Abstract
Acute pancreatitis (AP) is a common abdominal acute inflammatory disorder and the leading cause of hospital admission for gastrointestinal disorders in many countries. Clinical manifestations of AP vary from self-limiting local inflammation to devastating systemic pathological conditions causing significant morbidity and mortality. To date, despite extensive efforts in translating promising experimental therapeutic targets in clinical trials, disease-specific effective remedy remains obscure, and supportive care has still been the primary treatment for this disease. Emerging evidence, in light of the current state of pathophysiology of AP, has highlighted that strategic initiation of nutrition with appropriate nutrient supplementation are key to limit local inflammation and to prevent or manage AP-associated complications. The current review focuses on recent advances on nutritional interventions including enteral versus parenteral nutrition strategies, and nutritional supplements such as probiotics, glutamine, omega-3 fatty acids, and vitamins in clinical AP, hoping to advance current knowledge and practice related to nutrition and nutritional supplements in clinical management of AP.
Keywords: clinical management of acute pancreatitis, nutritional interventions, probiotics, prebiotics, vitamins, amino acids, omega-3 fatty acids
Introduction
Acute pancreatitis is the leading cause of acute hospital admission for gastrointestinal disorders in many countries, and its incidence continues to raise worldwide (1–3). The annual incidence of AP ranges from 13 to 45 cases per 100,000 population with the global estimate of 33.74 cases per 100,000 population, causing uneven burden across the globe. The health-care cost in the United States is reported to be $2.5 billion (1, 4, 5). Gallstones and alcoholism are the long-established two most common etiological factors, and other risk factors such as genetic predisposition, drugs, smoking, type 2 diabetes, and endoscopic retrograde cholangiopancreatography play a part (1, 3, 6). Clinical manifestations of AP vary from a mild edematous form to severe fulminant pancreatitis with potential devastating complications (7). Severity of AP is stratified into three categories: mild, moderately severe, and severe (Table 1). The overall mortality ranges from 5 to 20% depending on severity (8, 9). In patients who develop severe necrotizing pancreatitis, mortality is approximately 15%. In cases of infection of pancreatic necrosis and multi-organ failure, mortality can be as high as 30% (8). In China, the overall mortality rate of severe AP patients was estimated to be 11.8% (7). Up to date, a major challenge in search of targeted pharmacological therapy specific to AP, despite extensive efforts, is due to heterogeneous etiological factors and varying clinical manifestations associated with this condition (9, 10).
Table 1.
AP classification.
| Classification | Severity | Local complications | Systemic complications |
Reference | ||
|---|---|---|---|---|---|---|
| TOF | POF | EPC | ||||
| Atlanta 2012a | Mild | × | × | × | × | (10, 11) |
| Moderate | √ | √ | × | √ | ||
| Severe | √ | × | √ | √/× | ||
| Determinant basedb | Mild | × | × | × | N/A | |
| Moderate | Sterile | √ | × | N/A | ||
| Severe | Infected | √ | √ | N/A | ||
| Critical | Infected | × | √ | N/A | ||
AP, acute pancreatitis; EPC, exacerbation of preexisting comorbidity; N/A, not applicable; POF, persistent organ failure; TOF, transient organ failure; √, yes; ×, no.
aIn Atlanta 2012, local complications are subcategorized (interstitial edematous, necrotizing pancreatitis, infected necrotizing pancreatitis, other local complications, etc.), whereas systemic complications are defined as TOF or POF or an EPC (organ failure persisting for >48 h; three organ systems = renal, respiratory, cardiovascular; Marshal score ≥2).
bSepsis-related organ failure assessment scoring system is used to define organ failure, and for severe pancreatitis, either POF or infected necrosis is mandatory.
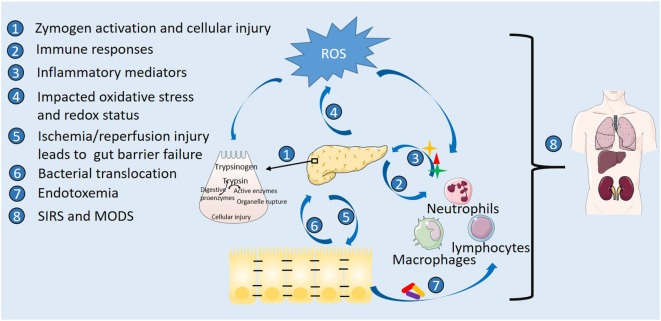
Pathophysiology of AP encompasses complex cascaded events of acinar cell inflammation, involvement of immune system, and systemic pathological outcomes (12) (Figure 1). Premature activation of intra-acinar digestive zymogens is one of the early hallmarks of AP. The resultant autodigestion of pancreas leads to release of pro-inflammatory mediators such as tumor necrosis factor-α, interleukin (IL)-1β, IL-6, which intermingle with microcirculation, causing increased vascular permeability, edema, hemorrhage, and necrosis of pancreas (13–15). Profound acinar cell injury and amplified inflammatory responses give rise to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), ultimately responsible for AP-associated mortality (16–18). The immune system is thought to play an important role in the disease pathogenesis of AP. Complex immunological events underlie progression of AP (12, 19). Dysregulated immune responses during AP include increased leukocyte counts, migration and activation of pro-inflammatory innate immune cells (neutrophils and macrophages) as well as depletion of T-lymphocytes and raised levels of plasma pro-inflammatory cytokines (12). Innate immune cells and derived inflammatory mediators as potential therapeutic targets have thus drawn much attention.
Figure 1.
Pathophysiology of acute pancreatitis highlighting sites of action by nutrition. Etiological stress triggers premature activation of digestive zymogens and intra-acinar cellular injury with accompanying oxidative stress. Involvement of immune cells with released inflammatory mediators and amplified oxidative stress exacerbate the inflammatory cascade. Gut inflammation and barrier failure occur following systemic inflammatory responses, vascular disturbance, and ischemia/reperfusion injury secondary to pancreatic inflammation. Disrupted barrier function further leads to bacterial translocation, pancreatic infection and necrosis, and endotoxemia, ultimately responsible for multiple organ dysfunction syndrome (MODS) and death.
Better understanding of the pathophysiology of AP has drawn research efforts to reestablish the immune and organ/tissue homeostasis in clinical AP and toward the development of new intervention strategies (20). With still obscure disease-specific pharmacological therapies, developing managing strategies from randomized clinical trials are critical in the prevention of systemic complications during severe AP. Nutrition support and intervention is an important part of clinical management of patients with AP (21, 22).
Nutritional Intervention in Clinical AP
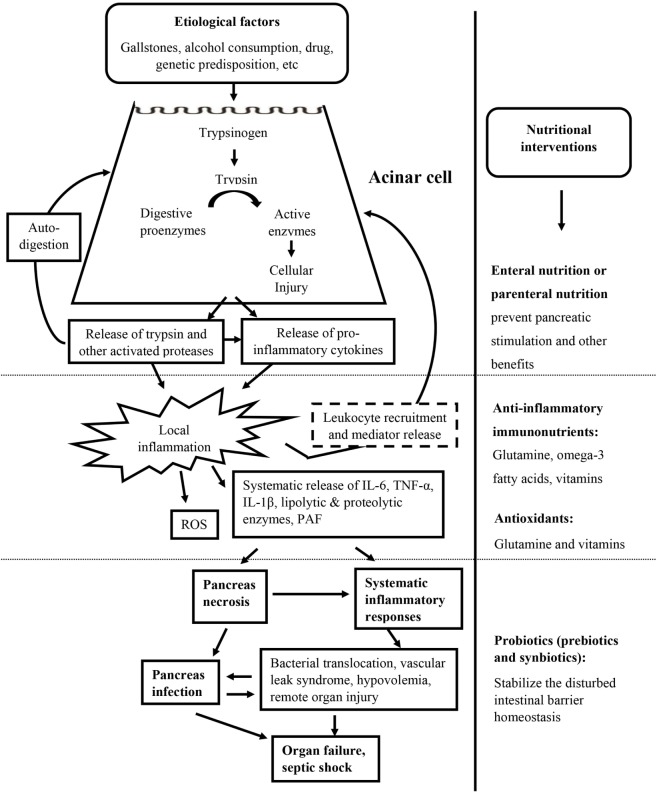
Nutrition and nutritional supplements have demonstrated necessity and importance not only in restoring energy balance but also in maintaining gut barrier function and providing important immunomodulatory and antioxidant effects (Figure 2). The gut is an important secondary organ and also a site of starting severe systemic complications during AP. Intestinal barrier dysfunction is associated with translocation of bacteria and their inflammatory and toxic products, responsible for infection of the necrotic pancreas and systemic inflammatory responses. Therefore, maintaining the integrity of the gut barrier in the small intestine is one of the main goals in early-phase treatment of severe AP (23). Optimal nutritional support in AP has been under debate for decades. Bowl at rest (nothing by mouth) strategy has been implemented conventionally to treat AP (24, 25). However, dietary restrictions exacerbate patient’s malnutrition due to imbalance between reduced food intake and higher nutritional requirements, leading to further catabolism, bacterial translocation (26), and ultimate mortality (27). Evidence of clinical trials has demonstrated parenteral nutrition (PN) in preventing pancreatic stimulation and many benefits of enteral nutrition (EN). However, in daily practice, it remains challenging to predict whether EN will be tolerated in patients with AP (8).
Figure 2.
Targeted nutritional interventions during the whole episode of acute pancreatitis. Targeted nutritional interventions: enteral or parental nutrition and nutritional supplements including anti-inflammatory immunonutrients, antioxidants, and probiotics are presented at the administration stage.
Strategic approaches to include nutritional supplements have also been attempted to provide additional immune regulatory and antioxidative effects. Probiotics and prebiotics have been shown to stabilize the disturbed intestinal barrier homeostasis and be beneficial in reducing the infection rate in primary clinical trials (28–31). Due to the immunosuppressive and inflammatory nature of the disease, immunonutrients like glutamine and omega-3 fatty acids (ω-3 FAs) have been added to parenteral or enteral formulas to modulate immune functions, suppress the hyper inflammatory responses, and reestablish tissue and organ homeostasis in clinical practice (21, 32, 33). Supplements with antioxidative properties like glutamine and vitamin C have also been suggested to provide additional beneficial effects (34).
The review aims to provide a comprehensive chronological review on latest clinical trials on EN versus PN strategies and nutritional supplements including probiotics (prebiotics and synbiotics), glutamine, ω-3 FAs, and vitamins, hoping to provide the basis for future development of nutritional strategies in clinical AP.
EN versus PN
Traditionally, AP patients were maintained on nil per os or nothing per mouth treatment until resolution of pain or normalization of pancreatic enzymes to allow the pancreas to rest (35). Currently, it is widely accepted that early EN may be critical to improve AP-associated malnutrition and the overall outcomes, as bowel rest is associated with intestinal mucosal atrophy and increased infectious complications (9). Gut barrier dysfunction is found in approximately 60% of patients with AP (8, 36). Importantly, EN exerts immunomodulatory effects to preserve gut mucosa integrity, stimulate intestinal motility, and reduce bacterial overgrowth (8, 37). A randomized clinical study demonstrated that immediate oral feeding in patients with mild AP was feasible and safe and accelerated recovery without adverse gastrointestinal events (38). Another randomized controlled trial supported early-stage introduction of initial oral nutrition with either a clear liquid diet or a low-fat solid diet for patients who developed mild AP (39). In these patients, if oral intake is not tolerated, enteral feeding is recommended (9). In patients with severe AP or predicted severe AP, EN with oral or tube feeding thought to preserve the gut barrier function to prevent bacterial translocation is preferred over PN. A multicenter randomized study in the New England Journal of Medicine demonstrated that early tube feeding and oral diet after 72 h are equivalent in reducing infection rates or death in AP patients at high risk for complications (40). A Cochrane meta-analysis of eight randomized controlled studies found that EN reduced mortality, systemic infections, and multiorgan failure among patients with AP as compared to PN (41). Another meta-analysis of 381 patients confirmed the benefit of EN versus PN support in patients with severe AP with lower mortality, fewer infectious complications, decreased organ failure and surgical intervention rate (42). Over the optimal route of EN, several trials have suggested the nasogastric route as an alternative to nasoduodenal or nasojejunal routes (43). Multiple randomized controlled trials involving 157 patients with predicted severe AP demonstrated that nasogastric feeding was safe and well tolerated compared with nasojejunal feeding (44). Given its demonstrated beneficial outcomes, it remains challenging to predict whether EN will be tolerated in patients with AP (8). However, as shown by multiple randomized trials that have associated total PN (TPN) with risks of infection and other complications (35), PN should still be minimized unless the enteral route is not available, not tolerated, or not meeting caloric requirements.
Nutritional Supplements
Probiotics, Prebiotics, and Synbiotics
Changes in intestinal motility and microbiome, immune response, and mucosal barrier function during AP lead to bacterial translocation and subsequent pancreatic necrosis infection, which is one of the principal causes of complications and death in severe AP patients (45). Potential roles of probiotics have been proposed for immunomodulatory and health-promoting benefits to restore the gut integrity, modulate immune responses against invading pathogens, and prevent proliferation of harmful bacteria beyond those of basic nutrition, which have been evaluated in a number of clinical trials (Table 2).
Table 2.
Characteristics of clinical trials on probiotic treatment in AP.
| Reference | Probiotic(s) or prebiotic(s) tested | Comparison groups | Gut barrier permeability |
Systemic complications |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Methods | Results | Infected necrosis | SIRS | MODS | Infection | Mortality | |||
| Olah et al. (46) |
Lactobacillus plantarum 299 plus oat fiber (109 × 2/daily dose) |
EN + symbiotic + fibers versus EN + heat-inactivated symbiotic + fibers | – | – | No difference | No difference | No difference | ↓ pancreatic infection requiring operation in the probiotic arm | No difference |
| Kecskes et al. (47) |
L. plantarum 299 plus oat fiber |
EN + symbiotic + fibers versus EN + heat-inactivated symbiotic + fibers | – | – | ↓ in symbiotic arm | – | – | – | |
| Olah et al. (48) | Multistrain (40 × 109/daily dose) and multifibers | EN + fibers versus EN + fibers + symbiotic | – | – | ↓ in symbiotic arm | ↓ SIRS + MODS in symbiotic arm | ↓ surgical interventions in the probiotic arm | No difference | |
| Qin et al. (49) | L. plantarum (unspecified strain) (1010/daily dose) | TPN versus partial PN + EN + probiotics | Lactulose/rhamnose urinary excretion | ↓ in the probiotic arm | – | ↓ SIRS in the probiotic arm | ↓ MODS in the probiotic arm | ↓ infective complications in the probiotic arm | No difference |
| Karakan et al. (50) | Multifibers | EN + multifibers versus EN | – | – | – | No difference | No difference | – | No difference |
| Besselink et al. (51) | Multistrain product (1010/daily dose) plus maltodextrins and cornstarch | EN + placebo versus EN + probiotics | – | – | No difference | – | ↑ MODS in the probiotic arm | No difference | ↑ in the probiotic arm due to NOMI |
| Besselink et al. (52) | Multistrain product (1010/daily dose) | EN + placebo versus EN + probiotics | PEG urinary excretion | No difference | – | – | – | – | – |
| Sharma et al. (53) | Multistrain product (1010/daily dose) | Placebo versus probiotics (through the current mode of feeding) | Lactulose/rhamnose urinary excretion | No difference | – | – | No difference | ↓ endotoxin core antibody IgG, IgM in the probiotic arm | No difference |
| Cui et al. (54) | Multistrain product 1 × 1011/12 h | PN versus EN versus EN + probiotics (PN) | – | – | ↓ in the EN arm and EN + probiotics arm | – | – | – | No difference |
AP, acute pancreatitis; EN, enteral nutrition; MODS, multiple organ dysfunction syndrome; PN, parenteral nutrition; SIRS, systemic inflammatory response syndrome; TPN, total parenteral nutrition.
An early indication of beneficial effects of synbiotics on severe AP-associated endotoxemia came from a randomized, double-blind clinical trial with 45 patients receiving either live or heat-inactivated Lactobacillus plantarum 299 with oat fiber supplement as early EN. The results suggested that supplementary combined pre- and probiotics was effective in reducing infected pancreatic necrosis and surgical interventions (46, 47). The findings were subsequently supported and extended by a larger study with 62 patients on the Synbiotic 2000 formulated early EN with four different types of prebiotics (inulin, beta-glucan, resistant starch, and pectin) and probiotics (four different Lactobacilli preparations). Patients receiving synbiotic therapy had reduced total incidence of SIRS and lower rates of organ failure, supporting that early EN with synbiotics may prevent organ dysfunctions in the late phase of severe AP (48). The effects of L. plantarum only enteral feeding were evaluated in 76 patients with AP. Overall, the patients with ecoimmunonutrition showed attenuated disease severity, improved intestinal permeability, and better clinical outcomes (49). Prebiotic fiber alone supplementation with EN assessed in a randomized, double-blind study with 30 consecutive severe AP patients was found to shorten hospital stay, duration of nutrition therapy, and reduce the acute phase response and overall complications compared to standard EN therapy (50). Probiotic prophylaxis in severe AP has been contraindicated. The Dutch Acute Pancreatitis Study Group reported in PROPATRIA, a multicenter, randomized, double-blind, placebo-controlled trial with in a total of 200 patients with predicted severe AP that multispecies probiotic (Ecologic 641: six probiotic strains) prophylaxis did not reduce the risk of infectious complications and was associated with an increased risk of mortality (55, 56), although overall this combination of probiotic strains reduced bacterial translocation (52). Following studies involving multispecies probiotic supplementation with EN early abandoned after the publication of PROPATRIA study seemed to support the results that no significant trend was identified for an effect of probiotics on gut permeability or endotoxemia in AP (53, 57), although a positive effect was observed with reduced endotoxin levels (57). Recently, a local study of 70 patients with severe AP comparing PN, EN and EN with addition of the probiotic Bifidobacterium found that early EN with Bifidobacterium resulted in lower levels of pro-inflammatory cytokines, improved gastrointestinal function, reduced complications, and shorter hospital stay in patients with severe AP (54). These data suggest the potential of single specific probiotic strains supplemented, which however should be further evaluated by validated clinical trials before their beneficial effects could be confirmed.
Glutamine
Glutamine is an important constituent of intra and extracellular amino acid pool, with immune modulatory and antioxidant effects, and its depletion has been demonstrated in critical illness (58). Glutamine improves immune cell functions and contributes to antioxidative defenses. It can also support the intestinal integrity and decrease bacterial translocation; hence reduce systemic inflammatory responses and sepsis, which are important in critical illnesses such as AP (33).
An early randomized, controlled study with 28 AP patients received either a standard TPN or an isonitrogen, isocaloric TPN containing 0.3 g/kg l-alanine-l-glutamine demonstrated that glutamine supplementation with TPN was associated with a significant increase of cholinesterase, albumin, and lymphocyte count in AP as well a decrease of C-reactive protein compared to standard TPN. AP patients receiving glutamine was associated with a reduced length of TPN and a trend of reduced length of hospital stay, suggesting that glutamine substitution in TPN is beneficial in patients with AP (59). The effects of glutamine enriched (0.3 g/kg/day) TPN when further evaluated in 40 patients with AP. Beneficial effects of glutamine supplementation to TPN were found on acute pancreatic responses with serum lipase, amylase activities, and C-reactive protein levels decreased and the prevention of complications in patients with AP (59). Later, the effect of parenteral glutamine on recovery from severe AP was more thoroughly investigated in a randomized trial with 44 patients. l-alanyl-l-glutamine-supplemented PN increased serum IL-10 levels, improved nitrogen balance, and decreased infectious morbidity in patients with severe AP (60). Enterally, supplementation of glutamine and arginine in patients diagnosed of AP and predicted to develop a severe course was found to improve gut barrier function by reducing the gut permeability and decreasing plasma endotoxin level in the early stage of severe AP (61). Other than glutamine supplemented with TPN and EN, intravenously administered glutamine with early nasojejunal nutrition was also evaluated. In a randomized study, 45 patients with severe AP received glutamine or normal amino acid solution together with nasojejunal nutrition. The results demonstrated that the glutamine-receiving group showed signs of improvement in all end-point measurements including the rate of pancreas-specific infectious complications, organ failure, length of hospital stay, and mortality rate; and statistical significant difference was noted only in the length of hospital stay (62). Furthermore, a randomized trial compared early versus late intravenous infusion of alanylglutamine dipeptide in 76 patients with severe AP and demonstrated that early-stage intervention achieved a better clinical outcome: shortened duration of hospitalization, reduced rate of infection, organ dysfunction, need for surgery, and mortality, compared to the late treatment (63). More recently, glutamine supplemented in combination with normal saline and hydroxyethyl starch in resuscitation fluids were more efficient in relieving inflammation and sustaining the intestinal barrier in patients with severe AP (64). Two recent meta-analysis studies of randomized controlled trials demonstrated that glutamine supplementation resulted in significantly reduced mortality and complications (65, 66). Further analysis suggested a clear advantage for glutamine supplementation in patients who received TPN. In contrast, patients with AP who received EN did not require glutamine supplementation (65). Finally, oral glutamine supplementation did not seem to confer any significant effect on gut permeability and endotoxemia in severe AP (67). Characteristics of clinical studies on glutamine supplementation included in this review have been summarized in Table 3. Together, while glutamine supplementation with TPN shows promising clinical outcomes, enteral glutamine supplementation needs to be investigated in future.
Table 3.
Characteristics of clinical trials on glutamine as the nutritional supplement in AP.
| Reference | Subjects/regions | Dosage (g/kg BW/day) | Method of assessment | AD-EN or PN interval (h) | Duration of EN or PN (days) | Infectious complication (n/N) | Mortality (n/N) | DOS (median or days mean ± SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | |||||
| Ockenga et al. (59) | 28/Germany | 0.3 | APACHE CT severity index |
<72 | 10–18 | 6–16 | 5/14 | 4/14 | 1/14 | 0/14 | 25 (19–40) | 21 (14–32) |
| Fuentes-Orozco et al. (60) | 44/Mexico | 0.4 | APACHE CT severity index |
24–48 | 17.5 ± 7.9 | 19.31 ± 12.62 | 16/22 | 9/22 | 5/22 | 2/22 | 26.59 ± 13.3 | 30.18 ± 10.42 |
| Huang et al. (61) | 32/China | 0.099 | APACHE | <72 | – | – | 2/18 | 2/14 | 0/18 | 0/14 | 20 ± 5 | 22 ± 5 |
| Hajdu et al. (62) | 45/Hungarian | 0.5 | – | 48 | – | – | – | – | 3/21 | 0/24 | 15.9 | 10.6 |
| Xue et al. (63) | 76/China | 20 g/day/person | APACHE CT severity index |
<24 | – | – | 10/38 | 3/38 | 8/38 | 2/38 | 45.2 ± 27.1 | 28.8 ± 9.4 |
| Singh et al. (67) | 80/India | 20 g/day/person | APACHE CT severity index |
<120 | 7 | 7 | 19/39 | 21/41 | 6/39 | 5/41 | 11 (2–36) | 12 (1–101) |
AD, the interval between admittance to ICU and start of enteral or parenteral nutrition; AP, acute pancreatitis; APACHE, acute physiology and chronic health evaluation; Cont., control; DOS, duration of hospital stay; EN, enteral nutrition; Interv., intervention; PN, parenteral nutrition.
Omega-3 Fatty Acids
Dietary polyunsaturated fatty acids have known immunomodulatory and other beneficial health-promoting effects. A prospective cohort study on the association of fish consumption and non-gallstone-related AP has suggested that total fish (fatty fish and lean fish combined) consumption may be associated with decreased risk of non-gallstone-related AP (68). A randomized prospective clinical trial assessing enteral formula enriched with ω-3 FAs in the treatment of AP suggested that EN supplemented with ω-3 FAs seemed to have clinical benefits based upon the shortened time of jejunal feeding and hospital stay (69). Subsequently, independent studies evaluated the effects of PN with ω-3 FA supplementation on severe AP. Wang et al. compared in a randomized, double-blind trial a total of 40 severe AP patients receiving PN with the same basal nutrients but different lipid compositions: soybean oil-/fish oil-based fat solutions. The study showed that patients with ω-3 FAs-supplemented PN had increased eicosapentaenoic acid concentrations and decreased pro-inflammatory cytokines, together with improved respiratory function and shortened continuous renal replacement therapy time, suggesting attenuated systemic responses to pancreatic and organ injury (70). A parallel study by the same group enrolling 56 patients who received isocaloric and isonitrogenous PN with fats of all ω-6 FAs or 4:1 ω-6:ω-3 FAs demonstrated that ω-3 FAs-supplemented PN elevated the IL-10 level and human leukocyte antigen-DR expression in severe AP patients (71). In accordance, during the initial stage of severe AP, parenteral supplementation with ω-3 fish oil emulsion was found to suppress SIRS, modulate the balance of pro-/anti-inflammatory cytokines and thus improve AP-associated severe conditions (72). Clinical studies on ω-3 FA supplementation have been summarized in Table 4. Although polyunsaturated FAs remain potential beneficial supplements with EN/PN, further larger trials are needed for formulations and confirmatory beneficial clinical effects.
Table 4.
Characteristics of clinical trials on ω-3 FAs as the nutritional supplements in AP.
| Reference | Subjects/regions | Dosage (g/kg BW/day) | Method of assessment | AD-EN or PN interval (h) | Duration of EN or PN (days) | Infectious complication (n/N) | Mortality (n/N) | DOS (days mean ± SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | |||||
| Lasztity et al. (69) | 28/Hungary | 3.3 g/day | APACHE CT severity index |
<24 | 17.57 ± 10.52 | 10.57 ± 6.70 | – | – | 1/14 | 2/14 | 19.28 ± 7.18 | 13.07 ± 6.70 |
| Wang et al. (70) | 40/China | 0.2 | APACHE | <72 | 5 | 5 | 5/20 | 3/20 | 2/20 | 0/20 | 70.5 ± 9.1 | 65.2 ± 7.3 |
| Wang et al. (71) | 28/China | 0.2 | APACHE CT severity index |
<72 | 5 | 5 | 9/28 | 6/28 | 2/28 | 0/28 | – | – |
AD, the interval between admittance to ICU and start of enteral or parenteral nutrition; AP, acute pancreatitis; APACHE, acute physiology and chronic health evaluation; Cont., control; DOS, duration of hospital stay; EN, enteral nutrition; Interv., intervention; PN, parenteral nutrition; ω-3 FAs, omega-3 fatty acids.
Vitamins
Oxidative stress is involved in the onset of AP and also in the development of the systemic inflammatory responses, being glutathione depletion, xanthine oxidase activation, and thiol oxidation in proteins critical features of the disease in the pancreas. Vitamins as important immunonutrients and antioxidants have been inversely associated with AP (73). Plasma concentrations of vitamin A and vitamin C were found significantly lower in AP patients than in healthy controls (P < 0.05) (74). Recently, vitamin D, mainly from the milk products, has been inversely associated with gallstone-related AP (73). Vitamin supplementation assessed in combination with other antioxidants or in vitamin-only therapy has been evaluated earlier and yielded mixed outcomes. A multicenter randomized, double-blind, placebo clinical trial by Siriwardena et al. concluded that use of intravenous combination antioxidant therapy containing vitamin C (N-acetylcysteine, selenium, vitamin C) was not justified to continue in clinical severe AP (75). Subsequently, another group comparing vitamin C, N-acetylcysteine, antoxyl forte antioxidant combination with standard medical treatment in early AP patients suggested that antioxidant supplementation could decrease the length of hospital stay and complications in patients with early AP, but this hypothesis needed to be supported by a larger clinical trial (76). With respect of vitamin-only antioxidant therapies, a study involving 84 AP patients and 40 healthy subjects in China on high-dose vitamin C has demonstrated that it has therapeutic efficacy on the disease and proposed the potential mechanisms to be promoting anti-oxidizing capability in patients, blocking lipid peroxidation and improving cellular immune function (77). In contrast, multiple vitamins-based antioxidant therapy (vitamin A, vitamin C, and vitamin E) in a single-center randomized study involving 39 patients has not been proven beneficial in patients with established severe AP (78). Collectively, data so far on vitamin therapy in AP (Table 5) have been mixed and should be carefully evaluated for dosing and timing of intervention for potential promising outcomes in clinical use.
Table 5.
Characteristics of clinical trials on vitamins as the nutritional supplements in AP.
| Reference | Subjects/region | Vitamin(s) tested | Dosage (g/kg BW/day) | Method of assessment | Duration of EN or PN (days) | Mortality (n/N) | DOS (days mean ± SD) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | |||||
| Siriwardena et al. (75) | 43/UK | Vitamin C + N-acetylcysteine, selenium | For vitamin C, 2 g/day for 2 days, 1 g/day (continued for up to day 7) | APACHE | 7 | 7 | 0/21 | 4/22 | 14.3 (15.7) | 20.4 (24.4) |
| Sateesh et al.(76) | 53/India | Vitamin C, N-acetyl cysteine, and antoxyl forte | Vitamin C 500 mg, N-acetyl cysteine 200 mg 8 hourly and antoxyl forte 1 capsule hourly | APACHE CT severity index |
– | – | 0/30 | 1/23 | 10.3 ± 7 | 7.2 ± 5 |
| Du et al. (77) | 84/China | Vitamin C | 10 or 1 g/day (con) | Detection of clinical, biochemical, and immunological markers | 5 | 5 | – | – | 13.45 ± 3.21 | 9.34 ± 4.24 |
| Bansal et al. (78) | 39/India | Vitamin A, vitamin E, vitamin C | Vitamin C (1,000 mg in 100 ml saline), vitamin E (200 mg oral), and vitamin A (10,000 IU) | APACHE CT severity index |
14 | 14 | 2/20 | 0/19 | 15.1 ± 5.43 | 12.8 ± 3.9 |
AP, acute pancreatitis; APACHE, acute physiology and chronic health evaluation; Cont., control; DOS, duration of hospital stay; EN, enteral nutrition; Interv., intervention; PN, parenteral nutrition.
Conclusion and Future Perspectives
In most patients, an oral soft or solid diet can be beneficial if tolerated. When oral feeding is not tolerated for a few days, enteral feeding through a nasogastric or nasojejunal feeding tube should be attempted within the first 72 h of administration. PN should be minimized for its risks of infection and other complications. Only if enteral route is not available or tolerated, PN may be considered. Overall, nutritional support plays a critical role in clinical management of severe AP, although the optimal timing remains unclear. Predicting the nutritional tolerance of patients with AP remains challenging as the current evaluation system needs to be improved. Various nutritional supplement(s) together with PN or EN with currently mixed clinical outcomes is a subject of interest for future evaluation and may lead to promising outcomes. In addition, given its heterogeneous etiological factors and varying clinical manifestations, precision medicine, although not much applied in the condition, remains as a temping approach to optimize clinical outcomes on classified individuals based on susceptibility to the condition and its systemic complications.
Author Contributions
JS designed the subject content of the review article. L-LP, JL, MS, and JS conducted initial search of literature, drafted the manuscript, and prepared the figures and tables. MB gave the constructive comments and critically reviewed the manuscript. JS had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Prof. Yi Miao from Pancreas Center, The First Affiliated Hospital of Nanjing Medical University, for critical reading of the manuscript and useful suggestions.
Footnotes
Funding. The work was supported by funds from the National Natural Science Foundation of China (Grant nos: 91642114, 31570915, 81573420, and 31400779), Key Program of Fundamental Research Funds for the Central Universities (Grant no: JUSRP51613A), free exploration funding from State Key Laboratory of Food Science and Technology (SKLF-ZZB-201702), “Zhuo Xue” Talent Plan of Fudan University, and Royal Society of New Zealand’s “Catalyst: Leaders New Zealand-China Scientist Exchange Programme.”
Abbreviations
AP, acute pancreatitis; ERCP, endoscopic retrograde cholangiopancreatography; SIRS, systemic inflammatory response syndrome; MODS, multiple organ dysfunction syndrome; ω-3 FAs, omega-3 fatty acids; PN, parenteral nutrition; EN, enteral nutrition; TPN, total parenteral nutrition.
References
- 1.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet (2015) 386:85–96. 10.1016/S0140-6736(14)60649-8 [DOI] [PubMed] [Google Scholar]
- 2.Forsmark CE, Swaroop Vege S, Wilcox CM. Acute pancreatitis. N Engl J Med (2016) 375:1972–81. 10.1056/NEJMra1505202 [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology (2013) 144:1252–61. 10.1053/j.gastro.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology (2015) 149:1731–41.e3. 10.1053/j.gastro.2015.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol (2016) 1:45–55. 10.1016/S2468-1253(16)30004-8 [DOI] [PubMed] [Google Scholar]
- 6.Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, et al. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology (2009) 9:601–14. 10.1159/000212097 [DOI] [PubMed] [Google Scholar]
- 7.Bai Y, Liu Y, Jia L, Jiang H, Ji M, Lv N, et al. Severe acute pancreatitis in China: etiology and mortality in 1976 patients. Pancreas (2007) 35:232–7. 10.1097/MPA.0b013e3180654d20 [DOI] [PubMed] [Google Scholar]
- 8.Lodewijkx PJ, Besselink MG, Witteman BJ, Schepers NJ, Gooszen HG, van Santvoort HC, et al. Nutrition in acute pancreatitis: a critical review. Expert Rev Gastroenterol Hepatol (2016) 10:571–80. 10.1586/17474124.2016.1141048 [DOI] [PubMed] [Google Scholar]
- 9.Janisch N, Gardner T. Recent advances in managing acute pancreatitis. F1000Res (2015) 4:F1000 Faculty Rev–1474. 10.12688/f1000research.7172.1 [DOI] [Google Scholar]
- 10.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut (2013) 62:102–11. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 11.Dellinger EP, Forsmark CE, Layer P, Levy P, Maravi-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity an international multidisciplinary consultation. Ann Surg (2012) 256:875–80. 10.1097/SLA.0b013e318256f778 [DOI] [PubMed] [Google Scholar]
- 12.Shamoon M, Deng Y, Chen YQ, Bhatia M, Sun J. Therapeutic implications of innate immune system in acute pancreatitis. Expert Opin Ther Targets (2016) 20:73–87. 10.1517/14728222.2015.1077227 [DOI] [PubMed] [Google Scholar]
- 13.Ramnath RD, Sun J, Bhatia M. PKC delta mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med (2010) 88:1055–63. 10.1007/s00109-010-0647-9 [DOI] [PubMed] [Google Scholar]
- 14.Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs (2001) 2:496–501. [PubMed] [Google Scholar]
- 15.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy (2002) 1:343–51. 10.2174/1568010023344517 [DOI] [PubMed] [Google Scholar]
- 16.Bhatia M. Acute pancreatitis as a model of SIRS. Front Biosci (2009) 14:2042–50. 10.2741/3362 [DOI] [PubMed] [Google Scholar]
- 17.McKay CJ, Buter A. Natural history of organ failure in acute pancreatitis. Pancreatology (2003) 3:111–4. 10.1159/000070078 [DOI] [PubMed] [Google Scholar]
- 18.Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep (2004) 6:99–103. 10.1007/s11894-004-0035-0 [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology (2013) 144:1230–40. 10.1053/j.gastro.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol (2013) 29:523–30. 10.1097/MOG.0b013e328363e399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson A, Subramaniam K, Davies A. Nutritional therapy in acute pancreatitis – time to take stock. Nutrition (2012) 28:731–2. 10.1016/j.nut.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 22.DiMagno MJ. Clinical update on fluid therapy and nutritional support in acute pancreatitis. Pancreatology (2015) 15:583–8. 10.1016/j.pan.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, et al. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol (2012) 46(Suppl):S46–51. 10.1097/MCG.0b013e3182652096 [DOI] [PubMed] [Google Scholar]
- 24.Singh VK, Moran RA, Afghani E, de-Madaria E. Treating acute pancreatitis: what’s new? Expert Rev Gastroenterol Hepatol (2015) 9:901–11. 10.1586/17474124.2015.1048225 [DOI] [PubMed] [Google Scholar]
- 25.Janisch NH, Gardner TB. Advances in management of acute pancreatitis. Gastroenterol Clin North Am (2016) 45:1–8. 10.1016/j.gtc.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg (2010) 200:111–7. 10.1016/j.amjsurg.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 27.Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis a meta-analysis of randomized trials. Arch Surg (2008) 143:1111–7. 10.1001/archsurg.143.11.1111 [DOI] [PubMed] [Google Scholar]
- 28.Muftuoglu MA, Isikgor S, Tosun S, Saglam A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutr (2006) 60:464–8. 10.1038/sj.ejcn.1602338 [DOI] [PubMed] [Google Scholar]
- 29.Karen M, Yuksel O, Akyurek N, Ofluoglu E, Caglar K, Sahin TT, et al. Probiotic agent Saccharomyces boulardii reduces the incidence of lung injury in acute necrotizing pancreatitis induced rats. J Surg Res (2010) 160:139–44. 10.1016/j.jss.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 30.Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients a meta-analysis of randomized controlled trials. Chest (2013) 143:646–55. 10.1378/chest.12-1745 [DOI] [PubMed] [Google Scholar]
- 31.Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Crit Care (2014) 18:R57. 10.1186/cc13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei QC, Wang XY, Xia XF, Zheng HZ, Bi JC, Tian F, et al. The role of omega-3 fatty acids in acute pancreatitis: a meta-analysis of randomized controlled trials. Nutrients (2015) 7:2261–73. 10.3390/nu7042261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jafari T, Feizi A, Askari G, Fallah AA. Parenteral immunonutrition in patients with acute pancreatitis: a systematic review and meta-analysis. Clin Nutr (2015) 34:35–43. 10.1016/j.clnu.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 34.Perez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol (2015) 5:1–14. 10.1016/j.redox.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology Practice guidelines in acute pancreatitis. Am J Gastroenterol (2006) 101:2379–400. 10.1111/j.1572-0241.2006.00856.x [DOI] [PubMed] [Google Scholar]
- 36.Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg (2014) 101:1644–56. 10.1002/bjs.9665 [DOI] [PubMed] [Google Scholar]
- 37.Hermsen JL, Sano Y, Kudsk KA. Food fight! Parenteral nutrition, enteral stimulation and gut-derived mucosal immunity. Langenbecks Arch Surg (2009) 394:17–30. 10.1007/s00423-008-0339-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckerwall GE, Tingstedt BBA, Bergenzalun PE, Andersson RG. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery – a randomized clinical study. Clin Nutr (2007) 26:758–63. 10.1016/j.clnu.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 39.Jacobson BC, Vander Vliet MB, Hughes MD, Maurer R, McManus K, Banks PA. A prospective, randomized trial of clear liquids versus low-fat solid diet as the initial meal in mild acute pancreatitis. Clin Gastroenterol Hepatol (2007) 5:946–51; quiz 886. 10.1016/j.cgh.2007.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med (2014) 371:1983–93. 10.1056/NEJMoa1404393 [DOI] [PubMed] [Google Scholar]
- 41.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev (2010) 1:CD002837. 10.1002/14651858.CD002837.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, et al. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med (2012) 51:523–30. 10.2169/internalmedicine.51.6685 [DOI] [PubMed] [Google Scholar]
- 43.Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology (2013) 144:1272–81. 10.1053/j.gastro.2013.01.075 [DOI] [PubMed] [Google Scholar]
- 44.Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta-analysis. Crit Care (2013) 17:R118. 10.1186/cc12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg (2003) 10:415–8. 10.1007/s00534-002-0727-5 [DOI] [PubMed] [Google Scholar]
- 46.Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific Lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg (2002) 89:1103–7. 10.1046/j.1365-2168.2002.02189.x [DOI] [PubMed] [Google Scholar]
- 47.Kecskes G, Belagyi T, Olah A. [Early jejunal nutrition with combined pre- and probiotics in acute pancreatitis – prospective, randomized, double-blind investigations]. Magy Seb (2003) 56:3–8. [PubMed] [Google Scholar]
- 48.Olah A, Belagyi T, Poto L, Romics L, Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology (2007) 54:590–4. [PubMed] [Google Scholar]
- 49.Qin HL, Zheng JJ, Tong DN, Chen WX, Fan XB, Hang XM, et al. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr (2008) 62:923–30. 10.1038/sj.ejcn.1602792 [DOI] [PubMed] [Google Scholar]
- 50.Karakan T, Ergun M, Dogan I, Cindoruk M, Unal S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: a prospective randomized double-blind study. World J Gastroenterol (2007) 13:2733–7. 10.3748/wjg.v13.i19.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. [Probiotic prophylaxis in patients with predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial]. Ned Tijdschr Geneeskd (2008) 152:685–96. [PubMed] [Google Scholar]
- 52.Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg (2009) 250:712–9. 10.1097/SLA.0b013e3181bce5bd [DOI] [PubMed] [Google Scholar]
- 53.Sharma B, Srivastava S, Singh N, Sachdev V, Kapur S, Saraya A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: a double-blind randomized controlled trial. J Clin Gastroenterol (2011) 45:442–8. 10.1097/MCG.0b013e318201f9e2 [DOI] [PubMed] [Google Scholar]
- 54.Cui LH, Wang XH, Peng LH, Yu L, Yang YS. [The effects of early enteral nutrition with addition of probiotics on the prognosis of patients suffering from severe acute pancreatitis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue (2013) 25:224–8. 10.3760/cma.j.issn.2095-4352.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 55.Besselink MG, Timmerman HM, Buskens E, Nieuwenhuijs VB, Akkermans LM, Gooszen HG, et al. Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]. BMC Surg (2004) 4:12. 10.1186/1471-2482-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet (2008) 371:651–9. 10.1016/S0140-6736(08)60207-X [DOI] [PubMed] [Google Scholar]
- 57.Lata J, Jurankova J, Stiburek O, Pribramska V, Senkyrik M, Vanasek T. [Probiotics in acute pancreatitis – a randomised, placebo-controlled, double-blind study]. Vnitr Lek (2010) 56:111–4. [PubMed] [Google Scholar]
- 58.Monfared SSMS, Vahidi H, Abdolghaffari AH, Nikfar S, Abdollahi M. Antioxidant therapy in the management of acute, chronic and post-ERCP pancreatitis: a systematic review. World J Gastroenterol (2009) 15:4481–90. 10.3748/wjg.15.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr (2002) 21:409–16. 10.1054/clnu.2002.0569 [DOI] [PubMed] [Google Scholar]
- 60.Fuentes-Orozco C, Cervantes-Guevara G, Mucino-Hernandez I, Lopez-Ortega A, Ambriz-Gonzalez G, Gutierrez-de-la-Rosa JL, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. JPEN J Parenter Enteral Nutr (2008) 32:403–11. 10.1177/0148607108319797 [DOI] [PubMed] [Google Scholar]
- 61.Huang XX, Wang XP, Ma JJ, Jing DD, Wang PW, Wu K. [Effects of enteral nutrition supplemented with glutamine and arginine on gut barrier in patients with severe acute pancreatitis: a prospective randomized controlled trial]. Zhonghua Yi Xue Za Zhi (2008) 88:2407–9. 10.3321/j.issn:0376-2491.2008.34.009 [DOI] [PubMed] [Google Scholar]
- 62.Hajdu N, Belagyi T, Issekutz A, Bartek P, Gartner B, Olah A. [Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis – a prospective randomized clinical study]. Magy Seb (2012) 65:44–51. 10.1556/MaSeb.65.2012.2.2 [DOI] [PubMed] [Google Scholar]
- 63.Xue P, Deng LH, Xia Q, Zhang ZD, Hu WM, Yang XN, et al. Impact of alanyl-glutamine dipeptide on severe acute pancreatitis in early stage. World J Gastroenterol (2008) 14:474–8. 10.3748/wjg.14.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao G, Zhang JG, Wu HS, Tao J, Qin Q, Deng SC, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol (2013) 19:2044–52. 10.3748/wjg.v19.i13.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asrani V, Chang WK, Dong Z, Hardy G, Windsor JA, Petrov MS. Glutamine supplementation in acute pancreatitis: a meta-analysis of randomized controlled trials. Pancreatology (2013) 13:468–74. 10.1016/j.pan.2013.07.282 [DOI] [PubMed] [Google Scholar]
- 66.Jeurnink SM, Nijs MM, Prins HA, Greving JP, Siersema PD. Antioxidants as a treatment for acute pancreatitis: a meta-analysis. Pancreatology (2015) 15:203–8. 10.1016/j.pan.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 67.Singh N, Mishra SK, Sachdev V, Sharma H, Upadhyay AD, Arora I, et al. Effect of oral glutamine supplementation on gut permeability and endotoxemia in patients with severe acute pancreatitis: a randomized controlled trial. Pancreas (2014) 43:867–73. 10.1097/MPA.0000000000000124 [DOI] [PubMed] [Google Scholar]
- 68.Oskarsson V, Orsini N, Sadr-Azodi O, Wolk A. Fish consumption and risk of non-gallstone-related acute pancreatitis: a prospective cohort study. Am J Clin Nutr (2015) 101:72–8. 10.3945/ajcn.113.076174 [DOI] [PubMed] [Google Scholar]
- 69.Lasztity N, Hamvas J, Biro L, Nemeth E, Marosvolgyi T, Decsi T, et al. Effect of enterally administered n-3 polyunsaturated fatty acids in acute pancreatitis – a prospective randomized clinical trial. Clin Nutr (2005) 24:198–205. 10.1016/j.clnu.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Li W, Li N, Li J. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr (2008) 32:236–41. 10.1177/0148607108316189 [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: a randomized controlled trial. Inflammation (2009) 32:304–9. 10.1007/s10753-009-9136-0 [DOI] [PubMed] [Google Scholar]
- 72.Xiong J, Zhu S, Zhou Y, Wu H, Wang C. Regulation of omega-3 fish oil emulsion on the SIRS during the initial stage of severe acute pancreatitis. J Huazhong Univ Sci Technolog Med Sci (2009) 29:35–8. 10.1007/s11596-009-0107-3 [DOI] [PubMed] [Google Scholar]
- 73.Setiawan VW, Pandol SJ, Porcel J, Wei PC, Wilkens LR, Le Marchand L, et al. Dietary factors reduce risk of acute pancreatitis in a large multiethnic cohort. Clin Gastroenterol Hepatol (2017) 15(2):257–65.e3. 10.1016/j.cgh.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musil F, Zadak Z, Solichova D, Hyspler R, Kaska M, Sobotka L, et al. Dynamics of antioxidants in patients with acute pancreatitis and in patients operated for colorectal cancer: a clinical study. Nutrition (2005) 21:118–24. 10.1016/j.nut.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 75.Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut (2007) 56:1439–44. 10.1136/gut.2006.115873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sateesh J, Bhardwaj P, Singh N, Saraya A. Effect of antioxidant therapy on hospital stay and complications in patients with early acute pancreatitis: a randomised controlled trial. Trop Gastroenterol (2009) 30:201–6. [PubMed] [Google Scholar]
- 77.Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ, Shen DM, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol (2003) 9:2565–9. 10.3748/wjg.v9.i11.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bansal D, Bhalla A, Bhasin DK, Pandhi P, Sharma N, Rana S, et al. Safety and efficacy of vitamin-based antioxidant therapy in patients with severe acute pancreatitis: a randomized controlled trial. Saudi J Gastroenterol (2011) 17:174–9. 10.4103/1319-3767.80379 [DOI] [PMC free article] [PubMed] [Google Scholar]