Abstract
Objective
To obtain a summary positive predictive value (sPPV) of contrast-enhanced CT in determining resectability.
Methods
The MEDLINE and EMBASE databases from JAN2005 to DEC2015 were searched and checked for inclusion criteria. Data on study design, patient characteristics, imaging techniques, image evaluation, reference standard, time interval between CT and reference standard, and data on resectability/unresectablity were extracted by two reviewers. We used a fixed-effects or random-effects approach to obtain sPPV for resectability. Several subgroups were defined: 1) bolus-triggering versus fixed-timing; 2) pancreatic and portal phases versus portal phase alone; 3) all criteria (liver metastases/lymphnode involvement/local advanced/vascular invasion) versus only vascular invasion as criteria for unresectability.
Results
Twenty-nine articles were included (2171 patients). Most studies were performed in multicentre settings, initiated by the department of radiology and retrospectively performed. The I2-value was 68%, indicating heterogeneity of data. The sPPV was 81% (95%CI: 75-86%). False positives were mostly liver, peritoneal, or lymphnode metastases. Bolus-triggering had a slightly higher sPPV compared to fixed-timing, 87% (95%CI: 81-91%) versus 78% (95%CI: 66-86%) (p = 0.077). No differences were observed in other subgroups.
Conclusions
This meta-analysis showed a sPPV of 81% for predicting resectability by CT, meaning that 19% of patients falsely undergo surgical exploration.
Key points
• Predicting resectability of pancreatic cancer by CT is 81% (95%CI: 75-86%).
• The percentage of patients falsely undergoing surgical exploration is 19%.
• The false positives are liver metastases, peritoneal metastases, or lymph node metastases
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-016-4708-5) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic Neoplasms, Computed Tomography, Positive Predictive Value, Systematic Review, Surgery
Introduction
Contrast-enhanced computed tomography (CT) plays a central role in staging of pancreatic cancer [1]. The staging system depends on the tumour size and location, extension beyond the confinement of the pancreas, adjacent vessels contact or encasement/occlusion, and the presence of distant metastatic disease (e.g. lungs, liver, bones, lymph nodes, and peritoneum). The presence of distant metastatic disease excludes patients from curative resection intent. In addition, based on which vessel is involved and the degree of involvement, patients are excluded or included from curative resection intent. In two recently published reviews [1, 2], one comparing CT with EUS showed lower diagnostic values for CT in evaluating vascular invasion (sensitivity 63% and 72% and specificity 92% and 89%) and the other review summarizing CT findings showed high sensitivity and specificity of 77% and 81%, respectively. Although the sensitivity value in the review by Yang et al is low, CT remains the most commonly used modality, because of the evaluation of distant metastases in a one-stop-shop strategy [3–6].
However, there are concerns that in patients with (borderline) resectable tumours on CT, a substantial number of patients (40%) are found to be unresectable during surgical exploration, based on the presence of either vascular invasion or metastatic disease [7]. This means that unnecessary surgical exploration/laparotomy is performed in 40% of these patients. In a recent review the additional role of laparoscopy before surgical exploration can reduce this percentage to 20% [7]. Several studies also showed the significant role of additional PET-CT to reduce the number of unnecessary surgical explorations [8–11]. However, both PET-CT and laparoscopy are operationally high cost and invasive methods. Therefore, CT remains playing a major initial role in the overall staging of pancreatic cancer
The most important outcome in determining resectability by CT is the positive predictive value for resectability, as in general the patients with (borderline) resectable tumours on CT will undergo surgical exploration followed by resection.
In patients found to be unresectable on CT it is not ethical to perform an invasive technique, and in addition abovementioned reviews [1, 2] showed that CT has a low number of patients with false positive vascular invasion. As this is an important feature in determining unresectablity, the number of patients falsely judged to be unresectable by CT is very low with high predictive values for unresectablity between 89-100% [3]. Therefore, summarizing the predictive values for unresectablity seems not to be relevant.
The aim of this study is to obtain a summary positive predictive value of contrast-enhanced CT in determining the resectability and to report the incidence of contra-indicative factors in the false positive patients (patients judged to be resectable on CT while on reference standard these patients were found to be unresectable).
Materials and methods
Search strategy
Computerized searches in MEDLINE and EMBASE databases from JAN 2005 to JUN 2015 were performed to identify relevant abstracts. For MEDLINE the following keywords were used: "Pancreatic Neoplasms"[Mesh] ”AND ("Tomography, X-Ray Computed"[Mesh] OR "Multidetector Computed Tomography"[Mesh] OR "Four-Dimensional Computed Tomography"[Mesh] OR "Spiral Cone-Beam Computed Tomography"[Mesh] OR "Cone-Beam Computed Tomography"[Mesh] OR "Tomography Scanners, X-Ray Computed"[Mesh] OR "Tomography, Emission-Computed"[Mesh] OR "Tomography, Spiral Computed"[Mesh] OR "Tomography, Emission-Computed, Single-Photon"[Mesh]).
For EMBASE the following text words were used: pancreatic cancer (tw) AND computed tomography (tw) or *computer assisted tomography (tw).
Selection of eligible articles
All retrieved hits were evaluated by a reviewer (X1), with experience in data-extraction of 20 meta-analyses on diagnostic accuracy studies. All titles and abstracts were screened in four steps.
-
Step 1:
duplicate papers, letters/comments/editorial/conference abstracts, not pancreatic cancer relevant papers and papers describing other type of pancreatic cancer were excluded.
-
Step 2:
case reports, studies on animals/cell lines/phantoms/children, reviews/guidelines, studies only evaluating treatment, studies evaluating prognosis/survival (not imaging related), studies clearly evaluating other imaging (biopsy, FNA), and studies reported in Chinese, Korean, Japanese, and Russian were subsequently excluded.
-
Step 3:
subsequently studies were excluded if other parameters based on CT were evaluated; CT was only used for screening or response monitoring or recurrent/follow-up or prognosis/association.
-
Step 4:
To identify additional studies, reference lists of relevant articles were checked manually and additional search was performed between JUN 2015 and DEC 2015.
Inclusion and exclusion criteria
All potentially eligible articles were double-checked on inclusion and exclusion criteria by the same reviewer with a delay of 4 weeks. Inclusion criteria were: (a) more than 25 patients with pancreatic adenocarcinoma, (b) patients undergoing contrast-enhanced CT, (c) contrast-enhanced CT for determining resectability, (d) histopathology (surgery, biopsy, or cytology), laparoscopy/laparoscopic ultrasound, follow-up, or consensus were used as reference test, (e) absolute numbers of true positive and false positive results available or could be extracted. Exclusion criteria were: (a) data on same outcome of same study population (study with the largest population was included) and (b) results on combination of different imaging modalities presented and cannot be differentiated for contrast-enhanced CT.
Data extraction
Of the included papers, data were extracted by two reviewers independently (X1 and X2, radiologist with experience in MRI imaging of the pancreas) and discrepancies were resolved by consensus. The following data (including quality assessment) were extracted:
Study design
Year of publication, study period, country of origin, setting (single-centre/multicentre), department of first author, type of data-collection (prospective/retrospective/unclear), and whether ethical approval was obtained (yes/no/unclear).
Patient characteristics
Patient population (suspected/diagnosed/underwent surgery), inclusion/exclusion criteria, number of patients with pancreatic carcinoma included/analysed, number of patients included in total in study, distribution of total number of patients (carcinoma, other malignant lesions, benign lesions), age of patients (mean + SD or median + range), sex ratio (male: female), number of patients undergoing surgery/resection.
Quality criterion was whether consecutive/random sample of patients were enrolled (yes/no/unclear) [12].
Imaging techniques
Bowel preparation, intravenous contrast agent (type, concentration, and amount), phased used (scan delay and reconstructed slice width if available).
Quality criterion was whether the execution of CT was described in sufficient detail to permits its replication (yes/no/unclear) [12]. The execution of CT was described in sufficient detail if type of scanner and type, amount and concentration of contrast agent and phases with scan delay were described
Image evaluation
Method of reconstructions (e.g. multiplanar reconstruction), observers (number, experience, and data on interobserver analysis), and CT criteria used for resectability/unresectablity. Quality criteria were: 1) whether the interpretation of CT was described in sufficient detail to permit its replication (yes/no/unclear). The interpretation of CT was described in sufficient detail if method of reconstruction, number/experience of observers, and criteria for resectability/unresectablity were given; 2) whether CT results were interpreted without knowledge of the results of the reference standard (yes/no/unclear); and 3) if resectability/unresectability criteria were pre-specified (yes/no/unclear) [12].
Reference standard
Data on composition of the reference standard (surgery, resection/histopathology, biopsy/aspiration, laparoscopy with or without ultrasound, follow- up, consensus) were extracted. Quality criterion was whether the reference standard was likely to correctly classify the target condition (yes/no/unclear). In case of consensus including CT, the reference standard was assessed as not correctly classifying the target condition.
Time interval between CT and reference standard
The time interval between CT and reference standard was recorded. Quality criterion was whether there was an appropriate interval [<1 month for surgery, resection/histopathology, biopsy/aspiration, laparoscopy with or without ultrasound, and < 12 months for follow-up between CT and reference standard (yes/no/unclear)] [12].
Data on resectability/unresectability
We extracted data by using CT resectability categories as were reported in the articles versus the two reference standard categories. The data was, therefore, expressed as follows:
2 × 2 table (resectable OR unresectable on CT vs. resectable OR unresectable on references standard).
1 × 2 table (resectable on CT vs. resectable OR unresectable on references standard).
True positive is defined as resectable on both CT and reference standard.
False positives are defined as resectable on CT, while unresectable on reference standard.
Of all false positive resectable tumours (judged to be resectable on CT while unresectable on reference standard), the contraindicative factors were recorded if available.
Data-analysis
Publication bias
To study publication bias, we constructed funnel plot for the positive predictive value (PPV) and the Egger regression test was used to examine funnel plot asymmetry [13]. PPV was defined as TP (resectable on both CT and reference standard)/TP + FP (total number of resectable patients on CT). We placed the PPV on the x-axis and the sample size on the y-axis. A p-value of < 0.05 was considered as showing significant publication bias.
Summary positive predictive value
The I 2 statistic, including 95% confidence intervals (CI), was used for quantification of heterogeneity of the PPV [14, 15]. We used either nonlinear fixed-effects (I 2 ≤ 25%) or random-effects (I 2 > 25%) approach to obtain summary PPV. Mean logit PPV with corresponding standard errors were obtained, and then antilogit transformation was performed to calculate summary estimates of PPV (sPPV) including 95% confidence intervals (95%CI) [16, 17].
Exploratory analysis
To study effect of several quality items on the summary PPV (sPPV), we incorporated these items in the model: (1) publication year; (2) design (multicentre vs. single-centre); (3) department of first authors (radiology vs. other); (4) data collection (prospective vs. retrospective/unclear); (5) patient selection (consecutive vs. not consecutive/unclear); (6) detailed description of CT techniques (yes vs. no/unclear); (7) detailed description of CT interpretation (yes vs. no/unclear); (8) blinded interpretation of CT (yes vs. no/unclear); (9) CT criteria described (yes vs. no/unclear); (10) appropriate interval between CT and reference standard (yes vs. no/unclear); and (11) reference standard correctly classify target condition (yes vs. no/unclear). Year of publication was explored as continuous factor, and all other factors were explored as binomial. We considered factors to be explanatory if the corresponding regression coefficients were significantly different from zero, meaning that p-values were less than 0.05.
Subgroup analysis
The following subgroups were defined a priori:
Studies using bolus triggering versus studies using fixed timing.
Studies including both pancreatic and portal phases versus studies including only portal phase.
Studies taking all criteria (liver metastases, other distant metastases, lymph node involvement, local advanced and vascular invasion) into account as criteria for unresectablity versus studies taking only vascular invasion as criteria for unresectablity.
The z test was performed to analyse differences in logit PPV estimates between subgroups. All data analyses were performed by using software (Microsoft Excel 2000, Microsoft, Redmond, Wash; SPSS 10.0 for Windows, SSPS, Chicago, IL, USA; SAS 9.3, SAS Institute, Cary, NC, USA).
Results
Search strategy and selection of eligible articles
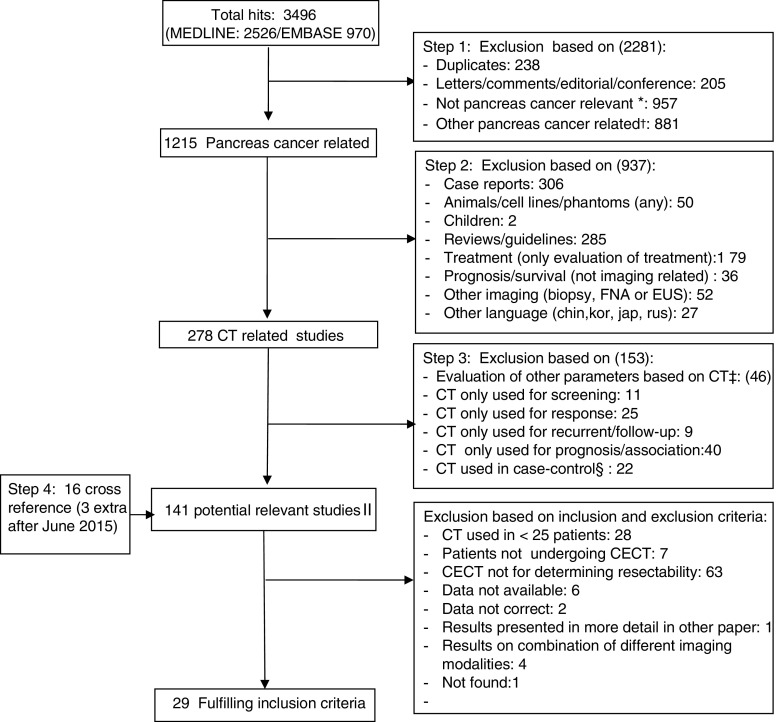
The search strategy resulted in 3496 articles. After excluding all non-relevant papers, 125 articles were found to be potentially relevant. An additional 16 articles were found after manually cross-checking, resulting in 141 relevant articles. These articles were checked on inclusion and exclusion criteria. Finally, 29 [18–46] fulfilled the inclusion criteria and data-extraction was performed. The search strategy and selection of eligible articles are shown in Supplement 1 and Fig. 1. The excluded articles are presented in Supplement 1.
Fig. 1.
Search, selection and inclusion of relevant papers. *Not relevant (other disease, pancreatitis, lung cancer, ovarian cancer, lipoma, neuroendocrine, insulinoma, melanoma, colon carcinoma, renal cell carcinoma, myeloma, colorectal cancer, pancreatitis, endocrine, intracranial, colitis, hernia, polyposis.). †Other type of pancreatic cancer: pseudopapillary, cystadenoma, acinar. ‡Evaluation of other parameters using CT (e.g. qualitative analysis, tumour volume, interobserver). §Case-control studies: evaluation of techniques in patients with pancreatic cancer vs. control (healthy or pancreatitis or other type of tumour, predefined). II Potentially relevant studies: evaluation of CT in patients with suspected, diagnosed pancreatic cancer
Study design
Most studies were performed in multicentre setting, initiated by the department of radiology and retrospectively performed. In case a study was prospectively designed [22, 30, 37, 41], resectability by CT was mostly retrospectively analysed. All data on study design characteristics are shown in Table 1.
Table 1.
Study design characteristics of the included studies
| Study author | Year of publication/Journal | Study period | Country of origin* | Type of study† | Department first author | Type of data collection | Ethical approval |
|---|---|---|---|---|---|---|---|
| Ellsmere [18] | 2005/Surg Endosc | Jan 1999-March 2002 | USA | Multi-centre | Surgery | Retrospective | UNCLEAR |
| Imbriaco [19] | 2005/AJR Am J Roentgenol | Sept 2001-Feb 2003 | Italy | Multi-centre | Radiology | Prospective | YES (informed consent) |
| Karmazanovsky [20] | 2005/Abdom Imaging | 1994-2003 | Russia | Single-centre | Radiology | Retrospective | UNCLEAR |
| Li [21] | 2005/J Comput Assist Tomogr | Dec 2001-Feb 2004 | China | Single-centre | Radiology | Retrospective | UNCLEAR |
| Phoa [22] | 2005/J Surg Oncol | Feb 1997-Jul 1999 | The Netherlands | Single-centre | Radiology | Prospective study (retrospective evaluation) | UNCLEAR |
| Imbriaco [23] | 2006/Radiol Med | Sept 2001-March 2004 | Italy | Single-centre | Radiology | Unclear | UNCLEAR |
| Tamm [24] | 2006/Abdom Imaging | Not available | USA | Single-centre | Radiology | Retrospective | YES |
| Kala [25] | 2007/Eur J Radiol | Not available | Czech Republic | Multi-centre | Surgery | Retrospective | UNCLEAR |
| Olivie [26] | 2007/JOP | Feb 2003-Jun 2004 | Canada | Multi-centre | Radiology | Prospective | YES (informed consent) |
| Smith [27] | 2007/Pancreas | March 2002-March 2005 | UK | Single-centre | Radiology | Retrospective | UNCLEAR |
| Zamboni [28] | 2007/Radiology | March 2003-March 2006 | USA | Single-centre | Radiology | Retrospective | YES |
| Furukawa [29] | 2008/Arch Surg | Sept 2002-March 2005 | Japan | Single-centre | Radiology | Prospective | YES |
| Klauss [30] | 2008/Pancreatology | March 2005-Aug 2006 | Germany | Multi-centre | Radiology | Prospective (retrospective evaluation) | YES (informed consent) |
| Shah [31] | 2008/J Surg Res | NA | USA | Single-centre | Surgery | Retrospective | YES |
| Manak [32] | 2009/Abdom Imaging | Jan 2000-Jul 2005 | Germany | Multicentre | Radiology | Retrospective | UNCLEAR |
| Park [33] | 2009/J Magn Reson Imaging | Jan 2004-Jul 2008 | Korea | Multi-centre | Radiology | Retrospective | YES |
| Satoi [34] | 2009/Hepatogastroenterology | Jan 2000-Apr 2005 | Japan | Single-centre | Surgery | Retrospective | YES (informed consent) |
| Croome [35] | 2010/Can J Surg | Jan 2005-Dec 2006 | Canada | Multi-centre | Surgery | Retrospective | YES |
| Grieser [36] | 2010/Acta Radiologica | Jan 2002- Jan 2007 | Germany | Single-centre | Radiology | Retrospective | YES |
| Grossjohann [37] | 2010/Scand J Gastroenterology | Dec 2005-Dec 2007 | Denmark | Multi-centre | Radiology | Prospective (retrospective evaluation) | YES |
| Kaneko [38] | 2010/J Comput Assist Tomogr | Jan 2000-March 2009 | USA | Multi-centre | Radiology | Retrospective | YES |
| Lee [39] | 2010/Eur J Radiol | Jan 2003- Jun 2005 | Korea | Multi-centre | Radiology | Retrospective | UNCLEAR |
| Koelblinger [40] | 2011/Radiology | Sept 2006-Nov 2007 | Austria | Multi-centre | Radiology | Prospective (IC) | YES |
| Fang [41] | 2012/Pancreatology | Nov 2008-Aug 2010 | China | Multi-centre | Surgery | Prospective (retrospective evaluation) | YES |
| Khattab [42] | 2012/The Egyptian Journal of Radiology and Nuclear Medicine | Dec 2009-Aug 2011 | Egypt | Single-centre | Radiology | Prospective (IC) | YES |
| Yao [43] | 2012/ANZ J Surg | Dec 2006-Jul 2009 | Australia | Multi-centre | Surgery | Retrospective | UNCLEAR |
| Cieslak [44] | 2014/Pancreatology | Jan 2007-Dec 2010 | The Netherlands | Multi-centre | Gastroenterology | Retrospective | UNCLEAR |
| Hassanen [45] | 2014/The Egyptian Journal of Radiology and Nuclear Medicine | Oct 2010-March 2013 | Egypt | Single-centre | Radiology | Prospective (IC) | YES |
| Iscanli [46] | 2014/Turk J Gastroenterol | NA | Turkey | Single-centre | Radiology | Retrospective | YES |
*Country of origin of first author. † Implies involvement of authors form different centers, not particular the involvement of patients form different centers
Patient characteristics
In most studies, patients with known pancreatic adenocarcinoma were included. In total, 2171 patients were included (fulfilling inclusion and exclusion criteria) or formed the study group, with ages ranging from 15 to 84 years with a pooled mean of 63.5 years. In 25 studies the distribution of male:female ratio was given as 1088:800.
In some studies [19, 28, 30, 36, 40, 41, 43, 44] data on other malignancies were also given; however, as most of the patients had adenocarcinoma, we did not exclude these studies. All patient characteristics per study are given in Table 2.
Table 2.
Patient characteristics of the included studies
| Study author | Patient Population (suspected or known) | Selection criteria (inclusion and/or exclusion criteria) | Number of total patients included* | Distribution of patients (adenocarcinoma; other malignancies; benign lesion) | Age (mean or median and SD or range) in years | Sex ratio (male: female) | Consecutive or random selection |
|---|---|---|---|---|---|---|---|
| Ellsmere [18] | Known | Inclusion: Patients with a diagnosis of pancreatic head adenocarcinoma who underwent a gastrointestinal procedure. Exclusion: Patients who did not undergo a thin-section dual-phase MDCT and attempted pancreaticoduodenectomy at the institution |
44 | Adenocarcinoma: 44 | Not available | Not available | YES |
| Imbriaco [19] | Suspected | Inclusion: Patients with a suspected pancreatic mass based on clinical symptoms, laboratory findings, and results of ERCP or sonography | 71 | Adenocarcinoma: 37 Other malignancy: 3 Benign lesions: 31 |
Mean: 63 ± 12 Range:29-80 |
41:30 | YES |
| Karmazanovsky [20] | Known | Inclusion: Patients who had morphologically confirmed pancreatic head adenocarcinoma by using spiral CT with bolus intravenous contrast enhancement; Only patients who underwent intraoperative surgical exploration were included in this investigation. | 89 | Adenocarcinoma:89 | Mean: 60 Range: 23-80 |
52:37 | UNCLEAR |
| Li [21] | Suspected | Inclusion: Patients with presumed pancreatic cancer underwent surgery |
54 | Adenocarcinoma: 54 | Mean: 61.2 Range: 40-79 |
37:17 | YES |
| Phoa [22] | Suspected | Inclusion: Patients suspected of having pancreatic carcinoma who underwent a spiral CT for staging; Patients with pancreatic head carcinoma eventually who underwent surgery for attempted resection with curative intent. Exclusion: patients with cholangiocarcinomas, cystic tumours or endocrine tumours. |
71 | Adenocarcinoma: 71 | Median: 62 Range: 42-76 |
33:38 | YES |
| Imbriaco [23] | Suspected | Patients with suspected pancreatic cancer based on clinical and laboratory findings and on results of endoscopic retrograde cholangiopancreatogram (ERCP) or ultrasonography (US). | 78 | Adenocarcinoma:46 Benign lesions: 32 |
Mean ± SD: 64 ± 12 | 42:36 | UNCLEAR |
| Tamm [24] | Known | Inclusion: Patient with biopsy-proven adenocarcinoma of the pancreas who underwent MDCT imaging using a dual-phase pancreas protocol and endoscopic ultrasound. Exclusion: patients with other histology or who underwent CT scanning using other imaging regimens were not included. Also excluded were patients who were identified as having resectable disease but refused surgery, were lost to follow-up or had significant comorbid disease that precluded surgery. |
55 | Adenocarcinoma: 55 | Mean: 67 Range: 46–86 |
31:24 | UNCLEAR |
| Kala [25] | Known | Inclusion: Patients with pancreatic cancer. | 55 (undergoing CT) | Adenocarcinoma: 55 | Not available | Not available | UNCLEAR |
| Olivie [26] | Suspected or known | Inclusion: Patients referred with a known or suspected diagnosis of cancer of the head and patients found to be resectable. | 28 (study group) | Adenocarcinoma:28 (study group) | Mean: 63 Range: 40-76 |
14:14 | YES |
| Smith [27] | Known | Inclusion: Patients with pancreatic ductal adenocarcinoma. Exclusion: Patients with ampullary tumours, tumours of the body and tail of the pancreas, and benign disease |
33 (underwent surgery) | Adenocarcinoma: 33 (study group) | Mean: 67.2 Range: 28- 88 (33 patients) |
20:13 (33 patients) |
YES |
| Zamboni [28] | Known | Inclusion: Patients who underwent surgical staging or attempt at curative resection for pancreatic carcinoma at our institution; Patients who underwent scanning in our institution with multiphase multidetector CT with multiplanar reconstructions and two- and three-dimensional CT angiography. Exclusion: all patients who underwent scanning at other institutions or with different CT protocols. |
114 | Adenocarcinoma: 110 Other malignancies: 4 |
Mean:65.9 Range: 33-85 |
52: 62 | UNCLEAR |
| Furukawa [29] | Known | Inclusion: Patients were referred for treatment of invasive ductal carcinoma of the pancreas. Exclusion: patients with other histologic types of pancreatic tumour such as endocrine tumour, intraductal papillary mucinous tumour, and solid and pseudopapillary tumour. Patients who already received surgical or medical treatment at another hospital. |
213 | Adenocarcinoma: 213 | Mean: 64 Range: 32-82 |
136: 77 | YES |
| Klauss [30] | Suspected | Inclusion: Patients with strong clinical suspicion of pancreatic carcinoma based on icterus, a preceding CT or ultrasound scan, unspecific weight loss, or an elevated CA19-9. Exclusion: Patients with renal impairment with creatinine values 12 mg/dL, manifest hyperthyroidism, known contrast medium allergy, and failure to consent to the study. |
80 | Adenocarcinoma: 36 Other malignancy: 9 Benign lesions: 35 |
Mean: 64.9 Range: 37–89 | 43:37 | UNCLEAR |
| Shah [31] | Known | Inclusion: Patients with pancreatic adenocarcinoma Exclusion: Patients presented with other pancreatic neoplasms, such as ampullary adenocarcinoma, pancreatic endocrine tumours, or cystic neoplasms. | 88 | Adenocarcinoma: 88 | Not available | Not available | YES |
| Manak [32] | Known | Inclusion: Patients with pathologically proven pancreatic adenocarcinoma underwent MDCT and who underwent surgery. Excluded: Patients who received a course of radio-chemotherapy before surgery. |
48 | Adenocarcinoma: 48 | Mean: 64.7 Range: 43–89 |
26:22 | UNCLEAR |
| Park [33] | Known | Inclusion: Patients with pancreatic cancer who had undergone curative or palliative surgery; Patients with both preoperative contrast-enhanced MRI including MRCP and triple phase MDCT within a month before surgery; Patients with diagnosed pancreatic ductal carcinoma on pathology examination of a surgical specimen. | 54 | Adenocarcinoma: 54 | Mean: 63.1 Range: 28–83 | 32:22 | UNCLEAR |
| Satoi [34] | Known | Inclusion: Patients with ductal adenocarcinoma after clinical; diagnosis of pancreatic cancer using ultrasonography, CT, MRCP, ERCP, endoscopic ultrasonography, cytological examination of the bile juice and/or biopsy of the bile duct mucosa. Exclusion: endocrine tumour of the pancreas, intraductal papillary mucinous cancer, acinar cell carcinoma, or anaplastic cancer. |
80 (patients undergoing multislice CT) | Adenocarcinoma: 80 | Mean: 65 Range 39-83 | 37:43 | YES |
| Croome [35] | Suspected | Inclusion: Patients referred to pancreatic surgeons because of suspected cancer of the pancreatic head. | 96 (undergoing CT) | Not available | Not available | Not available | UNCLEAR |
| Grieser [36] | Known | Inclusion: Patients who underwent surgical exploration or resection of a pancreatic mass at our medical center; patients who underwent a preoperative triphasic MDCT examination (4–64-slice MDCT) performed at our clinic; a detailed report of the operation and a histopathological analysis available. | 105 | Adenocarcinoma: 60 Other malignancies: 10 Benign lesions: 35 |
Mean ± SD: 58 ± 15 | 73:32 | YES |
| Grossjohann [37] | Known/suspected | Inclusion: patients with pancreatic head tumours and with suspected pancreatic head tumours. | 49 | Adenocarcinoma: 44 Benign lesions: 5 |
Mean: 66 years Range: 42–83 |
26:23 | YES |
| Kaneko [38] | Known | Inclusion: Patients presenting to our institution with adenocarcinoma of the pancreatic head were included. Exclusion: located in the pancreatic body or tail were excluded, as well as all patients with other neoplastic/inflammatory processes of the pancreas. |
109 (underwent surgery) | Adenocarcinoma: 109 | Mean: 65.1 Range: 39-86 |
56:53 | YES |
| Lee [39] | Known | Inclusion: Patients with newly diagnosed pancreatic ductal adenocarcinoma and who underwent surgery. | 56 | Adenocarcinoma: 56 | Mean: 60.9 Range: 37-76 |
30: 26 | UNCLEAR |
| Koelblinger [40] | Suspected | Inclusion: Patients suspected of having pancreatic cancer referred to hepatobiliary-pancreatic surgeons; suspected of having pancreatic cancer on the basis of findings from clinical examination (e.g., jaundice, increased CA 19-9 levels, rapid weight loss or previous US or CT studies performed). | 89 | Adenocarcinoma: 43 Other malignancies: 8 Benign lesions: 38 |
Mean ± SD: 65.5 ± 10.7 | 41:48 | YES |
| Fang [41] | Diagnosed | Inclusion: Patients with confirmed pancreatic and periampullary neoplasms. | 80 | Adenocarcinoma: 57 Other malignancy: 23 |
Mean ± SD: 57.9 ± 1.7 Range: 15-91 |
49:31 | UNCLEAR |
| Khattab [42] | Suspected | Inclusion: Patients with clinical and sonographic findings that raised suspicions of pancreatic cancer were included in our study. | 39 | Adenocarcinoma: 39 | Mean: 58.3 Range: 44-73 | 29:10 | UNCLEAR |
| Yao [43] | Known | Inclusion: Patients with potentially resectable pancreatic tumours detected on contrasted CT imaging and who also had preoperative PET/CT scans. | 36 | Adenocarcinoma: 30 Other malignancies: 3 Benign lesions: 4 1 patient had both adenocarcinoma and other malignancy |
Median: 71 Range: 32–84 | 24:12 | UNCLEAR |
| Cieslak [44] | Suspected | Inclusion: Patient who underwent exploratory laparotomy for a suspected pancreatic or periampullary malignancy; Patients who underwent both preoperative contrast enhanced CT and endoscopic ultrasonography. | 86 | Adenocarcinoma: 37† Other malignancy: 30† Benign lesions: 9† |
Mean ± SD: 65 ± 10.5 | 49:37 | YES |
| Hassanen [45] | Suspected | Inclusion: patients with suspected pancreatic carcinoma underwent biphasic MDCT for pancreatic examination. | 47 (study group) | Adenocarcinoma: 47 | Mean: 63.5 Range: 45-82 |
32:15 | YES |
| Iscanli [46] | Known | Inclusion: Patients with pancreatic adenocarcinoma confirmed by surgery-pathology or clinical follow-up. | 124 | Adenocarcinoma: 124 | Mean: 60.2 Range: 28-84 |
83: 41 | YES |
*Fulfilling inclusion criteria or forming the study group.
†Number of patients who were found to be resectable
CT technical features
The number of detectors ranged from 1 to 64. In several studies the specification of CT [18, 35, 43] was not reported. In most of studies, no information on oral contrast administration was given. Intravenous contrast was clearly given in most studies ranging from 90-200 mL. The timing of scanning was either fixed (mostly old studies) or bolus triggering. Pancreatic (late arterial, early portal) and portal (late portal) phases were performed in almost all studies, except in four studies [19, 22, 23, 44]. In ten studies execution of the CT was not described in sufficient detail due to missing information on type of scanner, the type/amount/concentration of iv contrast, and the different phases with scan delay [18, 20, 22, 25, 31, 34, 35, 37, 43, 44]. All details on CT technical features per study are given in Table 3.
Table 3.
CT technical features of the included studies
| Study author | Type of scanner | Bowel preparation | Intravenous contrast agent | Phases | Execution described in detail* |
|---|---|---|---|---|---|
| Ellsmere [18] | Not available | Not available | 100–150-mL Ultravist | Pancreatic phase: 40-s post-contrast delay, reconstruction slice thickness 3 mm. Portal venous phase: 70-s post-contrast delay, reconstruction slice thickness 5 mm. |
NO |
| Imbriaco [19] | 4-slice | 10-15 min before CT exam: 500 mL water orally | 150 mL Ultravist 370 (Iopromide) | Portal venous phase: 60-s post-contrast delay, 1.25-mm reconstruction interval | YES |
| Karmazanovsky [20] | Single-slice | Not available | 100 mL |
Bolus contrast enhancement
Arterial phase: 25-s delay Portal vein phase: 80-s delay |
NO |
| Li [21] | 4-slice | Not available | 120 mL Ultravist 350 (Iopromide) | Arterial phase: 20-s post-contrast delay, 2.5 mm collimation Pancreatic phase: 45-s post-contrast delay, 2.5 mm collimation Hepatic phase: 80-s post-contrast delay, 2.5 mm collimation |
YES |
| Phoa [22] | 2-slice | Not available | 130 mL Omnipaque 300 | Pancreatic phase: 50-s post-contrast delay, collimation 2.5 mm | NO |
| Imbriaco [23] | 4-slice | 500 mL water orally | 150 mL (370 mg I/mL) | Portal venous phase: 60-s post-contrast delay, reconstruction interval 1.25 mm | YES |
| Tamm [24] | 4-slice | Not available | 150 mL Optiray 300 (Ioversol) | Pancreatic phase: 25-s post-contrast delay, 2.5 mm slice thickness and reconstruction to 1.25 mm contiguous images. Portal phase: 55-s post-contrast delay, 5 mm slice thickness and reconstruction to 2.5 mm contiguous images. |
YES |
| Kala [25] | single-slice | Not available | 125 mL Optiray 350 | Not available | NO |
| Olivie [26] | 16-slice | 500 mL of water orally | 150 mL Omnipaque 350 | Arterial phase: 20-s post-contrast delay, section on width of 2.5 mm and an interval reconstruction of 1.25 mm. Late arterial phase: 40-s post-contrast delay, section on width of 2.5 mm and an interval reconstruction of 1.25 mm Portal phase: 60-s post-contrast delay, section on width of 2.5 mm and an interval reconstruction of 1.25 mm |
YES |
| Smith [27] | 4- or 8-slice | Not available | 100 mL Omnipaque 300 | Pancreatic phase: 40-s post-contrast delay, collimation 2.5 mm; reconstruction overlap, 1.25 mm Portal-venous phase: 65-s post-contrast delay, collimation 2.5 mm, reconstruction overlap 1.25 mm |
YES |
| Zamboni [28] | 4-, 8-, 16-, 64- slice | Not available | 150–200 mL Optiray 350 (Ioversol) |
Bolus tracking
: 150-HU threshold of enhancement abdominal aorta at the origin of the celiac axis Late arterial–early portal venous phase: 15- s post-threshold delay, collimation 1.25 mm for 4- and 8-slice CT, 0.625 mm for 16-slice or 0.5 mm for 64-slice CT. Venous phase: 25-s after arterial phase, collimation 1.25 mm for 4- and 8-slice CT, 0.625 mm for 16-slice, or 0.5 mm for 64-slice CT. |
YES |
| Furukawa [29] | 16-slice | Not available | 150 mL Iopamiron (Iopamidol) | Early arterial phase: 20-s post-contrast delay Late arterial phase: 40-s post-contrast delay Pancreatic phase: 70-s post-contrast delay Delayed phase: 120-s post-contrast delay 1-mm section thickness and reconstructed at 1-mm intervals (0.5-mm overlap) |
YES |
| Klauss [30] | 16-slice | 1.5 L still water | 120 mL Ultravist 370 |
Bolus threshold
: 100-HU enhancement in the aorta at the level of the superior mesentery artery Arterial phase: 8-s post-threshold delay, reconstructed slice thickness 2/1 Portal venous phase: 35-s post-threshold delay, reconstructed slice thickness (3/3 first and 1/0.5 s) |
YES |
| Shah [31] | 64-slice | Water | Intravenous contrast (type/concentration not available | Arterial phase Pancreatic phase Hepatic venous phase |
NO |
| Manak [32] | 4- and 16-slice | 800 mL water orally with 40 mg butylscopolamin or 1 mg glucagon | 120 mL (300–370 mg iodine/mL) | Pancreatic phase: 35-s post-contrast delay, section thickness 3.0 mm Portal venous phase: 70-s post-contrast delay, section thickness 3.0 mm |
YES |
| Park [33] | 4-, 8-, 16-, and 64- slice | Not available | 120 mL Ultravist 370 (Iopromide) |
Bolus tracking
: 100 HU at abdominal aorta 64-slice/16-slice/8-slice Early arterial phase: 6-s post-threshold (23-s post-contrast delay), slice thickness 3 mm. Late arterial phase: 5–9-s interscan delay between early and late phases, (37-45-s post-contrast delay); slice thickness of 3 mm. Venous phase: 70-s post-threshold: slice thickness of 3 mm 4-slice Early arterial phase: 6-s post-threshold (23-s post-contrast delay), slice thickness of 3.2 mm. Late arterial phase: 5–9-s interscan delay between early and late phases, (37-45-s post-contrast delay); slice thickness of 3.2 mm. Venous phase: 70-s post-threshold: slice thickness of 3.2 mm. |
YES |
| Satoi [34] | 4-slice | Not available | Not available | Arterial and portal phase; slice thickness 1.25 mm | NO |
| Croome [35] | Not available | Not available | Not available | Not available | NO |
| Grieser [36] | 4-, 8-, 16-, and 64-slice |
Not available | 100 mL Ultravist 370 (Iopromide) | Bolus threshold: aortic enhancement above 100 HU Early arterial phase: 4-s post-threshold delay (20-s post-contrast delay) Early portal venous phase: 20 s delay from the beginning of the arterial phase (40-s post-contrast delay) Venous phase: 60-s delay from the beginning of the arterial phase (80-s post-contrast delay) |
YES |
| Grossjohann [37] | 64-slice | Not available | Not available | Not available | NO |
| Kaneko [38] | 4-, 16-, and 64-slice | Not available | 120–150 mL Omnipaque 350 | 4-slice Pancreas phase delay: 45 s, Slice thickness 1.25 mm, Portal venous phase: 60-70s, Slice thickness 1.25 mm, 16 and 64 slice Pancreatic phase: bolus tracking with trigger at 150 HU; slice thickness: 0.75 mm Portal venous phase: 10-s delay after pancreatic phase, slice thickness: 0.75 mm |
YES |
| Lee [39] | 4-slice | No oral contrast agent | 150 mL Ultravist 370 (Iopromide) |
Bolus triggering
: 100-HU enhancement of the descending aorta Arterial phase: 5-s post-threshold delay; reconstruction interval of 5 mm Portal venous phase: 72-s post-contrast delay; reconstruction interval of 5 mm |
YES |
| Koelblinger [40] | 64–slice | 20 min before CT exam: 1000 mL water orally | 150 mL Iomeron 300 (Iomeprol) |
Bolus triggering
: threshold of 100 HU in the abdominal aorta. Pancreatic parenchymal: 25 –s post-threshold, slice thickness 3 mm Portal venous phase: 23-s after pancreatic phase, section thickness 3 mm |
YES |
| Fang [41] | 64 slice | Not available | 80-100 mL Iopamiron |
Bolus tracking: 100 HU in the diaphragmatic section of the abdominal aorta Arterial-phase: 8-s post-threshold slice thickness 0.67 mm Portal venous phase: 60-s post-threshold; slice thickness 0.67 mm |
YES |
| Khattab [42] | 64-slice | 1000 mL of mixed water and contrast orally | 100 mL Omnipaque 350 |
Bolus triggering
: 110 HU in the aorta at corresponding level of superior mesenteric artery. Pancreatic arterial phase: 20-s post-threshold delay; section width of 1.5 mm Delayed venous phase: 50-s post-threshold delay; section width of 1.5 mm |
YES |
| Yao [43] | Not available | Not available | Not available | Not available | NO |
| Cieslak [44] | Range: 16–64-slice | Oral contrast | 100-150 mL | Portal OR Arterial and portal; 5.0-mm slice thickness | NO |
| Hassanen [45] | 16-slice | 600–800 mL water or water soluble contrast agent orally | 100 mL Ultravist 370 (Iopromide) |
Bolus triggering
: 110 HU in the abdominal aorta at the level of the celiac axis Late arterial phase: 10- s post-threshold delay; 3.0 mm Portal venous phase: 35-s post-threshold delay; 3.0 mm |
YES |
| Iscanli [46] | 16- or 64- slice | 1000 mL water orally | 90-110 mL Visipaque 320 (Iodixanol) or Ultravist 370 (Iopromide) |
Bolus tracking: 180-HU enhancement on the proximal abdominal aorta Arterial phase: start automatically when maximum contrast reached Pancreatic phase: 45-s post-contrast delay: slice thickness: 1-1.25 mm, Portal phase: 65-s post-contrast delay: slice thickness: 1-1.25 mm |
YES |
*The execution of the CT was described in sufficient detail if type of scanner, the type, amount, and concentration of iv contrast and the different phases with scan delay were described
Interpretation of CT
Methods of reconstruction and the experience of observers were poorly described. However, the criteria used for unresectability was described in most of the papers, except in three studies [30, 35, 45]. In three studies [24, 25, 41], only vascular invasion was used as criteria for unresectability; in other studies all criteria were used. The interpretation of CT was not described in sufficient detail due to missing information on description of reconstruction methods, number and experience of observers and the criteria for resectability/unresectablity. Whether CT interpretation was blinded was not clear in most of the studies. All data in details are given in Table 4.
Table 4.
Interpretation of CT in included studies
| Study author | Method of reconstruction* | Observers (number and experience) | CT criteria for resectability or unresectability | Interpretation described in detail† | Blinded interpretation of CT‡ | Criteria defined§ |
|---|---|---|---|---|---|---|
| Ellsmere [18] | Not available | 4 abdominal radiologists independently | Criteria for resectability: 1. No distant metastasis; 2. A patent portal vein; 3. <50% arterial involvement |
NO | YES | YES |
| Imbriaco [19] | VR | 2 experienced abdominal radiologists independently | Criteria for unresectability: 1. Peripancreatic vascular invasion (defined as encasement of the celiac, hepatic, or superior mesenteric arteries or the portal or superior mesenteric vein) ¶; 2. Extrapancreatic invasion of adjacent tissues and organs other than duodenum; 3. Presence of hematogenous or distant lymph node metastases (other than peripancreatic nodes); 4. Signs of peritoneal carcinomatosis; 5. Distant lymph node exceeding 1.5 cm ¶ A vessel was considered to be involved if it showed a focal reduction in caliber, circumferential (>180°) encasement by tumour, or frank thrombosis. Involvement of the splenic artery or veins was not considered an absolute contraindication to resection unless the tumour extended into the splenoportal confluence or involved other splanchnic vessels. |
NO | YES | YES |
| Karmazanovsky [20] | MPR and VR | Not available | Criteria for unresectability: 1. Invasion of any of the vascular structures (portal vein, superior mesenteric vein, and artery) 2. Liver metastasis |
NO | UNCLEAR | YES |
| Li [21] | MPR and MIP and VR | 2 observers in consensus (10 and 5 years of experience in pancreatic imaging) | Criteria for unresectability: 1. Vascular invasion; 2. Hepatic metastasis 3. Other metastatic signs. |
YES | YES | YES |
| Phoa [22] | Not available | 2 experienced abdominal radiologists independently, followed by consensus |
Criteria for unresectability: 1. Tumour infiltration was present; 2. If any vessel (portal or superior mesenteric vein, hepatic artery, and the superior mesenteric artery) showed involvement of >180 degrees; 3. If tumour convexity was scored as Loyer grade D or E ¶ 4. Liver metastases; 5. Distant lymph nodes larger than 1 cm ¶ Vascular invasion according to Loyer grade 1 Grade A: fat plane visible between tumour and vessel; Grade B: normal pancreatic tissue between tumour and vessel; Grade C: tumour adjacent to vessel with a contour convex towards the vessel; Grade D: tumour adjacent to vessel with a contour concave towards the vessel, Grade E: circumferential involvement of vessel; Grade F: thrombosis or occlusion of vessel. |
NO | YES | YES |
| Imbriaco [23] | Not available | 2 experienced radiologists independently | Criteria for unresectability: 1. Peripancreatic vascular invasion (defined as infiltration of the coeliac trunk, hepatic artery, superior mesenteric artery or portal vein, and superior mesenteric vein) ¶; 2. Extrapancreatic invasion of adjacent tissues 3. Hematogenous or lymph node metastasis (>1.5 cm) or peritoneal carcinomatosis. ¶ The vessel was considered involved when its circumference was reduced more than >180° by the tumour or by evident thrombosis. Involvement of the splenic artery or vein was not considered an absolute contraindication to surgery unless the tumour extended to splenic-mesenteric-portal confluence. |
NO | YES | YES |
| Tamm [24] | Not available | 3 abdominal radiologists independently | Criteria for unresectability: 1. Primary tumour involving the superior mesenteric or celiac arteries, involvement of the common hepatic artery, M1 disease, or occlusion of the superior mesenteric vein and/or portal vein. |
NO | YES | YES |
| Kala [25] | Not available | Not available | Criteria for unresectablity: 1. Tumour invasion to coeliac trunk, to common hepatic artery, superior mesenteric artery, superior mesenteric vein, portal vein, inferior caval vein, and aorta. |
NO | UNCLEAR | Yes |
| Olivie [26] | MPR and curved MPR | Not available | Criteria for unresectability: 1. Liver metastases; 2. Peritoneal carcinomatosis; 3. Tumour infiltration in contact with more than 180° of the circumference of the walls of major arteries (celiac trunk, hepatic artery, superior mesenteric artery) and involvement of more than 180° of the circumference of the portal vein or the superior mesenteric vein. |
NO | YES | YES |
| Smith [27] | MPR and MIP and VR | 2 radiologist independently followed by consensus | Criteria for unresectability: 1. Metastases 2. Vascular encasement: Lu grade 3 or 4¶ 3. Vascular occlusion 4. Direct invasion of bowel, spleen, and mesentery (duodenum excluded) 5. Lymphadenopathy (nodes > 1 cm in short axis) outside the peripancreatic chain. ¶ Vascular involvement (for the superior mesenteric vessels, coeliac artery, and portal vein) was estimated using the Lu grade 2. Grade 0: no contiguity of tumour to vessel; Grade 1: tumour contiguous less than one quarter of circumference; Grade 2: between one quarter and one half; Grade 3: between one half and three quarters; Grade 4: greater than three quarters. |
NO | UNCLEAR | YES |
| Zamboni [28] (initial clinical evaluation) |
MPR and MIP and VR | 2 readers: one radiology resident/abdominal fellow (pool of 30 readers, with 2–6 years of experience) and one abdominal radiologist (pool of eight readers, with 8–20 years of experience). | Criteria for unresectability: 1. Distant disease (hepatic metastases, distant lymph node metastases, peritoneal metastases), 2. Invasion of adjacent organs other than the duodenum 3. Major vascular invasion: Grade 2, 3, or 4¶ 4. Lymph nodes were considered metastatic if the short-axis diameter was 1 cm or larger. ¶ Vascular invasion Grade 0: normal, with a fat plane or normal pancreas between tumour and vessel; Grade 1: loss of fat plane between tumour and vessel, with or without smooth displacement of the vessel); Grade 2: flattening and/or slight irregularity of one side of the vessel; Grade 3: encased vessel with tumour extending around at least two sides (i.e., two thirds of the perimeter), altering its contour and producing concentric or eccentric lumen narrowing; Grade 4: at least one major occluded vessel. |
YES | UNCLEAR | YES |
| Zamboni [28] (retrospective evaluation) |
2 gastrointestinal radiologists in consensus (>20 and 4 years of experience) | Same as for initial interpretation | YES | YES | YES | |
| Furukawa [29] | MPR | More than 1 diagnostic radiologist in conference | Criteria for unresectablity: 1. Extrapancreatic tumour spread, particularly into the celiac axis and the root of the superior mesenteric artery; 2. Para-aortic massive lymph node involvement [the short axis was greater than 1 cm in diameter or there were clusters of 3 or more smaller nodes (each < 1 cm)] 3. Presence of distant metastases and ascites; 4. Portal vein invasion (>90% direct contact). |
NO | YES | YES |
| Klauss [30] | Curved MPR | 2 radiologists in consensus (with 11 and 23 years of experience) | Not defined for resectability (only for vascular invasion) | NO | YES | NO |
| Shah [31] | Not available | Interpretated by an experienced multidisciplinary team |
Criteria for unresectablity: 1. Extra-pancreatic disease such as liver, omental, or peritoneal metastases; 2. Bulky (>2 cm) celiac adenopathy; 3. Malignant ascites; 4. Loss of a patent portosplenic confluence, or 360° encasement of the portal or superior mesenteric veins, or 5. Any contact between the tumour and the hepatic artery or superior mesenteric artery. |
NO | UNCLEAR | YES |
| Manak [32] | MPR | 2 radiologists in consensus | Criteria for unresectability 1. Vascular involvement ¶ 2. Liver and peritoneal metastases 3. Distant lymph nodes metastases. Enlarged lymph nodes (>10 mm) were considered as metastatic. Distant lymph nodes metastases beyond the peripancreatic chains indicated unresectability ¶ Arterial invasion (celiac trunk, superior mesenteric artery and common hepatic artery) was defined as any direct contiguity tumour to artery with complete obliteration of a fat plane even if the contiguity was less than 50%. ¶ Venous invasion (superior mesenteric vein, splenic vein, and portal vein) was defined when the tumour showed contiguity to more than 50% of vein circumference. |
NO | YES | YES |
| Park [33] | Not defined | 2 abdominal radiologists independently (8 and 7 years CT and MRI experience) | Criteria for unresectability: 1. Distant metastasis: liver, peritoneum, para-aortic lymph nodes, or lung; 2. Invasion into peripancreatic arteries: celiac trunk, hepatic artery, or SMA; 3. Massive venous invasion of portal vein and/or SMV, i.e., tumour infiltration with thrombosis and obliteration of the vessel lumen involving a segment longer than 2 cm; and 4. Infiltration in adjacent organs: stomach, spleen, or colon. |
YES | NO | YES |
| Satoi [34] | MPR | 1 experienced hepatopancreatolobiliary surgeon and 1 consultant radiologist in consensus | Criteria for potential resectable: 1. Patients with no distant metastases or no tumour extension to a major peripancreatic artery defined as tumour ingrowth with > 50% vessel contiguity in the celiac trunk, common or proper hepatic artery, or superior mesenteric artery 2. Patients with tumours invading the portal vein but in the absence of extended obstruction of the portal vein to distal branches of the superior mesenteric vein 3. Patients with cancer in pancreatic body and tail with celiac trunk invasion and without SMA invasion |
NO | UNCLEAR | YES |
| Croome [35] | Not available | Not available | Not available | NO | UNCLEAR | NO |
| Grieser [36] | MPR and curved MPR and MIP and VR and additional 3D | 2 experienced radiologists independently (4 and 6 years of experience in abdominal MDCT) | Criteria for unresectability: 1. Arterial infiltration (celiac trunk, superior mesenteric artery, common hepatic artery), 2. Distant metastases, and 3. Peritoneal carcinomatosis 4. Additional criterion comprised non-manageable venous infiltration or thrombosis (portal vein, superior mesenteric vein). |
YES | YES | YES |
| Grossjohann [37] | Not available | Not available | Criteria for resectability: 1. No invasion into larger surrounding vessels or surrounding organs; 2. Not included in a resected Whipple’s specimen; 3. No detection of liver metastases 4. No distant lymph node metastases 5. No peritoneal seeding. |
NO | UNCLEAR | YES |
| Kaneko [38] | MPR | Experienced radiologists specializing in gastrointestinal radiology |
Criteria for unresectability: 1. distant metastases to liver, peritoneum, omentum, or adjacent organs; 2. Invasion of a major peripancreatic vessel (celiac artery, hepatic artery, portal vein, and superior mesenteric artery and vein (Lu grade of 3 or greater) ¶ 3. Venous invasion causing thrombosis of the vein. ¶ Vascular involvement according to Lu 2: Grade 0: tumour not contiguous with vessel; Grade 1: tumour contiguous with less than a quarter of vessel circumference; Grade 2: between a quarter and one half; Grade 3: between one half and three quarters; Grade 4: greater than three quarters or with any evidence of focal vessel narrowing regardless of degree of contiguity. |
NO | UNCLEAR | YES |
| Lee [39] | MPR and MIP and VR | 2 radiologists independently (completed fellowship in gastrointestinal radiology) |
Criteria for unresectability: 1. Extrapancreatic invasion of the adjacent tissues or organs other than the duodenum and spleen, 2. Peritoneal metastasis, 3. Distant metastases, 4. Vascular invasion ¶. ¶ We used the criteria for arterial invasion constituted encasement, vessel margin irregularity, or tumour incursion into the periarterial fat plane with the tumour lying in juxtaposition to the vessel. ¶ Venous invasion was considered present when the tumour caused venous occlusion, flattening or narrowing, apposition with concavity toward the vessel lumen, or a circumferential apposition greater than 180°. |
YES | YES | YES |
| Koelblinger [40] | MPR and curved MPR | 2 gastrointestinal radiologists independently (>10 years of experience in abdominal CT and MR imaging) |
Criteria for unresectablity: 1. Vascular invasion: the main portal vein, the portal venous confluence, the superior mesenteric artery and vein, the celiac trunk, and the hepatic artery, Grade 3 or more ¶; 2. The presence of liver metastases, 3. Peritoneal carcinomatosis, 4. Adjacent organ infiltration, and 5. Pathologic lymph nodes ¶ Vascular invasion according to Li 3 Grade 0: no tumour contiguity; Grade 1: tumour contiguous with less than 90° vessel circumference; Grade 2: tumour contiguous between 90° and 180° circumference; Grade 3: tumour contiguous between 180° and 270° circumference; Grade 4: tumour contiguous with more than 270° circumference. |
YES | YES | YES |
| Fang [41] | VR | 2 radiologists for CTA 2 radiologists for 3D reconstructions |
CTA criteria for unresectablity: 1. A tumour intricately associated with the celiac trunk and its main branches, the abdominal aorta, the inferior vena cava, the portal vein, the superior mesenteric artery, the inferior mesenteric vein such that there is no apparent space in between them; 2. A low-density tumour completely surrounding its neighbouring blood vessels without causing lumen changes; 3. A low-density tumour that causes occlusion or stenosis by vascular invasion. 3D Criteria for unresectability: The following vessels were evaluated: portal vein, superior mesentery artery, inferior vena cava, superior mesenteric vein, left renal vein, right renal vein, hepatic artery, celiac trunk, and abdominal aorta. Type IV and V were classified as unresectable Type IV: the primary tumour was attached to and compressed the large vessels and the vessels had a “moth-eaten” instead of smooth appearance. Type V: the large blood vessels were surrounded by the primary tumour, distortion of the large vessels was evident and significant dilatation of the small pancreatic vein could be observed. |
NO | YES | YES |
| Khattab [42] | Curved MPR | 2 radiologists in consensus | Criteria of unresectability: 1. Tumours which were larger than 2 cm in size (tumours less than 2 cm may be associated with favorable outcome 2. Presence of local metastasis (such as enlarged lymph nodes outside peripancreatic draining chains) or direct invasion of the surrounding organs with exclusion of the duodenum, or more, 3. Distant metastases (liver or pulmonary metastasis), 4. Infiltration of the walls of major vessels including (celiac trunk, hepatic artery, splenic artery, superior mesenteric artery and portal vein or the superior mesenteric vein) ¶ ¶ Arterial vascular involvement was estimated using the criteria by Lu et al 2. Grade 0–2 was considered operable, whereas grades 3 and 4 were considered radiologically inoperable. Grade 0: no contiguity of tumour to vessel; Grade 1: tumour contiguous less than one quarter of circumference; Grade 2: between one quarter and one half; Grade 3: between one half and three quarters; Grade 4: greater than three quarters. ¶ Venous invasion was defined as tumour-to-vessel circumferential contiguity of 50% or more. Tumour-to-vein circumferential contiguity of less than 50% was not considered venous invasion |
NO | YES | YES |
| Yao [43] | Not available | Not available | Criteria for resectability: 1. No distant metastases; 2. Absence of tumour-induced occlusion of any aspect of the superior mesenteric-portal vein confluence; 3. Absence of tumour extension to the celiac axis, common hepatic artery and superior mesenteric artery. |
NO | UNCLEAR | YES |
| Cieslak [44] | Not available | 2 (1 surgeon and 1 expert radiologist) in consensus | Criteria for unresectability: 1. Vascular invasion: when exceeding 270 o of the circumference of the portal vein/superior mesenteric vein or exceeding 90 o of the circumference of the superior mesentery artery, celiac trunk, or hepatic artery; 2. Distant metastases |
NO | YES | YES |
| Hassanen [45] | Not available | 2 radiologist | Not available | NO | UNCLEAR | NO |
| Iscanli [46] | MPR and curved MPR MIP and VR | By one of two radiologists (5 and 12 years of experience in reading pancreatic imaging) |
Criteria for unresectability: 1. Distant metastases to the liver, peritoneum, or omentum; 2. Direct invasion of the adjacent organs (except the duodenum); 3. Vascular invasion of a major peripancreatic vessel, according to Lu. A Lu grade 0 to 2 was considered operable, whereas grade 3 and above were radiologically inoperable. A degree of contact of the vessel with the tumour of 180° was considered indeterminate¶. ¶ Lu criteria 2 evaluate vascular involvement by the degree of contact of the vessel with the tumour: Grade 0: no contiguity of tumour to vessels; Grade 1: tumour contiguous less than one quarter (<90°) of vessel circumference; Grade 2: tumour contiguous between one quarter and one half (90°-180°); Grade 3: between one half and three quarters (180°-270°); Grade 4: greater than three quarters (>270°) or any evidence of focal vessel narrowing or irregularity on the vessel wall, regardless of degree of contiguity. |
YES | UNCLEAR | YES |
*MPR: multiplanar reformation; MIP: maximum intensity projection; VR: volume rendering
†The interpretation of the CT was described in sufficient detail on number and experience of observers and the criteria for resectability/unresectablity were given
‡whether the CT results were interpreted without knowledge of the results of the reference standard
§If resectability/unresectability criteria were pre-specified (yes, no, unclear)
1Loyer EM, David CL, Dubrow RA, Evans DB, Charnsangavej C. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom.Imaging 1996; 21:202-20
2Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am. J Roentgenol. 1997; 168:1439-1443
3Li H, Zeng MS, Zhou KR, Jin DY, Lou WH. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput. Assist. Tomogr. 2005; 29:170-175
Reference standard and time interval between CT and reference standard
The interval time between CT and reference standard were only mentioned in ten studies [21, 26–28, 32, 36, 38, 42, 45, 46]. Most reference concerned surgery followed by resection. All details on reference standard and time interval between CT and reference standard are given in Supplement 2.
Data on resectability
As most studies were retrospectively performed, data on 2 x 2 tables or 1 x 2 tables were available, for details see Table 5.
Table 5.
Data on resectability by CT
| Study author | Resectable according to CT | Unresectable according to CT | PPV | FP distributions | ||
|---|---|---|---|---|---|---|
| Resectable according to reference standard (TP) | Unresectable according to reference standard (FP) | Resectable according to reference standard (FN) | Unresectable according to reference standard (TN) | TP/(TP + FP) | ||
| Ellsmere [18] | 22 | 14 | 1 | 7 | 61.1% (22/36) | Not available |
| Imbriaco [19] (Obs1) |
8 | 2 | 1 | 29 | 80.0% (8/10) | Not available |
| Imbriaco [19] (Obs 2) |
7 | 3 | 2 | 28 | 70.0% (7/10) | Not available |
| Karmazanovsky [20] (Potential resectable as resectable) |
52 | 18 | 4 | 15 | 74.3% (52/70) | Liver metastases (18) |
| Li [21] | 16 | 6 | 1 | 31 | 72.7% (16/22) | Invaded vessels (4)/Liver metastases (1) Peritoneal metastases (1) |
| Phoa [22] | 26 | 10 | 15 | 20 | 72.2% (26/36) | Not available |
| Imbriaco [23] (Obs 1) |
6 | 2 | 1 | 37 | 75.0% (6/8) | Not available |
| Imbriaco [23] (Obs 2) |
6 | 3 | 1 | 36 | 66.7% (6/9) | Not available |
| Tamm [24] (Obs 1) |
10 | 2 | 1 | 42 | 83.3% (10/12) | Liver metastases (1)/Peritoneal metastases (1) |
| Tamm [24] (Obs 2) |
10 | 3 | 1 | 41 | 76.9% (10/13) | Liver metastases (1)/Peritoneal metastases (1)/Vascular invasion (1) |
| Tamm [24] (Obs 3) |
10 | 6 | 1 | 38 | 62.5% (10/16) | Liver metastases (3)/Peritoneal metastases (1)/Vascular invasion (1)/Lung metastases (1) |
| Kala [25] | 14 | 14 | 2 | 19 | 50.0% (14/28) | Not available |
| Olivie [26] | 23 | 0 | 0 | 5 (palliative procedure) | 100.0% (23/23) | Not applicable |
| Smith [27] (Equivocal as resectable) |
10 | 14 | 1 | 8 | 41.7% (10/24) | Vascular invasion (9)/Infiltration beyond pancreas (3)/Liver metastases (1)/Other metastases (1) |
| Smith [27] (Equivocal as unresectable) |
9 | 7 | 2 | 15 | 56.3% (9/16) | Vascular invasion (3)/Infiltration beyond pancreas (3)/Other metastases (1) |
| Zamboni [28] (Clinical evaluation) |
78 | 10 | 0 | 26 | 88.6% (78/88) | Vascular invasion (1)/Liver metastases (5) Lymph nodes (2)/Peritoneal metastases (2) |
| Zamboni [28] (Retrospective evaluation) |
78 | 2 | 0 | 34 | 97.5% (78/80) | Not available |
| Furukawa [29] (Probably unresectable as unresectable) |
68 | 11 | 0 | 134 | 86.1% (68/79) | Peritoneal metastases (7)/Liver (3)/LN (1) |
| Klauss [30] | 21 | 0 | 1 | 6 | 100% (21/21) | NA |
| Shah [31] | 34 | 13 | NA | NA | 72.3% (34/47) | Metastases (9)/R2 resection (1)/Locally advanced (3) |
| Manak [32] | 44 | 4 | NA | NA | 91.7% (44/48) | Vascular invasion (1)/Liver metastases (2)/Peritoneal metastases and lymph nodes (1) |
| Park [33] (Obs 1) |
35 | 3 | 7 | 9 | 92.1% (35/38) | Not available |
| Park [33] (Obs 2) |
35 | 4 | 7 | 8 | 89.7% (35/39) | Not available |
| Satoi [34] | 29 | 3 | NA | NA | 90.6% (29/32) | Distant metastases (3) |
| Croome [35] | 24 | 16 | NA | NA | 60.0% (24/40) | Liver metastases (3)/Peritoneal metastases (1)/Omental seeding (1)/Vascular invasion (10)/Vascular invasion and lymph nodes (1) |
| Grieser) [36] (Without 3D) (Obs 1) |
32 | 6 | 1 | 31 | 84.2% (32/38) | Not available |
| Grieser [36] (With 3D) (Obs 1) |
32 | 4 | 1 | 33 | 88.9% (32/36) | Not available |
| Grieser [36] (Without 3D) (Obs 2) |
32 | 5 | 1 | 32 | 86.5% (32/37) | Not available |
| Grieser [36] (With 3D) (Obs 2) |
32 | 3 | 1 | 34 | 91.4% (32/35) | Not available |
| Grossjohann [37] | 10 | 9 | 2 | 8 | 53% (10/19) | Liver metastases (3)/Lymph node metastases (1)/Vascular invasion (5) |
| Kaneko [38] (Equivocal as resectable) |
67 | 20 | 0 | 22 | 77.1% (67/87) | Liver metastases (4)/Vascular invasion (10)/Peritoneal metastases (6) |
| Kaneko [38] (Equivocal as unresectable) |
67 | 12 | 0 | 30 | 84.8% (67/79) | Liver metastases (4)/Vascular invasion (4)/Peritoneal metastases (4) |
| Lee [39] (Obs 1) |
35 | 6 | 4 | 11 | 85.4% (35/41) | Peritoneal metastases (2)/Liver metastases (1)/Vascular invasion (1)/Adjacent organs (2) |
| Lee [39] (Obs 2) |
35 | 7 | 4 | 10 | 83.3% (35/42) | Not available |
| Koelblinger [40] (Obs 1) |
13 | 2 | 2 | 6 | 86.7% 13/15 | Carcinomatosis (1)/Lymph node (1) |
| Koelblinger [40] (Obs 2) |
13 | 3 | 2 | 5 | 81.3% 13/16 | Carcinomatosis (2)/Lymph node (1) |
| Fang [41] CTA |
30 | 2 | 8 | 17 | 93.8% (30/32) | Vascular invasion (2) |
| Fang [41] 3D |
38 | 0 | 0 | 19 | 100% (38/38) | Not Applicable |
| Khattab [42] | 15 | 3 | NA | NA | 83.3% (15/18) | Vascular invasion (2)/Liver and lymph nodes (1) |
| Yao [43] | 25 | 11 | NA | NA | 69.4% (25/36) | Bone metastases (2)/Lymph nodes (1)/Liver metastases (8) |
| Cieslak [44] | 75 | 8 | 1 | 2 | 90.4% (75/83) | Distant metastasis (4)/Locally advanced (3)/Liver metastases (1) |
| Hassanen [45] | 13 | 6 | 2 | 26 | 68.4% (13/19) | Vascular invasion (4)/Liver metastases (2) |
| Iscanli [46] (Equivocal as resectable) |
62 | 21 | 0 | 41 | 75.7% (62/83) | Liver metastases (9)/Peritoneal metastases (3)/Vascular invasion (9) |
| Iscanli [46] (Equivocal as unresectable) |
62 | 17 | 0 | 44 | 78.5% (62/79) | Liver metastases (9)/Peritoneal metastases (3)/Vascular invasion (5) |
NA: Not Applicable; Obs 1: Observer 1; Obs 2: Observers 2; Obs 3: Observer 3
Publication bias
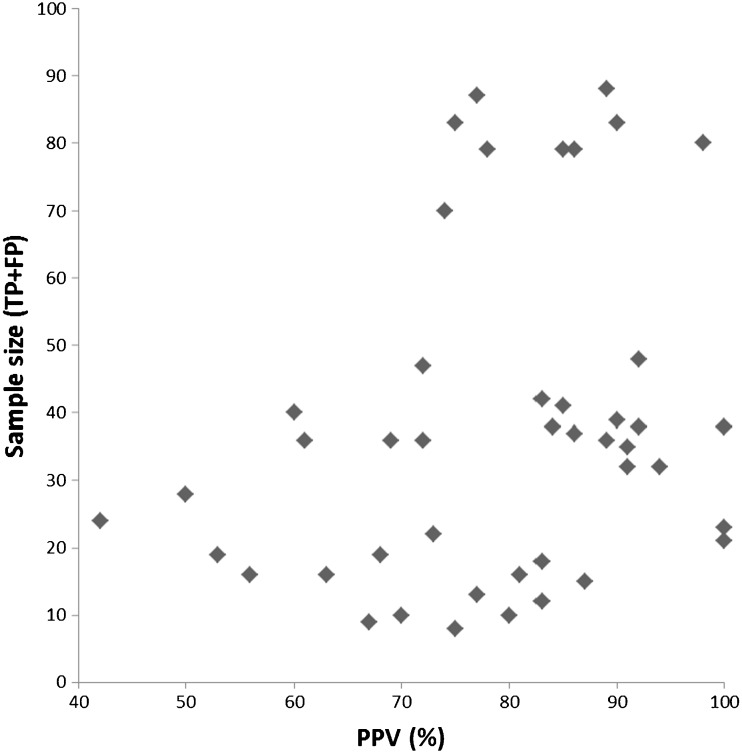
The regression coefficient showed no significant relationship between sample size and PPV. The coefficient was 0.47 (95%CI: -0.06-0.99) with a p-value of 0.08 (see also Fig. 2).
Fig. 2.
Funnel plot showing no relationship between sample size and PPV
Summary Positive Predictive Value (sPPV)
The I2 value was 68% (95%CI: 57-76%), indicating that data were heterogeneous. The sPPV was: 81% (95%CI: 75-86%). The false positives were mostly liver metastases, peritoneal metastases, or lymph node metastases. This means that these metastases were missed at CT (Table 7).
Exploratory analysis
Only description of CT technical features, description of interpretation of CT and blinded interpretation of CT had effect on summary PPV. All other items did not have an effect (all p > 0.05).
Studies where CT was described in details compared to studies where CT was not described in details: 85% (95%CI: 79-89%) and 72% (95%CI: 61-81%) respectively (p = 0.013).
Studied where CT interpretation was described in details compared to studies where CT interpretation was not described in details: 89% (95%CI: 83-93%) and 74% (95%CI: 67-80%), respectively (p = 0.004).
Studies where CT interpretation was blinded compared to studies where CT interpretation was not clear: 87% (95%CI: 81-91%) and 72% (95%CI: 63-80%), respectively (p = 0.001).
Subgroup analyses
Studies using bolus-triggering for CT scanning had a slightly higher sPPV compared to studies using fixed timing, respectively 87% (95%CI: 81-91%) and 78% (95%CI 66-86%); however, with a p-value of 0.077.
No significant difference was observed between studies including both pancreatic and portal phases vs. studies including only portal phase, respectively 84% (95%CI:78-88%) and 75% (95%CI: 60-85%), p = 0.153.
No difference was seen between studies taking all criteria into account compared to studies taking only vascular invasion as criteria for unresectablity, respectively 81% (95%CI: 76-86%) and 81% (95%CI: 45-96%), p = 0.984.
Discussion
This meta-analysis showed a sPPV of 81% (95%CI: 75-86%) for predicting resectability by CT. This means that the percentage of patients falsely undergoing surgical exploration is 19%. The false positives (resectable on CT while unresectable on reference standard) for resectability were mostly distant metastases such as liver metastases, peritoneal metastases, or lymph node metastases.
Surprisingly, when checking the subgroup analyses, no significant difference was found between studies including both pancreatic and portal phases (sPPV: 84%) vs. studies including only portal phase (sPPV: 75%). One would except that if both phases are combined, the number of false positive would be significantly reduced, as both phases has a complementary role in the staging of pancreatic cancer [3–6]
The pancreatic phase is the most important phase for detecting and staging a pancreatic tumour. In this phase there is optimal attenuation difference between the hypodense tumour and the normal enhancing pancreatic parenchyma. This phase is not only adequate in detecting primary tumour, but also in delineating periarterial tumour spread in relation to the celiac artery, celiac artery branches, and mesenteric arteries and veins as well (local staging).
The subsequent portal phase has a scan-delay of 70-80 s. At that moment the normal liver parenchyma will enhance optimally, because normal liver cells get 80% of their blood supply through the portal venous system. Liver metastases do not get their blood supply from the portal venous system and will be seen in this phase as hypodense lesions. This phase is, therefore, accurate for detection of liver lesions and is also used for the overall assessment of the abdomen to look for lymph nodes and peritoneal metastases.
We found no significant difference between both phases and only portal phase. This might be explained by the low number of studies using only portal phase, the heterogeneity within data or because the portal phase is also helpful for local staging of the tumour (primary goal of the pancreatic phase).
Based on the higher PPV, frequently used, and the complementary role of both phases, the use of both phases should be continued for evaluation of diagnosis, local staging (vascular invasion), and evaluation of metastatic disease.
Also, no differences were seen between studies taking all criteria (vascular invasion, liver metastases, peritoneal metastases, or lymph node metastases) into account compared to studies taking only vascular invasion as criteria for unresectablity. We expected less false positives when using all criteria, but the sPPV estimates were comparable 81% and 81%.
Most false positives for resectability in both subgroups were due to the presence of distant metastases such as liver metastases, peritoneal metastases, or lymph node metastases. This means that r distant metastases are missed by radiologists even if they are paying attention. In none of the studies, however, was the interpretation of liver or peritoneal metastases defined, and in general it is known that the role of imaging in detecting metastatic lymph nodes is still disappointing. This has also been shown in a recent published systematic review on the diagnostic accuracy of CT in assessing extra-regional lymph node metastases in pancreatic and peri-ampullary cancer, with a mean summary sensitivity of 25% [47].
Even though the distant metastases are missed even if paying attention, these features should be taken into account in the interpretation of CT for determining resectability and thereby defining criteria for especially the peritoneal and lymph node metastases, in order to further reduce the number of unnecessary surgical explorations.
Studies using bolus triggering had a slightly higher sPPV compared to studies using fixed timing, respectively, 87% and 78%; however, with a p-value of 0.077. In general study design and methodological criteria were poorly described. Most of them did not have effect on the outcome, except the description of CT technical features, description of CT interpretation and blinded interpretation of CT. For all these three items, it seems that in case the criteria were clearly described the sPPV raised. The role of these items also has been studied by different methodological group and was found to be relevant and, therefore, STARD 1 and STARD 2 have been developed on optimal reporting diagnostic accuracy studies [48, 49].
So far different reviews have been published on the diagnostic value of CT in evaluating vascular invasion [1, 2] and focusing on vascular invasion with promising results. Only one review published in 2005 evaluated the role of CT in determining resectability, however, they reported summary sensitivity and specificity [50] and not on the positive predictive value of CT. Several studies have been published on this topic after 2005.
We included all studies, hence also studies including a minor portion of patient with other cancer than adenocarcinoma. But most of the patients included were patients with adenocarcinoma. In addition, although the fixed scanning is an older method, there are still centres using this technique and, therefore, we also included these studies.
Most studies were retrospectively performed, and therefore, we were also able to include data on the patients with unresectable tumours on CT. But we did not summarize the negative predictive value (predictive value for unresectability), as it is known that these predictive values are high and in daily practice only patients with (potentially) resectable tumours on CT will undergo reference standard such as surgical exploration, and therefore, no data on reference standard by surgical exploration will be available in a prospective settings. Only one study was performed prospectively and all patients were verified by surgery/follow-up [19].
Summary positive predictive value (sPPV) of CT for determining resectability is high, however, still missing a significant number of patients with distant metastases such as liver metastases, peritoneal metastases, or lymph node metastases. In the paper of Allen et al [7], the false positive could be reduced from 40% to 17% using laparoscopy. Comparable findings are found in our meta-analysis when using only CT. However, a false positive rate of 19% is still high. In several studies the value of PET-CT has been studies in the staging of pancreatic cancer [8–11, 43, 51, 52] and showed to have an additional role in the staging, and thereby even reducing the number of unnecessary surgical exploration [11, 43, 51, 52].
Recommendation is to perform an additional imaging in the patients with (potentially) resectable pancreatic tumours to reduce the number of unnecessary surgical exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 76 kb)
Acknowledgements
The scientific guarantor of this publication is: Shandra Bipat
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding.
One of the authors has significant statistical expertise.
Institutional review board approval was not required because:
Study concerns a meta-analysis
Written informed consent was not required:
Study concerns a meta-analysis
Methodology: prospective, performed at one institution
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00330-016-4708-5) contains supplementary material, which is available to authorized users.
References
- 1.Yang R, Lu M, Qian X, Chen J, Li L, Wang J, et al. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: a systematic review. J Cancer Res Clin Oncol. 2014;140:2077–2086. doi: 10.1007/s00432-014-1728-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhao WY, Luo M, Sun YW, Xu Q, Chen W, Zhao G, et al. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobil Pancreat Dis Int. 2009;8:457–464. [PubMed] [Google Scholar]
- 3.Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291–304. doi: 10.1053/j.gastro.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hawary MM, Francis IR. Pancreatic ductal adenocarcinoma staging. Cancer Imaging. 2013;13:360–364. doi: 10.1102/1470-7330.2013.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hawary MM, Kaza RK, Wasnik AP, Francis IR. Staging of pancreatic cancer: role of imaging. Semin Roentgenol. 2013;48:245–252. doi: 10.1053/j.ro.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270:248–260. doi: 10.1148/radiol.13131184. [DOI] [PubMed] [Google Scholar]
- 7.Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2013;11 doi: 10.1002/14651858.CD009323.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich S, Goerres GW, Schafer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2003;242:235–243. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 10.Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 11.Wang XY, Yang F, Jin C, Guan YH, Zhang HW, Fu DL. The value of 18F-FDG positron emission tomography/computed tomography on the pre-operative staging and the management of patients with pancreatic carcinoma. Hepatogastroenterology. 2014;61:2102–2109. [PubMed] [Google Scholar]
- 12.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 13.Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31:88–95. doi: 10.1093/ije/31.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Leeflang MM, Deeks JJ, Rutjes AW, Reitsma JB, Bossuyt PM. Bivariate meta-analysis of predictive values of diagnostic tests can be an alternative to bivariate meta-analysis of sensitivity and specificity. J Clin Epidemiol. 2012;65:1088–1097. doi: 10.1016/j.jclinepi.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Ellsmere J, Mortele K, Sahani D, Maher M, Cantisani V, Wells W, et al. Does multidetector-row CT eliminate the role of diagnostic laparoscopy in assessing the resectability of pancreatic head adenocarcinoma? Surg Endosc. 2005;19:369–373. doi: 10.1007/s00464-004-8712-5. [DOI] [PubMed] [Google Scholar]
- 19.Imbriaco M, Megibow AJ, Ragozzino A, Liuzzi R, Mainenti P, Bortone S, et al. Value of the single-phase technique in MDCT assessment of pancreatic tumors. AJR AmJ Roentgenol. 2005;184:1111–1117. doi: 10.2214/ajr.184.4.01841111. [DOI] [PubMed] [Google Scholar]
- 20.Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zeng MS, Zhou KR, Jin DY, Lou WH (2005) Pancreatic adenocarcinoma: The different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomograph 29 [DOI] [PubMed]
- 22.Phoa SS, Tilleman EH, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS. Value of CT criteria in predicting survival in patients with potentially resectable pancreatic head carcinoma. J Surg Oncol. 2005;91:33–40. doi: 10.1002/jso.20270. [DOI] [PubMed] [Google Scholar]
- 23.Imbriaco M, Smeraldo D, Liuzzi R, Carrillo F, Cacace G, Vecchione D, et al. Multislice CT with single-phase technique in patients with suspected pancreatic cancer. Radiol Med. 2006;111:159–166. doi: 10.1007/s11547-006-0017-4. [DOI] [PubMed] [Google Scholar]
- 24.Tamm EP, Loyer EM, Faria S, Raut CP, Evans DB, Wolff RA, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging. 2006;31:568–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 25.Kala Z, Valek V, Hlavsa J, Hana K, Vanova A. The role of CT and endoscopic ultrasound in pre-operative staging of pancreatic cancer. Eur J Radiol. 2007;62:166–169. doi: 10.1016/j.ejrad.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Olivie D, Lepanto L, Billiard JS, Audet P, Lavallee JM. Predicting resectability of pancreatic head cancer with multi-detector CT. Surgical and pathologic correlation. JOP. 2007;8:753–758. [PubMed] [Google Scholar]
- 27.Smith SL, Basu A, Rae DM, Sinclair M. Preoperative staging accuracy of multidetector computed tomography in pancreatic head adenocarcinoma. Pancreas. 2007;34:180–184. doi: 10.1097/01.mpa.0000250133.87520.d9. [DOI] [PubMed] [Google Scholar]
- 28.Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770–778. doi: 10.1148/radiol.2453061795. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa H, Uesaka K, Boku N. Treatment decision making in pancreatic adenocarcinoma: multidisciplinary team discussion with multidetector-row computed tomography. Arch Surg. 2008;143:275–280. doi: 10.1001/archsurg.2007.78. [DOI] [PubMed] [Google Scholar]
- 30.Klauss M, Mohr A, von Tengg-Kobligk H, Friess H, Singer R, Seidensticker P, et al. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology. 2008;8:204–210. doi: 10.1159/000128557. [DOI] [PubMed] [Google Scholar]
- 31.Shah D, Fisher WE, Hodges SE, Wu MF, Hilsenbeck SG, Charles BF. Preoperative prediction of complete resection in pancreatic cancer. J Surg Res. 2008;147:216–220. doi: 10.1016/j.jss.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 32.Manak E, Merkel S, Klein P, Papadopoulos T, Bautz WA, Baum U. Resectability of pancreatic adenocarcinoma: assessment using multidetector-row computed tomography with multiplanar reformations. Abdom Imaging. 2009;34:75–80. doi: 10.1007/s00261-007-9285-2. [DOI] [PubMed] [Google Scholar]
- 33.Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30:586–595. doi: 10.1002/jmri.21889. [DOI] [PubMed] [Google Scholar]
- 34.Satoi S, Yanagimoto H, Toyokawa H, Tanigawa N, Komemushi A, Matsui Y, et al. Pre-operative patient selection of pancreatic cancer patients by multi-detector row CT. Hepatogastroenterology. 2009;56:529–534. [PubMed] [Google Scholar]
- 35.Croome KP, Jayaraman S, Schlachta CM. Preoperative staging of cancer of the pancreatic head: is there room for improvement? Can J Surg. 2010;53:171–174. [PMC free article] [PubMed] [Google Scholar]
- 36.Grieser C, Steffen IG, Grajewski L, Stelter L, Streitparth F, Schnapauff D, et al. Preoperative multidetector row computed tomography for evaluation and assessment of resection criteria in patients with pancreatic masses. Acta Radiol. 2010;51:1067–1077. doi: 10.3109/02841851.2010.520023. [DOI] [PubMed] [Google Scholar]
- 37.Grossjohann HS, Rappeport ED, Jensen C, Svendsen LB, Hillingso JG, Hansen CP, et al. Usefulness of contrast-enhanced transabdominal ultrasound for tumor classification and tumor staging in the pancreatic head. Scand J Gastroenterol. 2010;45:917–924. doi: 10.3109/00365521003702718. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko OF, Lee DM, Wong J, Kadell BM, Reber HA, Lu DS, et al. Performance of multidetector computed tomographic angiography in determining surgical resectability of pancreatic head adenocarcinoma. J Comput Assist Tomogr. 2010;34:732–738. doi: 10.1097/RCT.0b013e3181e5d6f7. [DOI] [PubMed] [Google Scholar]
- 39.Lee JK, Kim AY, Kim PN, Lee MG, Ha HK. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. Eur J Radiol. 2010;73:310–316. doi: 10.1016/j.ejrad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Koelblinger C, Ba-Ssalamah A, Goetzinger P, Puchner S, Weber M, Sahora K, et al. Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancer. Radiology. 2011;259:757–766. doi: 10.1148/radiol.11101189. [DOI] [PubMed] [Google Scholar]
- 41.Fang CH, Zhu W, Wang H, Xiang N, Fan Y, Yang J, et al. A new approach for evaluating the resectability of pancreatic and periampullary neoplasms. Pancreatology. 2012;12:364–371. doi: 10.1016/j.pan.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Khattab EM, AlAzzazy MZ, El Fiki IM, Morsy MM (2012) Resectability of pancreatic tumors: Correlation of multidetector CT with surgical and pathologic results. Egypt J Radiol Nucl Med 43
- 43.Yao J, Gan G, Farlow D, Laurence JM, Hollands M, Richardson A, et al. Impact of F18-fluorodeoxyglycose positron emission tomography/computed tomography on the management of resectable pancreatic tumours. ANZ J Surg. 2012;82:140–144. doi: 10.1111/j.1445-2197.2011.05972.x. [DOI] [PubMed] [Google Scholar]
- 44.Cieslak KP, van Santvoort HC, Vleggaar FP, van Leeuwen MS, ten Kate FJ, Besselink MG, et al. The role of routine preoperative EUS when performed after contrast enhanced CT in the diagnostic work-up in patients suspected of pancreatic or periampullary cancer. Pancreatology. 2014;14:125–130. doi: 10.1016/j.pan.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Hassanen O, Ghieda U, Eltomey MA. Assessment of vascular invasion in pancreatic carcinoma by MDCT. Egypt J Radiol Nucl Med. 2014;45:2014. [Google Scholar]
- 46.Iscanli E, Turkvatan A, Bostanci EB, Sakaotullari Z. Assessment of surgical resectability of pancreatic adenocarcinomas with multidetector computed tomography: What are the possibilities and problems? Turk J Gastroenterol. 2014;25:01. doi: 10.5152/tjg.2014.4973. [DOI] [PubMed] [Google Scholar]
- 47.Tseng DS, van Santvoort HC, Fegrachi S, Besselink MG, Zuithoff NP, Borel Rinkes IH, et al. Diagnostic accuracy of CT in assessing extra-regional lymphadenopathy in pancreatic and peri-ampullary cancer: a systematic review and meta-analysis. Surg Oncol. 2014;23:229–235. doi: 10.1016/j.suronc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Radiology. 2003;226:24–28. doi: 10.1148/radiol.2261021292. [DOI] [PubMed] [Google Scholar]
- 49.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology. 2015;277:826–832. doi: 10.1148/radiol.2015151516. [DOI] [PubMed] [Google Scholar]
- 50.Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438–445. doi: 10.1097/01.rct.0000164513.23407.b3. [DOI] [PubMed] [Google Scholar]
- 51.Barber TW, Kalff V, Cherk MH, Yap KS, Evans P, Kelly MJ. 18 F-FDG PET/CT influences management in patients with known or suspected pancreatic cancer. Intern Med J. 2011;41:776–783. doi: 10.1111/j.1445-5994.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoneyama T, Tateishi U, Endo I, Inoue T (2014) Staging accuracy of pancreatic cancer: Comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur J Radiol 83 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 76 kb)