Abstract
Brain is a rich environment where neurons and glia interact with neighboring cells as well as extracellular matrix in three-dimensional (3D) space. Astrocytes, which are the most abundant cells in the mammalian brain, reside in 3D space and extend highly branched processes that form microdomains and contact synapses. It has been suggested that astrocytes cultured in 3D might be maintained in a less reactive state as compared to those growing in a traditional, two-dimensional (2D) monolayer culture. However, the functional characterization of the astrocytes in 3D culture has been lacking. Here we cocultured neurons and astrocytes in 3D and examined the morphological, molecular biological, and electrophysiological properties of the 3D-cultured hippocampal astrocytes. In our 3D neuron-astrocyte coculture, astrocytes showed a typical morphology of a small soma with many branches and exhibited a unique membrane property of passive conductance, more closely resembling their native in vivo counterparts. Moreover, we also induced reactive astrocytosis in culture by infecting with high-titer adenovirus to mimic pathophysiological conditions in vivo. Adenoviral infection induced morphological changes in astrocytes, increased passive conductance, and increased GABA content as well as tonic GABA release, which are characteristics of reactive gliosis. Together, our study presents a powerful in vitro model resembling both physiological and pathophysiological conditions in vivo, and thereby provides a versatile experimental tool for studying various neurological diseases that accompany reactive astrocytes.
Keywords: 3D culture, reactive astrocyte, adenovirus, functional characterization, passive conductance, tonic GABA current
INTRODUCTION
In vitro cell culture systems has been widely utilized as a simple, fast, and cost-effective experimental tool in basic and applied sciences. In most cases, cells are cultured on flat, two-dimensional (2D) plastic or glass surfaces in monolayers. Although the conventional 2D culture system has several advantages and indeed has revealed key mechanistic insights, it is obvious that 2D cell culture does not adequately mimic the natural 3D environment in which cells reside, and the unnatural environment may alter cellular morphology, gene expression, and function [1]. In an effort to preserve the structural and functional complexity of cells, a number of 3D culture systems have been developed with the aid of various scaffold materials, such as porous gelatin sponge [2], agarose [3], collagen [4,5,6], etc. To date, several cell types have been cultured in 3D, including hepatocytes [2], fibroblasts [7], stem cells [8,9], neurons [5,10], and astrocytes [6,11].
Astrocytes are the most abundant cells in the mamamlian central nervous system and are recognized as important regulators of brain function [12,13]. Astrocytes become reactive in a number of pathological conditions, following injury and in many neurological disorders, such as Alzheimer's and Parkinson's diseases [14,15,16,17], to name a few. Although the concept of reactive astrogliosis and its molecular definition is still incomplete, astrocytes are considered reactive when they become hypertrophic and increase the expression of molecular markers for reactive astrocytes, such as GFAP (glial fibrillary acidic protein), nestin, and vimentin [14,15,17,18], Recently, increases in GABA content and tonic GABA release in astrocytes have also been suggested to indicate astrogliosis, especially in neurodegenerative diseases [16,19].
Developing a physiologically relevant 3D culture system is of particular importance for astrocyte studies, because it is well known that in a conventional 2D culture system, astrocytes do not preserve many of the structural and functional features observed in vivo, but rather exhibit properties of reactive astrocytes even without any stimuli. Although 2D cell culture system might help minimize large-scale and cost-intensive animal testing, high baseline activation raises a serious concern when investigating the changes of astrocytes in pathological conditions or in response to various stimuli [11,18,20]. Thus, several attempts have been made to develop suitable 3D culture systems and previous studies have shown that 3D culture allows astrocytes to maintain some important features that they exhibit in vivo, such as morphological complexity and low baseline activation [6,11]. However, the functional or physiological relevance has not been fully elucidated.
The aim of this study was to develop a physiologically relevant culture system that preserves important features of both resting and reactive astrocytes. By coculturing neurons and astrocytes in 3D and analyzing the morphological, molecular biological, and electrophysiological properties of the 3D-cultured astrocytes, here we show that astrocytes grown in 3D collagen matrix more closely resemble their in vivo counterparts. Moreover, we show that high-titer adenoviral infection induces morphological changes in astrocytes and increases GABA content as well as tonic GABA release, which are characteristics of reactive astrogliosis. Our culture presents a powerful in vitro model resembling both physiological and pathophysiological conditions in vivo, which can provide a versatile experimental tool for studying various neurological diseases and conditions that accompany reactive astrocytes.
MATERIALS AND METHODS
Co-culture of hippocampal neurons and astrocytes
Pregnant ICR mice (E18.5) were purchased from DBL (Eumseong, Korea) and sacrificed for primary culture of neurons and glia, following a previously described protocol with minor modifications [5]. Briefly, embryos were decapitated and the entire hippocampi were dissected out and were treated with papain (Worthington) and serially triturated. Dissociated cells were counted and seeded at a density of 1×105 cells ml-1 for 2D and 4×106 cells ml-1 in a collagen mixture for 3D. Cells were cultured in plating medium consisting of neurobasal media supplemented with 5% fetal bovine serum (GIBCO), 2% B27-supplement (Invitrogen), 2 mM Glutamax-I (GIBCO) and 1% penicillin-streptomycin (GIBCO). After 1 day, plating medium was replaced by serum-free medium and maintained at 37℃ in a 5% CO2 humidified incubator. One-half of the medium was replaced with fresh culture medium every 2~4 days. Cultured cells growing in 500 µl media were treated with 1 µl adeno-CMV-mCherry virus (viral stock titer: 4.5×1012 genome copies (GC) ml-1) for 4 days.
Preparation of collagen-based 3D culture mixture
Commercial type I collagen (8~11 mg ml-1 in acetic acid (0.1% [w/v] for custom-extracted collagen and 0.02 N for commercial collagen), rat tail; Corning or custom-extracted) was used and neutralized (pH 7.5) collagen solutions were prepared as reported previously [5]. Total of 500 µl of a 2.5 mg ml-1 collagen solution was prepared by adding 110~160 µl collagen stock (depending on the concentration of collagen stock solution), 50 µl of 10x Dulbecco Modified Eagle Medium (DMEM; Sigma-Aldrich). 10~20 µl of 0.5 N NaOH for neutralization, and 50 µl of cell-suspension in DMEM. Final volume of 500 µl was matched by adding 1x DMEM (Lonza). Cells were seeded in collagen at a density of 4×106 cell ml-1. All solutions were added on ice to minimize undesired gelation. Collagen seeded with cells was then immediately processed for gelation (37℃, 30 min), and media was added.
Immunofluorescence staining
To stain neurons and astrocytes, 3D constructs were fixed in 4% [w/v] paraformaldehyde and blocking was performed with 2% [w/v] BSA in PBS containing 0.1% [w/v] Triton X-100 for 2 hr. 3D constructs were then incubated sequentially with primary and secondary antibodies diluted in the blocking solution at 4℃ overnight. Following primary antibodies were used: mouse anti-βIII-tubulin (TuJ1) (1:1,000; Abcam), chicken anti-GFAP (1:500; Millipore). Alexa Fluor conjugates (Alexa Fluor 488 and 594) (1:1,000; Molecular Probes) were used for secondary antibodies. Nuclei were stained with Hoechst 33342 (1: 5,000; Molecular Probes). All samples were rinsed with PBS between the incubation steps.
Confocal laser scanning microscopy
Fluorescence images were acquired using an inverted confocal laser scanning microscope (LSM 700; Carl Zeiss) equipped with solid-state lasers (405, 488 and 555 nm). Post-image processing such as maximum intensity projection and 3D reconstruction was performed using ZEN 2012 software (Carl Zeiss). Z-stacked images (stack size, 17~176 µm; step size, 0.94~3.4 µm) were acquired with 10x and 20x objectives.
Electrophysiology
Electrophysiological recordings were performed with co-culture of hippocampal neurons and astrocytes at DIV (days in vitro) 7 and 14. Whole-cell patch-clamp recordings were achieved by applying collagenase as previously described [5]. For tonic GABA measurement, whole-cell recordings were made from neurons. The holding potential was -60 mV. Pipette resistance was typically 5~8 MΩ and the pipette was filled with an internal solution (in mM): 135 CsCl, 4 NaCl, 0.5 CaCl2, 10 HEPES, 5 EGTA, 2 Mg-ATP, 0.5 Na2-GTP, 10 QX-314, pH adjusted to 7.2 with CsOH. For passive conductance measurement, whole-cell recordings were made from astrocytes. The holding potential was -70 mV. Pipette resistance was typically 5~8 MΩ for granule cells and the pipette was filled with an internal solution (in mM): 140 K-gluconate, 10 HEPES, 7 NaCl, and 2 MgATP adjusted to pH 7.4 with CsOH. Electrical signals were digitized and sampled at 50 µS intervals with Digidata 1440A and Multiclamp 700B amplifier (Molecular Devices) using pCLAMP 10.2 software. Data were filtered at 2 kHz.
Virus injection
Mice (8~10 weeks old, C57BL/6 background) were anesthetized by intraperitoneal injection of 2% avertin (20 µl g-1) and placed into stereotaxic frames. Adenovirus containing mCherry was loaded into a micro dispenser (VWR, USA) and injected bilaterally into the hippocampal dentate gyrus (DG) region at a rate of 0.3 µl min-1 (total 2 µl) with a 25 µl syringe using a syringe pump KD Scientific, USA). The stereotaxic coordinates of the injection site were 1.7 mm away from the bregma and the depth was 2 mm beneath the skull. All experimental procedures described below were conducted according to the animal welfare guidelines approved by Institutional Animal Care and Use Committee of the Korea Institute of Science and Technology.
Statistical analysis
The significance of data for comparison was assessed by Student's two-tailed unpaired t-test between two groups and one-way ANOVA test between three groups. Analyses were performed with Prism (GraphPad Software, Inc.) and Clampfit software. The data distribution was assumed to be normal. Data are presented as mean±SEM (standard error of the mean). Levels of statistical significance are indicated as follows: *(p<0.05), **(p<0.01), ***(p<0.001).
RESULTS
3D-cultured astrocytes exhibit in vivo like morphologies
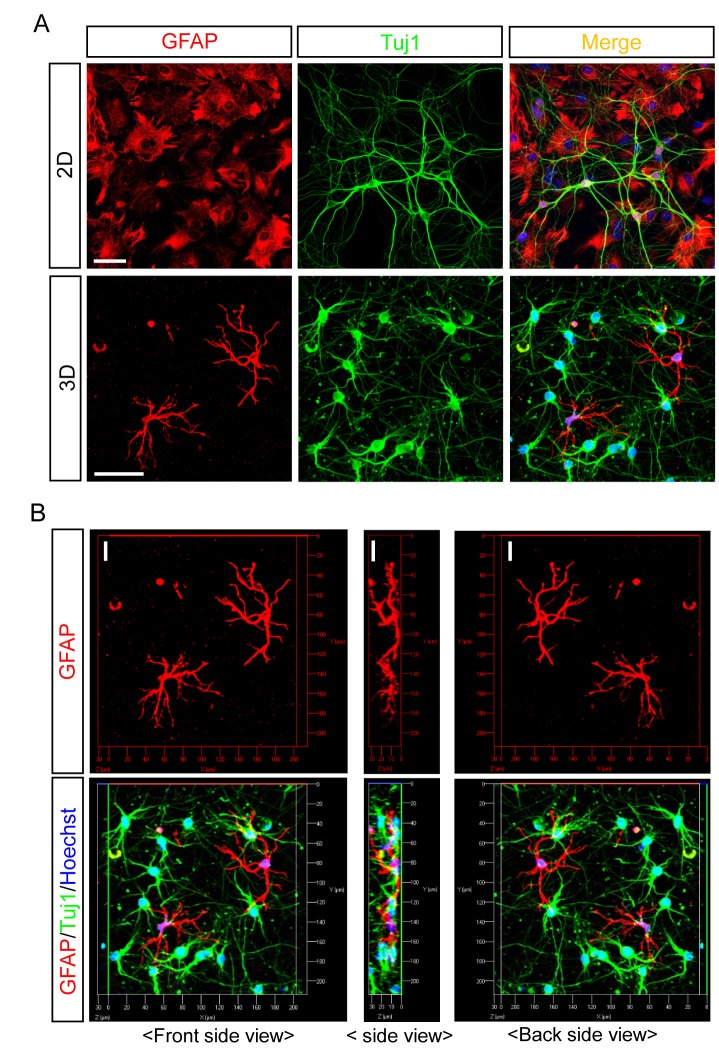
To assess the morphology of cells in the 3D neuron-astrocyte co-culture, we performed immunocytochemistry by using antibodies against Tuj1, a neuron-specific class III β tubulin, and GFAP, an astrocytic filament protein. In striking contrast to neurons that show similar morphology in 2D and 3D cultures, astrocytes in the two cultures exhibited a remarkable difference. In the standard 2D culture, astrocytes had a flat, polygonal morphology, which is very different from their in vivo counterparts. Collagen-based 3D culture supported more complex cellular morphology (Fig. 1A). Astrocytes possessed a small soma and several, clearly distinct processes, resembling a stellate morphology of resting-state astrocytes observed in vivo. With a side view of 3D-cultured astrocytes, we could readily identify processes branching out in several directions from soma (Fig. 1B). These morphological properties of 2D- and 3D-cultured astrocytes are similar with previous findings [11,21] and show that astrocytes can maintain key morphological features of the in vivo or ex vivo astrocytes when cultured in 3D.
Fig. 1. Two- and three-dimensional co-culture of neurons and astrocytes. (A) Representative images of primary mouse neuron-astrocyte co-culture immunostained with anti-GFAP and anti-TuJ1 antibodies at DIV 10 for 2D and at DIV 5 for 3D. Nuclei are stained with Hoechst. Scale bars: 50 µm. (B) Three dimensional (3D) reconstruction of astrocyte images of (A). Scale bars: 20 µm.
Adenovirus induces reactive astrocyte with an increase of GABA content in 3D culture
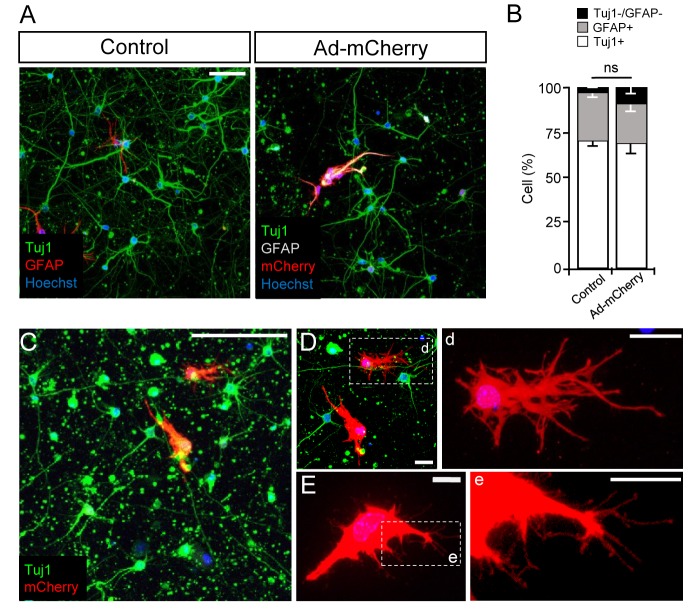
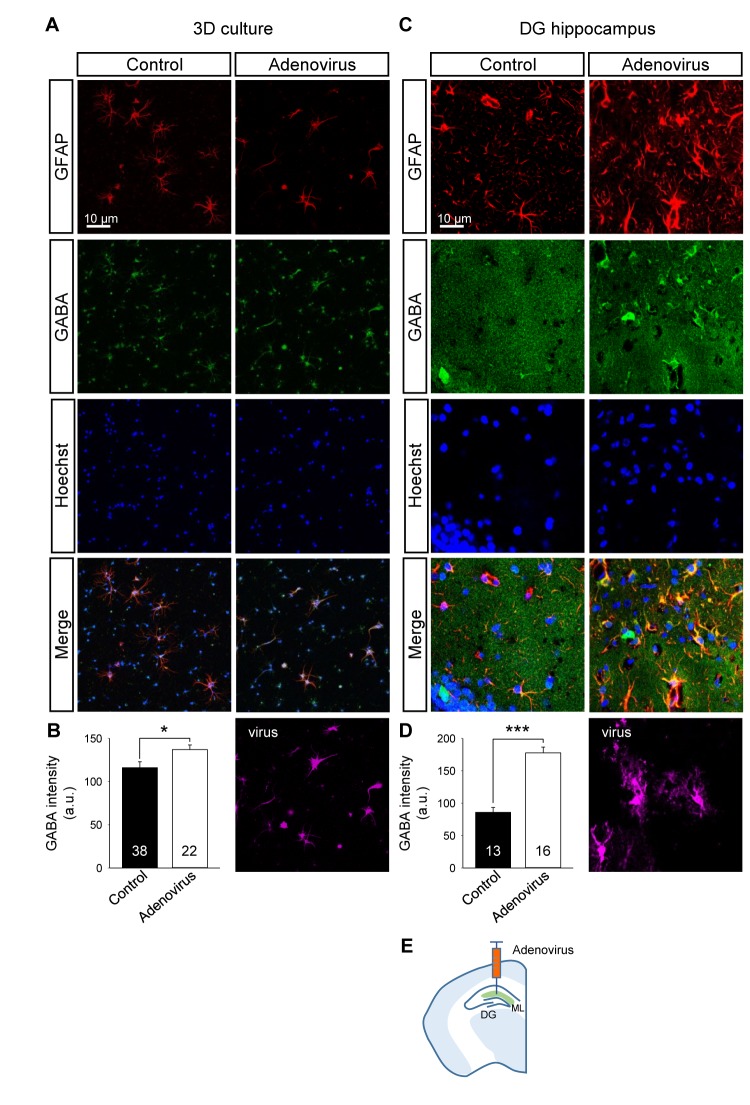
High-titer adenoviral transduction of astrocytes has been shown to cause local and selective virus-induced reactive astrocytosis in vivo [22]. Although it has been speculated that interactions between viral DNA or capsid with receptors expressed in astrocytes might lead to preferential astrogliosis, the mechanism underlying the induction of astrogliosis is not understood. To examine if we could mimic the induction of reactive astrocytes in our 3D culture system, we infected the culture with adenovirus expressing mCherry as a reporter gene under the control of a CMV promoter. Upon treatment of cells with high-titer virus (1 µl of 4.6×1012 GC ml-1 viral stock solution into a total of 500 µl of culture media), astrocytes displayed a hypertrophic morphology typical of reactive astrocytes (Fig. 2A, C, D and E). The cell population in neurons and astrocytes was not affected by adenoviral infection as compared to control condition (Fig. 2B). Increase in GABA contents has also been reported in hippocampal reactive astrocytes from Alzheimer's disease model mice [16,19]. In our 3D culture, we observed a significant increase of GABA signal in adenovirus-infected reactive astrocytes as compared to control astrocytes (Fig. 3A and B).
Fig. 2. Ad-CMV-mCherry infection induces cellular hypertrophy of astrocytes in 3D culture system. (A, B) Neuron-astrocyte 3D co-cultures were infected with Ad-CMV-mCherry at DIV 3 and fixed at DIV 7, followed by immunostaining with anti-TuJ1 and anti-GFAP antibodies. Nuclei are stained with Hoechst. Control indicates intact cultures that were not infected with any virus. Representative images (A) and quantification (B) of neurons and astrocytes from six independent experiments are shown. (C~E) Representative images of a neuron-astrocyte 3D co-culture infected with Ad-CMV-mCherry at DIV 3 and fixed at DIV 7. Note the cellular hypertrophy of astrocytes induced by viral infection. D and E, enlarged images of astrocytes expressing mCherry in C. Astrocyte image is rotated in E. Boxed regions in D and E are further enlarged in right (d and e) to clearly show the processes from reactive astrocytes. Scale bars: 50 µm in A, 100 µm in C, 20 µm in D, and 10 µm in E.
Fig. 3. GABA content was increased in reactive astrocytes from 3D culture and hippocampal brain slice. (A) Immunostaining of neuron-astrocyte 3D co-culture with anti-GFAP, anti-GABA antibodies and Hoechst. (B) Quantification of GABA immunoreactivity in control and adenovirus-infected astrocytes. *p<0.05, unpaired student's t-test. (C) Immunostaining of adenovirus-injected hippocampal brain slice with anti-GFAP, anti-GABA antibodies and Hoechst. (D) Quantification of GABA immunoreactivity in control and adenovirus-infected conditions. ***p<0.001, unpaired student's t-test. (E) Schematic for virus injection into DG hippocampus. DG, dentate gyrus; ML, molecular layer.
We next compared morphological and biochemical characteristics of reactive astrocytes in adenovirus-infected hippocampal brain slice with those in our 3D cell culture system. Similar to our 3D culture (see Fig. 1, 2, 3A and 3B), we found cellular hypertrophy and significant increase of GABA signal in reactive astrocytes from the DG region of hippocampal brain slice infected with adenovirus (Fig. 3C and 3D). These ex vivo results were reminiscent of a previous report from in vivo studies [22]. However, adenoviral induction of astrogliosis was rather less selective in hippocampal slice. In addition to astrocytes that were directly infected with adenovirus, as evidenced by the expression of mCherry, astrocytes in the vicinity of the infected cells showed hypertrophy and up-regulation of GFAP, which might be due to inflammatory responses resulting from physical injury caused by injection of adenovirus into the hippocampus.
Tonic GABA release is increased in adenovirus-induced reactive astrocytes
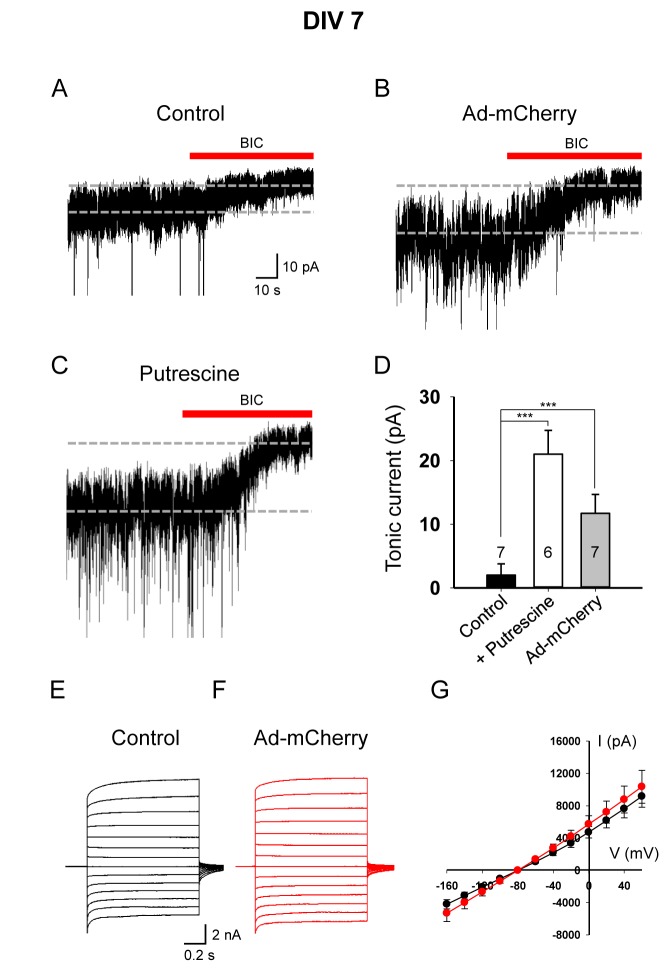
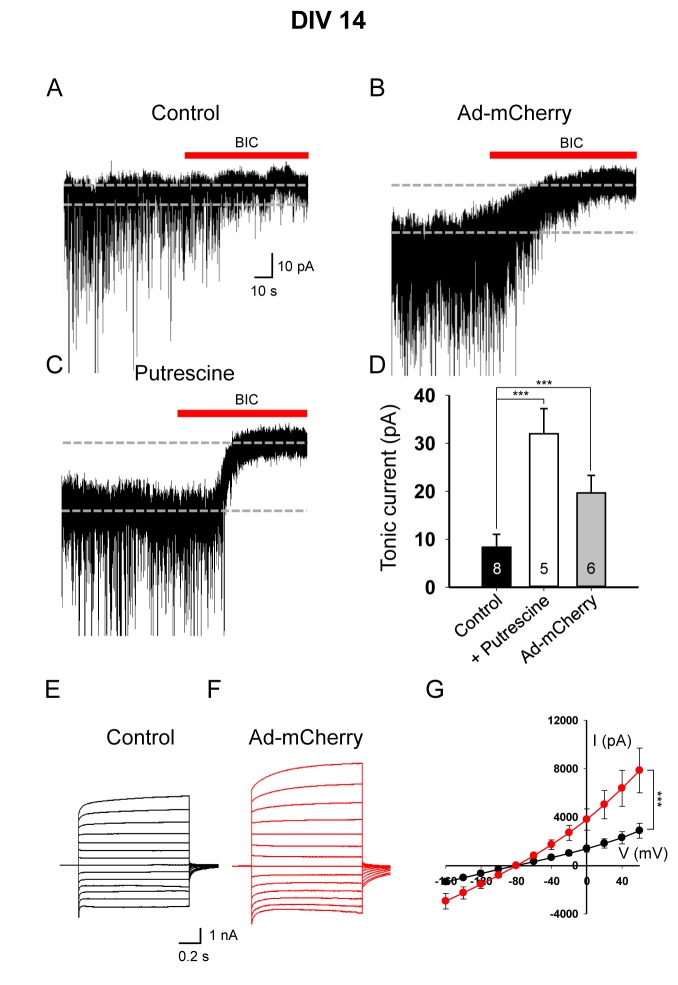
The increased intracellular GABA content in reactive astrocytes can be released tonically from astrocytes and detected in neighboring neurons in the form of tonic inhibition current [16,19]. To test this in 3D culture at DIV 7, we measured the tonic GABA current using the GABAA antagonist bicuculline from a neuron in the vicinity of viral infection (Fig. 4A). We found a significant increase in tonic GABA current in adenovirus treated condition compared to control condition (Fig. 4A, B), indicating an increase in tonic GABA release from reactive astrocytes. It has been suggested that astrocytes synthesize GABA through monoamine oxidase B (MAO-B) pathway with putrescine as an initial substrate [16,23,24]. To test if putrescine pathway is intact in our 3D culture system, we measured the tonic GABA current in normal 3D-cultured astrocytes treated with putrescine. We found a significant enhancement of tonic GABA current after putrescine treatment (Fig. 4C). We found a very similar trend in the DIV 14 culture, compared to DIV 7 condition, except that the magnitude of putrescine- and adenovirus-induced tonic GABA current was much higher at DIV 14 (Fig. 5A~D). These results suggest that tonic GABA release from reactive astrocytes is detected in adenovirus-infected reactive astrocytes.
Fig. 4. Tonic GABA current was increased by reactive astrocytes at DIV 7. (A~C) Representative traces of tonic currents from control (A), Ad-CMV-mCherry-infected (B), and putrescine-treated (C) conditions. (D) Magnitude of bicuculline (BIC, 50 µM)-sensitive tonic currents in control, putrescine-treated and viral infected conditions, as indicated. ***p<0.001, one-way ANOVA, Dunnetts's test. (E, F) Representative traces of passive conductance from control (E) and Ad-CMV-mCherry-infected (F) conditions. (G) Current-voltage relationship (I~V) from -160 mV to +60 mV.
Fig. 5. Tonic GABA current was increased by reactive astrocytes at DIV 14. (A~C) Representative traces of tonic currents from control (A), Ad-CMV-mCherry-infected (B), and putrescine-treated (C) and conditions. (D) Magnitude of bicuculline (BIC, 50 µM)-sensitive tonic current in control, putrescine-treated and viral infected conditions, as indicated. ***p<0.001, one-way ANOVA, Dunnetts's test. (E, F) Representative traces of passive conductance from control (E) and Ad-CMV-mCherry-infected (F) conditions. (G) Current-voltage relationship (I~V) from -160 mV to +60 mV. ***p<0.001, two-way ANOVA, Bonferroni's test.
Fully differentiated, mature astrocytes have been shown to display a leaky membrane property with extremely low membrane resistance (ranging from 1~10 MΩ), namely a passive conductance with a linear current-voltage relationship [25]. It has been recently demonstrated that the most of passive conductance is mediated by the heterodimer of two-pore potassium (K2P) channel subunits, TREK-1 and TWIK-1 [26]. Therefore, the presence of passive conductance can serve as a useful electrophysiological marker of astrocytes. To test whether our 3D-cultured astrocytes display this electrophysiological property, we measured the passive conductance from individual astrocytes by whole-cell patch-clamping. We found that the passive conductance was readily observed in 3D-cultured normal and reactive astrocytes (Fig. 4E-G, 5E-G). Interestingly, passive conductance was significantly increased in reactive astrocytes at DIV 14 (Fig. 5G), but not at DIV 7 (Fig. 4G). This result is consistent with previous reports that showed an enhancement of passive conductance in ischemic condition accompanying reactive astrocytes due to an increase in K2P channel expression [27,28]. Taken together, these results suggest that 3D-cultured reactive astrocytes display the electrophysiological features as evidenced by the increased tonic GABA release and passive conductance, resembling pathophysiological conditions in vivo.
DISCUSSION
Here we developed a collagen-based 3D coculture system composed of neurons and astrocytes and examined the morphological, molecular biological and electrophysiological properties. In basal condition, 3D-cultured astrocytes exhibited a distinct stellate morphology with a small soma and several branches and showed passive conductance, resembling resting astrocytes observed in vivo and ex vivo. In the adenovirus-treated condition, astrocytes became reactive, as evidenced by morphological changes as well as increases in GABA content, tonic GABA release, and passive conductance. Such morphological, molecular biological and electrophysiological features mimic those of reactive astrocytes in pathological conditions in vivo [16,19,27,28].
Biological functions of some types of cells are particularly difficult to study in vitro if they alter gene expression, cell morphology or function in culture. In vitro systems that allow experimentation on primary astrocytes, alone or with neurons or other cells, have been used for decades [21,29,30], and have provided insights in understanding the function and dysfunction of astrocytes. However, it is well documented that astrocytes lose morphological complexity as well as important biochemical features upon transfer from the in vivo to the traditional 2D culture environment. In particular, astrocytes cultured on flat surfaces exhibit highly reduced morphological complexity and show biochemical signs of undesired reactiveness, such as high levels of GFAP, vimentin, and nestin [6,11,18]. In the present study, we show that (i) astrocytes are not reactive in basal culture conditions, (ii) astrogliosis can be induced by viral infection in culture and (iii) reactive astrocytes display passive conductance and tonic current (Fig. 4, 5). On the basis of previous studies including ours and other groups reporting electrophysiological properties of reactive astrocytes in vivo, such as tonic GABA release and passive conductance [16,23,26], we conclude that the reactive astrocytes induced in our 3D culture system resemble their native counterparts not only morphologically and biochemically but also electrophysiologically and functionally.
Together with previous findings [6,11], this study further confirms the advantages and appropriateness of the 3D culture system for studying astrocytes. By minimizing baseline reactivity, it is possible to induce astrogliosis applying a whole range of experimental approaches. As an example, here we infected the culture with high-titer adenovirus and observed morphological and functional changes in astrocytes, in a way that mimics astrogliosis observed in in vivo and ex vivo conditions [16,19,27,28]. In contrast to intracranial injection of adenoviral vectors, which inevitably causes local physical injury and is usually accompanied by inflammation that can confound the development of astrocytosis [14,17,18], our 3D culture in the current form did not include microglia, and thereby provides a more direct and simplified system to study astrogliosis. With such properties, our 3D neuron-astrocyte coculture can be applied for identifying the molecular mechanisms and signaling pathways involved in astrogliosis, as well as for investigating methods to prevent or reverse such process. Our method is versatile in that cellular components can be readily adjusted, for example, by adding other cellular components (e.g. microglia), changing the types and density of cells obtained from various brain regions, combining cellular components from genetically-modified animals, or applying patient-derived stem cell technology to meet specific needs or model neurodegenerative diseases and other neurological conditions that accompany reactive astrocytes [14,15,16,17].
In summary, 3D coculture of neurons and astrocytes can be adapted to reconstruct normal and diseased conditions, thereby helping us to gain further insights towards understanding the multifaceted and complex roles of astrocytes.
ACKNOWLEDGEMENTS
This study was supported by KIST Institutional Grant (2E26662 to C.J.L., 2V05590 to E.M.H.), National Research Council of Science and Technology (NST) Grant by the Korean government (MSIP) (CRC-15-04-KIST), Creative Research Initiative Program (2015R1A3A2066619) and the Brain Research Program through the National R esearch Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016M3C7A1905074, 2015M3C7A1028396, NRF-2012M3C7A1055412).
References
- 1.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Lin KH, Maeda S, Saito T. Long-term maintenance of liver-specific functions in three-dimensional culture of adult rat hepatocytes with a porous gelatin sponge support. Biotechnol Appl Biochem. 1995;21:19–27. [PubMed] [Google Scholar]
- 3.Shiraha H, Koide N, Hada H, Ujike K, Nakamura M, Shinji T, Gotoh S, Tsuji T. Improvement of serum amino acid profile in hepatic failure with the bioartificial liver using multicellular hepatocyte spheroids. Biotechnol Bioeng. 1996;50:416–421. doi: 10.1002/(SICI)1097-0290(19960520)50:4<416::AID-BIT8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix. Exp Cell Res. 1996;223:357–371. doi: 10.1006/excr.1996.0091. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Im SK, Oh SJ, Jeong S, Yoon ES, Lee CJ, Choi N, Hur EM. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat Commun. 2017;8:14346. doi: 10.1038/ncomms14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.East E, Golding JP, Phillips JB. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J Tissue Eng Regen Med. 2009;3:634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster U, Büttner R, Hofstädter F, Knüchel R. A heterologous in vitro coculture system to study interaction between human bladder cancer cells and fibroblasts. J Urol. 1994;151:1707–1711. doi: 10.1016/s0022-5347(17)35349-1. [DOI] [PubMed] [Google Scholar]
- 8.Carlberg B, Axell MZ, Nannmark U, Liu J, Kuhn HG. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed Mater. 2009;4:045004. doi: 10.1088/1748-6041/4/4/045004. [DOI] [PubMed] [Google Scholar]
- 9.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leach MK, Feng ZQ, Gertz CC, Tuck SJ, Regan TM, Naim Y, Vincent AM, Corey JM. The culture of primary motor and sensory neurons in defined media on electrospun poly-L-lactide nanofiber scaffolds. J Vis Exp. 2011:pii: 2389. doi: 10.3791/2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puschmann TB, Zandén C, De Pablo Y, Kirchhoff F, Pekna M, Liu J, Pekny M. Bioactive 3D cell culture system minimizes cellular stress and maintains the in vivo-like morphological complexity of astroglial cells. Glia. 2013;61:432–440. doi: 10.1002/glia.22446. [DOI] [PubMed] [Google Scholar]
- 12.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 14.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langan TJ, Slater MC. Astrocytes derived from longterm primary cultures recapitulate features of astrogliosis as they re-enter the cell division cycle. Brain Res. 1992;577:200–209. doi: 10.1016/0006-8993(92)90275-e. [DOI] [PubMed] [Google Scholar]
- 21.Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res. 2012;37:2569–2588. doi: 10.1007/s11064-012-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon BE, Lee CJ. GABA as a rising gliotransmitter. Front Neural Circuits. 2014;8:141. doi: 10.3389/fncir.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Kimelberg HK. Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K(+)](o) uptake capabilities. J Neurophysiol. 2000;84:2746–2757. doi: 10.1152/jn.2000.84.6.2746. [DOI] [PubMed] [Google Scholar]
- 26.Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, Bae Y, Woo J, Kim D, Park M, Lee CJ, Park JY. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun. 2014;5:3227. doi: 10.1038/ncomms4227. [DOI] [PubMed] [Google Scholar]
- 27.Kucheryavykh LY, Kucheryavykh YV, Inyushin M, Shuba YM, Sanabria P, Cubano LA, Skatchkov SN, Eaton MJ. Ischemia increases TREK-2 channel expression in astrocytes: relevance to glutamate clearance. Open Neurosci J. 2009;3:40–47. doi: 10.2174/1874082000903010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Pagán AF, Rivera-Aponte DE, Melnik-Martínez KV, Zayas-Santiago A, Kucheryavykh LY, Martins AH, Cubano LA, Skatchkov SN, Eaton MJ. Up-regulation of TREK-2 potassium channels in cultured astrocytes requires de novo protein synthesis: relevance to localization of TREK-2 channels in astrocytes after transient cerebral ischemia. PLoS One. 2015;10:e0125195. doi: 10.1371/journal.pone.0125195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Zhou M, Jiang M. A protocol for primary dissociated astrocyte and neuron co-culture. Sheng Li Xue Bao. 2013;65:72–76. [PubMed] [Google Scholar]
- 30.He Y, Yao Y, Tsirka SE, Cao Y. Cell-culture models of the blood-brain barrier. Stroke. 2014;45:2514–2526. doi: 10.1161/STROKEAHA.114.005427. [DOI] [PMC free article] [PubMed] [Google Scholar]