volume of fat tissue should also be injected at the base of each finger, to give a uniform appearance to the whole hand [120].
6.6.3. Rhinoplasty
Patients who elect to undergo augmentation rhinoplasty often present with concerns of a low dorsum and a short nose [130]. Both autologous grafts and synthetic implants can result in acceptable outcomes of rhinoplasty. In general, synthetic implants are associated with higher rates of complications, such as displacement and extrusion. Coleman [2], [24] emphasized that structural fat grafting to regions with thin skin, such as the periorbital area, must involve the delivery of minute fat parcels. The nasal dorsum is characterized by relatively thin skin and limited space so the implantation of large fat parcels is more likely to lead to dislodgement of the implant, nodulation, and skin irregularities [130]. Therefore, often, autologous microfat transplantation is used to correct the profile of the nose [130].
6.6.4. Breast augmentation and asymmetry
Initially, fat grafting was considered to be a promising technique for breast augmentation and correction of breast asymmetry because of advantages such as easy availability of donor tissue, absence of a scar, and short recovery time. Moreover, because the procedure could be performed in an outpatient setting and avoided the complications of prostheses, it gained significant initial popularity [131]. However, the value of this technique in augmenting breasts and filling breast defects became controversial because the results are not always sustainable. In a historical review, Billings et al. [132] reported that the results of free fat transplantation are unpredictable, with wide variation in the bulk of the graft, the possibility of fat resorption, and the necessity of other lipofilling techniques. Although fat grafting for primary breast augmentation had a “bad reputation” in the past, the procedure itself has gained more popularity recently and is being performed more and more by plastic surgeons worldwide for primary breast augmentation [74], [133], [134]. There are adequate studies in the literature to support the efficacy and safety of fat grafting for primary breast augmentation [75], [132], [134], [135]. In fact, fat graft transplantation will probably replace most of the current techniques for the correction of breast asymmetry [133], [136], especially less-significant breast asymmetries. Breast augmentation with implants, mastopexy, and breast reduction will continue to play a role in the correction of significant breast asymmetry [133].
6.6.5. Gluteal augmentation
Gluteal augmentation is often performed by means of intramuscular implants, but lipofilling has begun to gain popularity in recent times. Indeed, fat grafting involves the harvesting of fat from unwanted areas, and this allows associating gluteal augmentation with body contouring surgery. Fat grafting will play an important role in gluteal augmentation and may replace implant-based gluteal augmentation if the patient has a great enough amount of fat as a donor material [133], [137], [138].
7. Complications
Every step in fat transplantation, i.e., harvesting, processing, and transplantation, is important, but viability of the harvested fat cells is crucial [139]. The chances of survival are higher the less the fat graft is manipulated and the more quickly it is reinjected [140]. Donor-site complications appear to be minimal and related to the liposuction technique. The possible complications include bruising, swelling, haematoma formation, paraesthesia or donor-site pain, infection, hypertrophic scarring, contour irregularities, and damage to the underlying structures for example due to the intraperitoneal or intramuscular penetration of the cannula [26], [73], [74], [141], [142], [143].
Lipofilling of the breast could cause in the recipient site fat necrosis, oil cyst formation, and calcification if large volumes of fat are injected into a single area or if fat is injected into poorly vascularized areas. These changes result in the failure of “graft take” and lead to palpable masses due to fat necrosis. Moreover, these masses may be difficult to distinguish clinically from local recurrence in breast cancer patients and lead to a need for additional imaging and needle biopsy in 3%–15% of patients [26], [73], [74], [141], [142], [143]. Post-lipofilling calcification can be found on mammograms in 0.7%–4.9% of patients [26]. Agha et al. [144] in a review of 24 studies reported 207 complications, 7,3% of 2832 treated breasts. Fat necrosis accounted for 62% of all complications and occurred in 17 of the 24 studies.
The complications of lipofilling for hand rejuvenation may include cellulitis at the donor site [120], transient digital numbness [145], infections at both the recipient and harvest sites [146], cyst formation [146], [147] in 10% of patients, temporary dysaesthesia [146], fat necrosis [146], [148], and reabsorption of the grafted fat [121], [147], which is the most common complication. In one study, 24% of patients required repeated fat injections [121].
The major complications of facial rejuvenation by lipofilling are possibly attributable to the injection of fat grafts in “dangerous” areas such as the glabella and nasolabial folds [117], [149]. In fact, fat grafts may cause cerebral or ocular artery thrombosis, with an increase in local pressure, resulting in a reflux of the fat into the ophthalmic artery and the internal carotid artery [117], [149]. To limit this risk and the risks of fat embolism and serious consequences, verification of an absence of blood reflux into the syringe prior to the injection, slow injection at low pressure, and the use of a blunt-tip cannula are recommended [117], [149]. When lipofilling is used to correct the dorsum of the nose, surgeons should prevent a spike in local pressure that could propel a fat parcel upstream to the ophthalmic artery where it could occlude the central retinal artery and cause visual disturbance or blindness [118], [149], [150], [151].
8. Discussion
Fat injection has been used for more than 20 years as a relatively low-risk and low-morbidity procedure to correct a variety of soft-tissue defects in the face, trunk, and extremities. As with any surgical procedure, the technique used, its execution, and the experience of the surgeon affect the outcomes. In the case of breast reconstruction, lipofilling can be used to improve soft-tissue coverage following prosthesis or tissue expander implantation and to achieve volume replacement of implants in patients with unsatisfactory outcomes of oncoplastic reconstruction. Fat grafts are easily available, biocompatible, associated with low donor-site morbidity, and provide a natural appearance. However, fat grafting is generally considered an unpredictable procedure [5], with long-term retention rates varying between 10% and 80% [152], [153]. Choi et al. [153] analysed volumetric data obtained using 3D imaging over a certain time course, and found that the breast tissue has a resolution similar to that of soft-tissue oedema, and that approximately 40%–50% of the injected fat volume was retained at 5 months after the procedure [153]. However adipose-derived stem cells (ADSC), either isolated from fresh adipose tissues before ADSC cryopreservation or isolated from cryopreserved fat tissues, showed unaltered capabilities of proliferation and differentiation after optimal cryopreservation protocols. This confirms the probability of ADSC in providing an important source for cell based therapy and tissue engineering [154], [155], [156]. Successful cryopreservation of adipose tissue and ADSC can lead to a new era in fat grafting and ADSC-related tissue regeneration therapy in plastic surgery [157], [158], [159], [160]. In cosmetic breast surgery, lipofilling is a popular procedure and will probably replace most of the current techniques for the correction of less-significant breast asymmetry [134]. However, breast augmentation with implants, mastopexy, and breast reduction will continue to play a role in the correction of important breast asymmetry [134], [137].
Rohrich et al. have classed it as number three in the list of top innovations in plastic surgery [161]. Agha et al. recently published a systematic review and meta-analysis of this topic and demonstrated the utility of this technique as assessed across six outcome domains; oncological, clinical, aesthetic and functional, patient-reported, process and radiological [162], [163]. Whilst conducting the review they noticed significant heterogeneity in the outcomes used by the various study authors. Further analysis of the 35 studies in follow-up work identified that there were a total of 51 different outcomes reported [162], [164]. Their objective is to develop a core outcome set (COS) for autologous fat grafting to the breast. It would result in improvements in the conduct and reporting of autologous fat grafting research and help to minimize outcome and reporting bias, standardise end-points, boost transparency, increase reproducibility and external validity, aid evidence synthesis and help guide clinical decision-making going forward [162].
Nasal lipofilling has become a particularly interesting alternative for the treatment patients who have previously undergone rhinoplasty, especially those who refuse secondary invasive surgery. The ideal indications for nasal lipofilling are small defects such as lateral osteotomy sequelae or inverted V deformations, particularly in young subjects. Injection of autologous fat is an interesting technique for the improvement of the quality of the skin on the nasal dorsum, especially if it is thin and cicatricial. Thus, another indication for microfat injection could be the augmentation of nasal cutaneous material with the intention of performing secondary rhinoplasty [165], [166].
Lipofilling can be effectively used to correct burn scars and radiodermatitis and for the rejuvenation of the face or hands, owing to the regenerative properties of ASCs [85], [167]. In fact, ASCs in fat grafts allow the regeneration of damaged tissues through their paracrine, immunomodulatory, chemotactic, and differentiating effects. When injected in burned or scarred areas, these cells reduce tissue contraction, improve skin texture, and relieve pain [85], [156]. In radiodermatitis, ASCs promote the regeneration of healthy tissue at the expense of the radio-damaged tissues [99]. ASCs also help reverse the effects of photoaging via regeneration of the microcirculation and collagen by means of the release of growth factors [156].
9. Conclusions
Lipofilling can be used in various fields of plastic surgery due to its filler and regenerative effects, with minimal discomfort for the patient. However, one of the fundamental limitations is that the amount of the fat graft is strictly linked to the amount of adipose tissue in the patients. The role of lipofilling could be more significant with the application of the findings of experimental research on tissue engineering and ASCs.
Ethical approval
Nothing to declare.
Sources of funding
Nothing to declare.
Author contribution
Dr. Simonacci Francesco, writing.
Dr. Bertozzi Nicolo', data collections.
Dr. Grieco Michele Pio, data collections.
Prof. Eugenio Grignaffini, data collections.
Prof. Raposio Edoardo, study design.
Conflicts of interest
The authors declares that there is no conflict of interest regarding the publication of this paper.
Guarantor
Dr. Simonacci Francesco.
Dr. Bertozzi Nicolo'.
Dr. Grieco Michele Pio.
Prof. Grignaffini Eugenio.
Prof. Raposio Edoardo.
Trial registry number
Nothing to declare.
References
- 1.Billings E., Jr., May J.W., Jr. Historical review and present status of free fat graft auto transplantation in plastic and reconstructive surgery. Plast. Reconstr. Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Coleman S.R. Structural fat grafting. Aesthet. Surg. J. 1998;18:386–388. doi: 10.1016/S1090-820X(98)70098-6. [DOI] [PubMed] [Google Scholar]
- 3.Coleman S.R. Long-term survival of fat transplants: controlled demonstrations. Aesthet. Plast. Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 4.Li B.W., Liao W.C., Wu S.H., Ma H. Cryopreservation of fat tissue and application in autologous fat graft: in vitro and in vivo study. Aesthet. Plast. Surg. 2012;36:714–722. doi: 10.1007/s00266-011-9848-z. [DOI] [PubMed] [Google Scholar]
- 5.Gir P., Brown S.A., Oni G., Kashefi N., Mojallal A., Rohrich R.J. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast. Reconstr. Surg. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 6.Leong D.T., Hutmacher D.W., Chew F.T., Lim T.C. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J. Dermatol. Sci. 2005;37:169–176. doi: 10.1016/j.jdermsci.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Tremolada C., Palmieri G., Ricordi C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transpl. 2010;19:1217–1223. doi: 10.3727/096368910X507187. [DOI] [PubMed] [Google Scholar]
- 8.Ferraro G.A., De Francesco F., Tirino V., Cataldo C., Rossano F., Nicoletti G., D'Andrea F. Effects of a new centrifugation method on adipose cell viability for autologous fat grafting. Aesthet. Plast. Surg. 2011;35:341–348. doi: 10.1007/s00266-010-9613-8. [DOI] [PubMed] [Google Scholar]
- 9.Katz A.J., Llull R., Hedrick M.H., Futrell J.W. Emerging approaches to the tissue engineering of fat. Clin. Plast. Surg. 1999;26:587–603. [PubMed] [Google Scholar]
- 10.Raposio E., Guida C., Baldelli I., Benvenuto F., Curto M., Paleari L., Filippi F., Fiocca R., Robello G., Santi P.L. Characterization and induction of human pre-adipocytes. Toxicol. In Vitro. 2007;21:330–334. doi: 10.1016/j.tiv.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Raposio E., Guida C., Coradeghini R., Scanarotti C., Parodi A., Baldelli I., Fiocca R., Santi P.L. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008;41:739–754. doi: 10.1111/j.1365-2184.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raposio E., Bertozzi N., Bonomini S., Bernuzzi G., Formentini A., Grignaffini E., Pio Grieco M. Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds. 2016;28:126–131. [PubMed] [Google Scholar]
- 13.Higuci A., Chuang C.W., Ling Q.D., Huang S.C., Wang L.M., Chen H., Chang Y., Wang H.C., Bingh J.T., Chang Y., Hsu S.T. Differentiation ability of adipose-derived stem cells separated from adipose tissue by a membrane filtration method. J. Memb. Sci. 2011;366:286–294. [Google Scholar]
- 14.Salibian A.A., Widgerow A.D., Abrouk M., Evans G.R. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch. Plast. Surg. 2013;40:666–675. doi: 10.5999/aps.2013.40.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caruana G., Bertozzi N., Boschi E., Pio Grieco M., Grignaffini E., Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann. Ital. Chir. 2015;86:1–4. [PubMed] [Google Scholar]
- 16.Kakagia D., Pallua N. Autologous fat grafting: in search of the optimal technique. Surg. Innov. 2014;21:327–336. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 17.Pu L.L., Coleman S.R., Cui X., Ferguson R.E., Jr., Vasconez H.C. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast. Reconstr. Surg. 2008;122:932–937. doi: 10.1097/PRS.0b013e3181811ff0. [DOI] [PubMed] [Google Scholar]
- 18.Fagrell D., Eneström S., Berggren A., Kniola B. Fat cylinder transplantation: an experimental comparative study of three different kinds of fat transplants. Plast. Reconstr. Surg. 1996;98:90. doi: 10.1097/00006534-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Qin W., Xu Y., Liu X., Xu S. Experimental and primary clinical research of core fat graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26:576–582. [PubMed] [Google Scholar]
- 20.Pu L.L., Cui X., Fink B.F., Cibull M.L., Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann. Plast. Surg. 2005;54:288–292. [PubMed] [Google Scholar]
- 21.Ozsoy Z., Kul Z., Bilir A. The role of cannula diameter in improved adipocyte viability: a quantitative analysis. Aesthet. Surg. J. 2006;26:287–289. doi: 10.1016/j.asj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Campbell G.-L., Laudenslager N., Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. Am. J. Cosmet. Surg. 1987;4:89–94. [Google Scholar]
- 23.Erdim M., Tezel E., Numanoglu A., Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J. Plast. Reconstr. Aesthet. Surg. 2009;62:1210–1214. doi: 10.1016/j.bjps.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Coleman S.R. Structural fat grafting: more than a permanent filler. Plast. Reconstr. Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 25.Crawford J.L., Hubbard B.A., Colbert S.H., Puckett C.L. Fine tuning lipoaspirate viability for fat grafting. Plast. Reconstr. Surg. 2010;126:1342–1348. doi: 10.1097/PRS.0b013e3181ea44a9. [DOI] [PubMed] [Google Scholar]
- 26.Hamza A., Lohsiriwat V., Rietjens M. Lipofilling in breast cancer surgery. Gland. Surg. 2013;2:7–14. doi: 10.3978/j.issn.2227-684X.2013.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein J.A. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast. Reconstr. Surg. 1993;92:1085–1098. [PubMed] [Google Scholar]
- 28.Illouz Y.G., de Villers Y.T. Churchill Livingstone; New York, NY: 1989. Body Sculpturing by Lipoplasty; pp. 120–126. [Google Scholar]
- 29.Kasem A., Wazir U., Headon H., Mokbel K. Breast lipofilling: a review of current practice. Arch. Plast. Surg. 2015;42:126–130. doi: 10.5999/aps.2015.42.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasiou-Plakida D. Fat injections for facial rejuvenation: 17 years experience in 1720 patients. J. Cosmet. Dermatol. 2003;2:119–125. doi: 10.1111/j.1473-2130.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 31.Mazzola R.F. Quality Medical Publishing; St. Louis, MO: 2009. Fat Injection: from Filling to Regeneration; pp. 373–422. [Google Scholar]
- 32.Tonnard P., Verpaele A., Peeters G., Hamdi M., Cornelissen M., Declercq H. Nanofat grafting: basic research and clinical applications. Plast. Reconstr. Surg. 2013;132:1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 33.Gause T.M., Kling R.E., Sivak W.N., Marra K.G., Rubin J.P., Kokai L.E. Particle size in fat graft retention: a review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 2014;3:273–279. doi: 10.4161/21623945.2014.957987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mojallal A., Foyatier J.L. The effect of different factors on the survival of transplanted adipocytes. Ann. Chir. Plast. Esthet. 2004;49:426–436. doi: 10.1016/j.anplas.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Sommer B., Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol. Surg. 2000;26:1159–1566. [PubMed] [Google Scholar]
- 36.Strong A.L., Cederna P.S., Rubin J.P., Coleman S.R., Levi B. The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast. Reconstr. Surg. 2015;136:897–912. doi: 10.1097/PRS.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condé-Green A., Wu I., Graham I., Chae J.J., Drachenberg C.B., Singh D.P., Holton L., 3rd, Slezak S., Elisseeff J. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: a pilot study. Aesthet. Surg. J. 2013;33:713–721. doi: 10.1177/1090820X13487371. [DOI] [PubMed] [Google Scholar]
- 38.P1 Smith, Adams W.P., Jr., Lipschitz A.H., Chau B., Sorokin E., Rohrich R.J., Brown S.A. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast. Reconstr. Surg. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 39.Minn K.W., Min K.H., Chang H., Kim S., Heo E.J. Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthet. Plast. Surg. 2010;34:626–631. doi: 10.1007/s00266-010-9525-7. [DOI] [PubMed] [Google Scholar]
- 40.Y1 Ramon, Shoshani O., Peled I.J., Gilhar A., Carmi N., Fodor L., Risin Y., Ullmann Y. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast. Reconstr. Surg. 2005;115:197–201. [PubMed] [Google Scholar]
- 41.Botti G., Pascali M., Botti C., Bodog F., Cervelli V. A clinical trial in facial fat grafting: filtered and washed versus centrifuged fat. Plast. Reconstr. Surg. 2011;127:2464–2473. doi: 10.1097/PRS.0b013e3182131d5d. [DOI] [PubMed] [Google Scholar]
- 42.Khater R., Atanassova P., Anastassov Y., Pellerin P., MartinotDuquennoy V. Clinical and experimental study of autologous fat grafting after processing by centrifugation and serum lavage. Aesthet. Plast. Surg. 2009;33:37–43. doi: 10.1007/s00266-008-9269-9. [DOI] [PubMed] [Google Scholar]
- 43.Pfaff M., Wu W., Zellner E., Steinbacher D.M. Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthet. Plast. Surg. 2014;38:224–229. doi: 10.1007/s00266-013-0261-7. [DOI] [PubMed] [Google Scholar]
- 44.Kurita M., Matsumoto D., Shigeura T., Sato K., Gonda K., Harii K., Yoshimura K. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast. Reconstr. Surg. 2008;121:1033–1041. doi: 10.1097/01.prs.0000299384.53131.87. [DOI] [PubMed] [Google Scholar]
- 45.Hoareau L., Bencharif K., Girard A.C., Gence L., Delarue P., Hulard O., Festy F., Roche R. Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiency. J. Plast. Reconstr. Aesthet. Surg. 2013;66:712–719. doi: 10.1016/j.bjps.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 46.Coleman S.R. Facial augmentation with structural fat grafting. Clin. Plast. Surg. 2006;33:567–577. doi: 10.1016/j.cps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Wilson A., Butler P.E., Seifalian A.M. Adipose-derived stem cells for clinical applications: a review. Cell Prolif. 2011;44:86–98. doi: 10.1111/j.1365-2184.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuin A.J., Domerchie P.N., Schepers R.H., Willemsen J.C., Dijkstra P.U., Spijkervet F.K., Vissink A., Jansma J. What is the current optimal fat grafting processing technique? A systematic review. J. Craniomaxillofac. Surg. 2016;44:45–55. doi: 10.1016/j.jcms.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Conde-Green A., de Amorim N.F., Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J. Plast. Reconstr. Aesthet. Surg. 2010;63:1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 51.Raposio E., Caruana G., Petrella M., Bonomini S., Grieco M.P. A standardized method of isolating adipose-derived stem cells for clinical applications. Ann. Plast. Surg. 2016;76:124–126. doi: 10.1097/SAP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 52.Raposio E., Caruana G., Bonomini S., Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014;133:1406–1409. doi: 10.1097/PRS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., Sato K., Inoue K., Nagase T., Koshima I., Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. J. Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 54.Lee R.H., Kim B., Choi I., Kim H., Choi H.S., Suh K., Bae Y.C., Jung J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow are proved to be safe and reliable, allowing surgeons and adipose tissue. Cell Physiol. Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 55.Coradeghini R., Guida C., Scanarotti C., Sanguineti R., Bassi A.M., Parodi A., Santi P.L., Raposio E. A comparative study of proliferation and hepatic differentiation of human adipose-derived stem cells. Cells Tissues Organs. 2010;191:466–477. doi: 10.1159/000273266. [DOI] [PubMed] [Google Scholar]
- 56.Aluigi M.G., Coradeghini R., Guida C., Scanarotti C., Bassi A.M., Falugi C., Santi P., Raposio E. Pre-adipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biol. Int. 2009;33:594–601. doi: 10.1016/j.cellbi.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Scanarotti C., Bassi A.M., Catalano M., Guida C., Coradeghini R., Falugi C., Aluigi M., Santi P., Raposio E. Neurogenic-committed human pre-adipocytes express CYP1A isoforms. Chem. Biol. Interact. 2010;184:474–483. doi: 10.1016/j.cbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Tang W., Zeve D., Suh J.M., Bosnakovski D., Kyba M., Hammer R.E., Tallquist M.D., Graff J.M. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapur S.K., Katz A.J. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222–2228. doi: 10.1016/j.biochi.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Salgado A.J., Reis R.L., Sousa N.J., Gimble J.M. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 61.Charrière G., Cousin B., Arnaud E., André M., Bacou F., Penicaud L., Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J. Biol. Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 62.Cousin B., André M., Casteilla L., Pénicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J. Cell Physiol. 2001;186:380–386. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Cousin B., Munoz O., Andre M., Fontanilles A.M., Dani C., Cousin J.L., Laharrague P., Casteilla L., Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto D., Sato K., Gonda K., Takaki Y., Shigeura T., Sato T., Aiba-Kojima E., Iizuka F., Inoue K., Suga H., Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 65.Hsia H.C., Thomson J.G. Differences in breast shape preferences between plastic surgeons and patients seeking breast augmentation. Plast. Reconstr. Surg. 2003;112:312–320. doi: 10.1097/01.PRS.0000066365.12348.A7. [DOI] [PubMed] [Google Scholar]
- 66.Raposio E., Belgrano V., Santi P., Chiorri C. Which is the ideal breast size?: Some social clues for plastic surgeons. Ann. Plast. Surg. 2016;76:340–345. doi: 10.1097/SAP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 67.Cigna E., Ribuffo D., Sorvillo V., Atzeni M., Piperno A., Calò P.G., Scuderi N. Secondary lipofilling after breast reconstruction with implants. Eur. Rev. Med. Pharmacol. Sci. 2012;16:1729–1734. [PubMed] [Google Scholar]
- 68.Simonacci F., Bertozzi N., Grieco M.P., Grignaffini E., Raposio E. Autologous fat transplantation for breast reconstruction: a literature review. Ann. Med. Surg. (Lond.) 2016;12:94–100. doi: 10.1016/j.amsu.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan J., Raposio E., Wang J., Nordström R.E. Development of the inframammary fold and ptosis in breast reconstruction with textured tissue expanders. Aesthet. Plast. Surg. 2002;26:219–222. doi: 10.1007/s00266-002-1477-0. [DOI] [PubMed] [Google Scholar]
- 70.Raposio E., Cicchetti S., Adami M., Ciliberti R.G., Santi P.L. Computer planning for breast reconstruction by tissue expansion: an update. Plast. Reconstr. Surg. 2004;113:2095–2097. doi: 10.1097/01.prs.0000121189.51406.12. [DOI] [PubMed] [Google Scholar]
- 71.Raposio E., Caregnato P., Barabino P., Gualdi A., Orefice A., Spagnolo A., Capello C., Santi P.L. Computer-based preoperative planning for breast reconstruction in the woman with unilateral breast hypoplasia. Miner. Chir. 2002;57:711–714. [PubMed] [Google Scholar]
- 72.Pinsolle V., Chichery A., Grolleau J.L., Chavoin J.P. Autologous fat injection in Poland's syndrome. J. Plast. Reconstr. Aesthet. Surg. 2008;61:784–791. doi: 10.1016/j.bjps.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 73.Coleman S.R., Saboeiro A.P. Fat grafting to the breast revisited: safety and efficacy. Plast. Reconstr. Surg. 2007;119:775–785. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 74.Gutowski K.A. ASPS fat graft task force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast. Reconstr. Surg. 2009;124:272–280. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 75.Chan C.W., McCulley S.J., Macmillan R.D. Autologous fat transfer–a review of the literature with a focus on breast cancer surgery. J. Plast. Reconstr. Aesthet. Surg. 2008;61:1438–1448. doi: 10.1016/j.bjps.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Peer L.A. Loss of weight and volume in human fat grafts: with postulation of a ‘‘cell survival theory’’. Plast. Reconstr. Surg. 1950;5:217–230. [Google Scholar]
- 77.Chajchir A. Fat injection: long-term follow-up. Aesthet. Plast. Surg. 1996;20:291–296. doi: 10.1007/BF00228458. [DOI] [PubMed] [Google Scholar]
- 78.Mikus J.L., Koufman J.A., Kilpatrick S.E. Fate of liposuctioned and purified autologous fat injections in the canine vocal fold. Laryngoscope. 1995;105:17–22. doi: 10.1288/00005537-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Peer L.A. The neglected free fat graft, its behavior and clinical use. Am. J. Surg. 1956;92:40–47. doi: 10.1016/s0002-9610(56)80009-3. [DOI] [PubMed] [Google Scholar]
- 80.Sattler G., Sommer B. Liporecycling: a technique for facial rejuvenation and body contouring. Dermatol. Surg. 2000;26:1140–1144. [PubMed] [Google Scholar]
- 81.Spear S.L., Wilson H.B., Lockwood M.D. Fat injection to correct contour deformities in the reconstructed breast. Plast. Reconstr. Surg. 2005;116:1300–1305. doi: 10.1097/01.prs.0000181509.67319.cf. [DOI] [PubMed] [Google Scholar]
- 82.Khouri R.K., Smit J.M., Cardoso E., Pallua N., Lantieri L., Mathijssen I.M., Khouri R.K., Jr., Rigotti G. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast. Reconstr. Surg. 2013;132:1280–1290. doi: 10.1097/PRS.0b013e3182a4c3a9. [DOI] [PubMed] [Google Scholar]
- 83.Klinger M., Marazzi M., Vigo D., Torre M. Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthet. Plast. Surg. 2008;32:465–469. doi: 10.1007/s00266-008-9122-1. [DOI] [PubMed] [Google Scholar]
- 84.Caviggioli F., Villani F., Forcellini D., Vinci V., Klinger F. Scar treatment by lipostructure. Update Plast. Surg. 2009;2:51–53. [Google Scholar]
- 85.Klinger M., Caviggioli F., Klinger F.M., Giannasi S., Bandi V., Banzatti B., Forcellini D., Maione L., Catania B., Vinci V. Autologous fat graft in scar treatment. J. Craniofac. Surg. 2013;24:1610–1615. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 86.Mojallal A., Lequeux C., Shipkov C., Breton P., Foyatier J.L., Braye F., Damour O. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast. Reconstr. Surg. 2009;124:765–774. doi: 10.1097/PRS.0b013e3181b17b8f. [DOI] [PubMed] [Google Scholar]
- 87.Atiyeh B.S., Costagliola M. Cultured epithelial autograft (CEA) in burn treatment: three decades later. Burns. 2007;33:405–413. doi: 10.1016/j.burns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Jones I., Currie L., Martin R. A guide to biological skin substitutes. Br. J. Plast. Surg. 2002;55:185–193. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C.P., Fu X.B. Therapeutic potential of stem cells in skin repair and regeneration. Chin. J. Traumatol. 2008;11:209–221. doi: 10.1016/s1008-1275(08)60045-0. [DOI] [PubMed] [Google Scholar]
- 90.Lataillade J.J., Doucet C., Bey E., Carsin H., Huet C., Clairand I., Bottollier-Depois J.F., Chapel A., Ernou I., Gourven M., Boutin L., Hayden A., Carcamo C., Buglova E., Joussemet M., de Revel T., Gourmelon P. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2007;2:785–794. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 91.Arno A., Smith A.H., Blit P.H., Shehab M.A., Gauglitz G.G., Jeschke M.G. Stem cell therapy: a new treatment for burns? Pharmaceuticals. 2011;4:1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Linares H.A., Larson D.L. Early differential diagnosis between hypertrophic and nonhypertrophic healing. J. Invest. Dermatol. 1974;62:514–516. doi: 10.1111/1523-1747.ep12681048. [DOI] [PubMed] [Google Scholar]
- 93.Bruno A., Delli Santi G., Fasciani L., Cempanari M., Palombo M., Palombo P. Burn scar lipofilling: immunohistochemical and clinical outcomes. J. Craniofac. Surg. 2013;24:1806–1814. doi: 10.1097/SCS.0b013e3182a148b9. [DOI] [PubMed] [Google Scholar]
- 94.Brongo S., Nicoletti G.F., La Padula S., Mele C.M., DʼAndrea F. Use of lipofilling for the treatment of severe burn outcomes. Plast. Reconstr. Surg. 2012;130:374e–376e. doi: 10.1097/PRS.0b013e3182590387. [DOI] [PubMed] [Google Scholar]
- 95.Gal S., Ramirez J.I., Maguina P. Autologous fat grafting does not improve burn scar appearance: a prospective, randomized, double-blinded, placebo-controlled, pilot study. Burns. 2016 Dec 29 doi: 10.1016/j.burns.2016.09.019. pii: S0305–4179(16)30393-X. [DOI] [PubMed] [Google Scholar]
- 96.Davis D.R., Stewart D.J. Autologous cell therapy for cardiac repair. Expert Opin. Biol. Ther. 2011;11:489–508. doi: 10.1517/14712598.2011.556615. [DOI] [PubMed] [Google Scholar]
- 97.William J., Berger T., Elston D. Andrews' diseases of the skin. Clin. Dermatol. 2005;10:789–790. [Google Scholar]
- 98.Bernier J., Bonner J., Vermorken J.B., Bensadoun R.J., Dummer R., Giralt J., Kornek G., Hartley A., Mesia R., Robert C., Segaert S., Ang K.K. Consensus guidelines for the management of radiation dermatitis and coexisting acne like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann. Oncol. 2008;19:142–149. doi: 10.1093/annonc/mdm400. [DOI] [PubMed] [Google Scholar]
- 99.Rigotti G., Marchi A., Galiè M., Baroni G., Benati D., Krampera M., Pasini A., Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 100.Mori A., Lo Russo G., Agostini T., Pattarino J., Vichi F., Dini M. Treatment of human immunodeficiency virus-associated facial lipoatrophy with lipofilling and submalar silicone implants. J. Plast. Reconstr. Aesthet. Surg. 2006;59:1209–1216. doi: 10.1016/j.bjps.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 101.Carr A., Samaras K., Chisholm D.J., Cooper D.A. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 102.Brinkman K., Smeitink J.A., Romijn J.A., Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 103.Fliers E., Sauerwein H.P., Romijn J.A., Reiss P., van der Valk M., Kalsbeek A., Kreier F., Buijs R.M. HIV-associated adipose redistribution syndrome as a selective autonomic neuropathy. Lancet. 2003;362:1758–1760. doi: 10.1016/s0140-6736(03)14858-1. [DOI] [PubMed] [Google Scholar]
- 104.Coleman S.R. Facial recontouring with lipostructure. Clin. Plast. Surg. 1997;24:347–367. [PubMed] [Google Scholar]
- 105.Gosain A.K., Klein M.H., Sudhakar P.V., Prost R.W. A volumetric analysis of soft-tissue changes in the aging midface using high-resolution MRI: implications for facial rejuvenation. Plast. Reconstr. Surg. 2005;115:1143–1152. doi: 10.1097/01.prs.0000156333.57852.2f. [DOI] [PubMed] [Google Scholar]
- 106.Le Louarn C. Midface region: functional anatomy, ageing process, indications and concentric malar lift. Ann. Chir. Plast. Esthet. 2009;54:411–420. doi: 10.1016/j.anplas.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Rohrich R.J., Pessa J.E. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast. Reconstr. Surg. 2007;119:2219–2227. doi: 10.1097/01.prs.0000265403.66886.54. [DOI] [PubMed] [Google Scholar]
- 108.Rohrich R.J., Arbique G.M., Wong C., Brown S., Pessa J.E. The anatomy of suborbicularis fat: implications for periorbital rejuvenation. Plast. Reconstr. Surg. 2009;124:946–951. doi: 10.1097/PRS.0b013e3181b17b76. [DOI] [PubMed] [Google Scholar]
- 109.Massry G.G., Azizzadeh B. Periorbital fat grafting. Facial Plast. Surg. 2013;29:46–57. doi: 10.1055/s-0033-1333842. [DOI] [PubMed] [Google Scholar]
- 110.Serra-Renom J.M., Serra-Mestre J.M. Periorbital rejuvenation to improve the negative vector with blepharoplasty and fat grafting in the malar area. Ophthal. Plast. Reconstr. Surg. 2011;27:442–446. doi: 10.1097/IOP.0b013e318224b0d5. [DOI] [PubMed] [Google Scholar]
- 111.Moscatello D.K., Schiavi J., Marquart J.D., Lawrence N. Collagenase-assisted fat dissociation for autologous fat transfer. DermatolSurg. 2008;34:1314–1321. doi: 10.1111/j.1524-4725.2008.34282.x. [DOI] [PubMed] [Google Scholar]
- 112.Piasecki J.H., Gutowski K.A., Lahvis G.P., Moreno K.I. An experimental model for improving fat graft viability and purity. Plast. Reconstr. Surg. 2007;119:1571–1583. doi: 10.1097/01.prs.0000256062.74324.1c. [DOI] [PubMed] [Google Scholar]
- 113.Tonnard P., Verpaele A., Peeters G., Hamdi M., Cornelissen M., Declercq H. Nanofat grafting: basic research and clinical applications. Plast. Reconstr. Surg. J. 2013;132:1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 114.Youn S., Shin J.I., Kim J.D., Kim J.T., Kim Y.H. Correction of infraorbital dark circles using collagenase-digested fat cell grafts. Dermatol. Surg. 2013;39:766–772. doi: 10.1111/dsu.12140. [DOI] [PubMed] [Google Scholar]
- 115.Stuzin J.M. Discussion: nanofat grafting: basic research and clinical applications. Plast. Reconstr. Surg. J. 2013;132:1027–1028. doi: 10.1097/PRS.0b013e31829fe246. [DOI] [PubMed] [Google Scholar]
- 116.Roh M.R., Kim T.K., Chung K.Y. Treatment of infraorbital dark circles by autologous fat transplantation: a pilot study. Br. J. Dermatol. 2009;160:1022–1025. doi: 10.1111/j.1365-2133.2009.09066.x. [DOI] [PubMed] [Google Scholar]
- 117.Boureaux E., Chaput B., Bannani S., Herlin C., De Runz A., Carloni R., Mortemousque B., Mouriaux F., Watier E., Bertheuil N. Eyelid fat grafting: indications, operative technique and complications; a systematic review. J. Craniomaxillofac. Surg. 2016;44:374–380. doi: 10.1016/j.jcms.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 118.Lin T.M., Lin T.Y., Chou C.K., Lai C.S., Lin S.D. Application of microautologous fat transplantation in the correction of sunken upper eyelid. Plast. Reconstr. Surg. Glob. Open. 2014;2:e259. doi: 10.1097/GOX.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le T.P., Peckinpaugh J., Naficy S., Amadi A.J. Effect of autologous fat injection on lower eyelid position. Ophthal. Plast. Reconstr. Surg. 2014;30:504–507. doi: 10.1097/IOP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 120.Hoang D., Orgel M.I., Kulber D.A. Hand rejuvenation: a comprehensive review of fat grafting. J. Hand Surg. Am. 2016;41:639–644. doi: 10.1016/j.jhsa.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 121.Teimourian B., Adham M. Rejuvenation of the hand: fat injection combined with TCA peel. Aesthet. Surg. J. 2000;20:70–71. [Google Scholar]
- 122.Bains R.D., Thorpe H., Southern S. Hand aging: patients' opinions. Plast. Reconstr. Surg. 2005;117:2212–2218. doi: 10.1097/01.prs.0000218712.66333.97. [DOI] [PubMed] [Google Scholar]
- 123.Jakubietz R., Grünert J.G., Kloss D.F., Meffert R., Schmidt K., Jakubietz M.G. Aging and aesthetic ideal of the hand. Hautarzt. 2009;60:220–225. doi: 10.1007/s00105-008-1696-7. [DOI] [PubMed] [Google Scholar]
- 124.Fabi S.G., Goldman M.P. Hand rejuvenation: our experience. Dermatol. Surg. 2012;38:1112–1127. doi: 10.1111/j.1524-4725.2011.02291.x. [DOI] [PubMed] [Google Scholar]
- 125.Streker M., Reuther T., Krueger N., Kerscher M. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face, hand, and décolletage. J. Drugs Dermatol. 2013;12:990–994. [PubMed] [Google Scholar]
- 126.Sadick N.A. 52-week study of safety and efficacy of calcium hydroxylapatite for rejuvenation of the aging hand. J. Drugs Dermatol. 2011;10:47–51. [PubMed] [Google Scholar]
- 127.Brandt F.S., Cazzaniga A., Strangman N., Coleman J., Axford-Gatley R. Long-term effectiveness and safety of small gel particle hyaluronic acid for hand rejuvenation. Dermatol. Surg. 2012;38:1128–1135. doi: 10.1111/j.1524-4725.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 128.Coleman S.R. Hand rejuvenation with structural fat grafting. Plast. Reconstr. Surg. 2002;110:1731–1744. doi: 10.1097/01.PRS.0000033936.43357.08. [DOI] [PubMed] [Google Scholar]
- 129.Redaelli A. Cosmetic use of polylactic acid for hand rejuvenation: report on 27 patients. J. Cosmet. Dermatol. 2006;5:233–238. doi: 10.1111/j.1473-2165.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 130.Kao W.P., Lin Y.N., Lin T.Y., Huang Y.H., Chou C.K., Takahashi H., Shieh T.Y., Chang K.P., Lee S.S., Lai C.S., Lin S.D., Lin T.M. Microautologous fat transplantation for primary augmentation rhinoplasty: long-term monitoring of 198 Asian patients. Aesthet. Surg. J. 2016;36:648–656. doi: 10.1093/asj/sjv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bircoll M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast. Reconstr. Surg. 1987;79:267–271. doi: 10.1097/00006534-198702000-00022. [DOI] [PubMed] [Google Scholar]
- 132.Billings E., May J.W. Historical review and present status of free fat graft auto transplantation in plastic and reconstructive surgery. Plast. Reconstr. Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 133.Pu L.L., Yoshimura K., Coleman S.R. Future perspectives of fat grafting. Clin. Plast. Surg. 2015;42:389–394. doi: 10.1016/j.cps.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Kling R.E., Mehrara B.J., Pusic A.L., Young V.L., Hume K.M., Crotty C.A., Rubin J.P. Trends in autologous fat grafting to the breast: a national survey of the American Society of Plastic Surgeons. Plast. Reconstr. Surg. 2013;132:35–46. doi: 10.1097/PRS.0b013e318290fad1. [DOI] [PubMed] [Google Scholar]
- 135.Veber M., Tourasse C., Toussoun G., Moutran M., Mojallal A., Delay E. Radiographic findings after breast augmentation by autologous fat transfer. Plast. Reconstr. Surg. 2011;127:1289–1299. doi: 10.1097/PRS.0b013e318205f38f. [DOI] [PubMed] [Google Scholar]
- 136.Delay E., Sinna R., Ho Quoc C. Tuberous breast correction by fat grafting. Aesthet. Surg. J. 2012;33:522–528. doi: 10.1177/1090820X13480641. [DOI] [PubMed] [Google Scholar]
- 137.Murillo W.L. Buttock augmentation: case studies of fat injection monitored by magnetic resonance imaging. Plast. Reconstr. Surg. 2004;114:1606–1614. doi: 10.1097/01.prs.0000138760.29273.5d. [DOI] [PubMed] [Google Scholar]
- 138.Cardenas-Camarena L., Arenas-Quintana R., RoblesCervantes J.A. Buttocks fat grafting: 14 years of evolution and experience. Plast. Reconstr. Surg. 2011;128:545–555. doi: 10.1097/PRS.0b013e31821b640b. [DOI] [PubMed] [Google Scholar]
- 139.Peltoniemi H.H., Salmi A., Miettinen S., Mannerström B., Saariniemi K., Mikkonen R. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J. Plast. Reconstr. Aesthet. Surg. 2013;66:1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 140.Smith P., Adams W.P., Jr., Lipschitz A.H., Chau B., Sorokin E., Rohrich R.J., Brown S.A. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast. Reconstr. Surg. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 141.Delay E., Garson S., Tousson G., Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet. Surg. J. 2009;29:360–376. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 142.Illouz Y.G., Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthet. Plast. Surg. 2009;33:706–715. doi: 10.1007/s00266-009-9377-1. [DOI] [PubMed] [Google Scholar]
- 143.Chan C.W., McCulley S.J., Macmillan R.D. Autologous fat transfer: a review of the literature with a focus on breast cancer surgery. J. Plast. Reconstr. Aesthet. Surg. 2008;61:1438–1448. doi: 10.1016/j.bjps.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 144.Agha R.A., Fowler A.J., Herlin C., Goodacre T.E., Orgill D.P. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J. Plast. Reconstr. Aesthet. Surg. 2015;68:143–161. doi: 10.1016/j.bjps.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 145.Bank J., Fuller S.M., Henry G.I., Zachary L.S. Fat grafting to the hand in patients with Raynaud phenomenon. Plast. Reconstr. Surg. 2014;133:1109–1118. doi: 10.1097/PRS.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 146.Butterwick K. Rejuvenation of the aging hand. Dermatol. Clin. 2005;23:515–527. doi: 10.1016/j.det.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 147.Fournier P.F. Fat transfer to the hand for rejuvenation. In: Shiffman M.A., editor. Autologous Fat Transfer: Art, Science, and Clinical Practice. Springer-Verlag Berlin Heidelberg; Berlin, Germany: 2010. pp. 273–280. [Google Scholar]
- 148.Vara A.D., Miki R.A., Alfonso D.T., Cardoso R. Hand fat grafting complicated by abscess. Hand (N Y) 2013;8:348–351. doi: 10.1007/s11552-013-9508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park S.H., Sun H.J., Choi K.S. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin. Ophthalmol. 2008;2:679–683. [PMC free article] [PubMed] [Google Scholar]
- 150.Chou C.K., Lin T.M., Chiu C.H. Influential factors in autologous fat transplantation - focusing on the lumen size of injection needle and the injecting volume. J. IPRAS. 2013;9:25–27. [Google Scholar]
- 151.Wu W.T. The oriental nose: an anatomical basis for surgery. Ann. Acad. Med. Singap. 1992;21:176–189. [PubMed] [Google Scholar]
- 152.Ersek R.A. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast. Reconstr. Surg. 1991;87:219–227. [PubMed] [Google Scholar]
- 153.Choi M., Small K., Levovitz C. The volumetric analysis of fat graft survival in breast reconstruction. Plast. Reconstr. Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 154.El-Sabbagh A.H. Modern trends in lipomodeling. GMS Interdiscip. Plast. Reconstr. Surg. DGPW. 2017;6 doi: 10.3205/iprs000108. Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cui X., Pu L.L. The search for a useful method for the optimal cryopreservation of adipose aspirates: part II. In vivo study. Aesthet. Surg. J. 2010;30:451–456. doi: 10.1177/1090820X10374100. [DOI] [PubMed] [Google Scholar]
- 156.Pu L.L., Coleman S.R., Cui X., Ferguson R.E., Jr., Vasconez H.C. Cryopreservation of autologous fat grafts harvested with the Coleman technique. Ann. Plast. Surg. 2010;64:333–337. doi: 10.1097/SAP.0b013e3181b022cb. [DOI] [PubMed] [Google Scholar]
- 157.Son D., Oh J., Choi T. Viability of fat cells over time after syringe suction lipectomy: the effects of cryopreservation. Ann. Plast. Surg. 2010;65:354–360. doi: 10.1097/SAP.0b013e3181bb49b8. [DOI] [PubMed] [Google Scholar]
- 158.Thirumala S., Gimble J.M., Devireddy R.V. Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose derived adult stem cells. Stem Cells Dev. 2010;19:513–522. doi: 10.1089/scd.2009.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thirumala S., Wu X., Gimble J.M., Devireddy R.V. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng. Part C Methods. 2010;16:783–792. doi: 10.1089/ten.TEC.2009.0552. [DOI] [PubMed] [Google Scholar]
- 160.Miyamoto Y., Oishi K., Yukawa H., Noguchi H., Sasaki M., Iwata H., Hayashi S. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transpl. 2012;21:617–622. doi: 10.3727/096368911X605556. [DOI] [PubMed] [Google Scholar]
- 161.Rohrich R.J., Rosen J., Longaker M.T. So you want to be an innovator? Plast. Reconstr. Surg. 2010;126:1107–1109. doi: 10.1097/PRS.0b013e3181e3b854. [DOI] [PubMed] [Google Scholar]
- 162.Agha R.A., Fowler A.J., Pidgeon T.E., Wellstead G., Orgill D.P. VOGUE steering group. Protocol for the development of a core outcome set for autologous fat grafting to the breast. Int. J. Surg. 2016;31:104–106. doi: 10.1016/j.ijsu.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 163.Agha R.A., Fowler A.J., Herlin C., Goodacre T.E., Orgill D.P. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J. Plast. Reconstr. Aesthet. Surg. 2015;68:143–161. doi: 10.1016/j.bjps.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 164.Agha R.A., Fowler A.J., Pidgeon T.E., Wellstead G., Orgill D.P. The need for core outcome reporting in autologous fat grafting for breast reconstruction. Ann. Plast. Surg. 2016;77:506–512. doi: 10.1097/SAP.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 165.Sarfati I., Ihrai T., Kaufman G., Nos C., Clough K.B. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2011;64:1161–1166. doi: 10.1016/j.bjps.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 166.Gerald O'Daniel T. Multimodal management of atrophic acne scarring in the aging face. Aesthet. Plast. Surg. 2011;35:1143–1150. doi: 10.1007/s00266-011-9715-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Derby B.M., Dai H., Reichensperger J., Cox L., Harrison C., Cosenza N., Yang M., Bueno R.A., Neumeister M.W. Adipose-derived stem cell to epithelial stem cell transdifferentiation: a mechanism to potentially improve understanding of fat grafting's impact on skin rejuvenation. Aesthet. Surg. J. 2014;34:142–153. doi: 10.1177/1090820X13515700. [DOI] [PubMed] [Google Scholar]