Abstract
Introduction
Our aim was to examine the frequency of various electrographic patterns including periodic discharges (PD), repetitive spike waves (RSW), rhythmic delta activities (RDA), nonconvulsive seizures (NCS) and nonconvulsive status epilepticus (NCSE) in continuous EEG monitoring (cEEG) of the critically ill patients with change of consciousness and the presence of specific clinical and laboratory findings associated with these important patterns in this study.
Methods
Patients with changes of consciousness in the neurological intensive care unit (NICU) were consecutively monitored with cEEG during 2 years. Their clinical, electrophysiological, radiological and laboratory findings were evaluated retrospectively.
Results
This sample consisted of 57 (25 men) patients with a mean age of 68.2 years. Mean duration of cEEG monitoring was 2532.6 minutes. The most common electrographic patterns were PD (33%) and NCS-NCSE (26.3%). The presence of NCS-NCSE was significantly associated with PD (57.9%, p<0.001). PD and NCS-NCSE were the mostly seen in patients with acute stroke and hypoxic encephalopathy. Duration of monitoring was significantly longer in the group with PD and NCS-NCSE (p:0.004, p:0.014). Detection of any electrographic pattern in EEG before monitoring was associated with the presence of any pattern in cEEG (59.3%, p<0.0001). Convulsive or nonconvulsive seizure during monitoring was common in patients with electrographic patterns (p<0.0001). 66.7% of NCS-NCSE was seen within the first 12 hours and 26.7% was seen within the 12–24 hours of the monitoring.
Conclusion
Detection of any electrographic pattern in EEG before monitoring was associated with the presence of any important pattern in cEEG monitoring. This association suggest that at least 24 hours-monitoring of these patients could be useful for the diagnosis of clinical and/or electrographic seizures.
Keywords: Intensive care unit, EEG, monitorization
INTRODUCTION
There is no consensus on the terminology for rhythmic and periodic patterns (RPP) detected by continuous electroencephalography (cEEG) monitoring of unconscious patients in the neurological intensive care unit (NICU) (1). The growth in cEEG utilization has led to an increase in the recognition of these EEG patterns of uncertain diagnostic and prognostic information. Furthermore, there is controversy regarding which electrographic patterns are associated with neuronal injury, which require treatment, and how aggressively to treat these patterns (2,3).
We aimed to examine the frequency of some electroencephalographic patterns including periodic discharges (PD), repetitive spike waves (RSW), rhythmic delta activities (RDA), nonconvulsive seizures (NCS), and nonconvulsive status epilepticus (NCSE) recorded by cEEG in critically ill patients with a change of consciousness. This study was also aimed to detect clinical and laboratory correlates of these patterns.
METHODS
We retrospectively identified all critically ill patients over the age of 18 years with a change of consciousness (Glasgow Coma Score [GCS] ≤14) who underwent cEEG monitoring in the NICU during two years. Clinical information was gathered from a review of inpatient medical notes, neuroimaging studies and reports, and discharge summaries. Baseline demographic data (age, gender), past medical history, duration of hospitalization in the NICU, clinical seizures at admission, in the emergency department, or during hospitalization prior to cEEG; treatment with continuous IV or intermittent antiepileptic drugs (AED) and other treatments such as antibiotics, sedatives and anesthetics, all radiology and laboratory findings were investigated. Premonitoring and postmonitoring scores including GCS, modified Rankin Scale score of the patients, and also National Institutes of Health Stroke Scale score for those with acute ischemic stroke were recorded. The presence of an acute or chronic ischemic, hemorrhagic lesion and vascular occlusion detected in the neuroimaging of the patients that were performed before, and if any, during the monitoring were also recorded.
During cEEG monitoring, the presence of an infection, the use of antibiotics, the presence of respiratory disease (noninvasive ventilator support or the presence of an orotracheal intubation) were recorded. Presence of hypertension (blood pressure >130/80 mmHg), hypotension (blood pressure systolic <90 mmHg, mean blood pressure <60 mmHg), tachycardia (heart rate >100 bpm), bradycardia (heart rate <40/minutes), hyperthermia (body temperature >37°C), and hypothermia (body temperature <35°C) were also noted. The arterial blood gas (hypoxia PaO2 <60 mmHg, hypocapnia PaCO2 <30 mmHg, hypercapnia PaCO2 >45 mmHg), blood glucose (hypoglycemia <50 mg/dL, hyperglycemia >180 mg/dL), hypernatremia-hyponatremia (Na 136-145 meq/L), hypokalemia-hyperkalemia (K 3.3–5.1 meq/L), hypomagnesemia (1.6–2.40 mg/dL), hypocalcemia-hypercalcemia (8.8–10.2 mg/dL), hyperuricemia (2.4–5.7 mg/dL), hyperammonemia (19–60 mg/dL), increased AST-ALT level (AST >40 U/L, ALT >41 U/L) were also recorded.
The local ethics committee approved this study.
cEEG Monitoring Protocol and Analysis
cEEG was recorded using 21 MR-compatible silver/silver chloride electrodes, affixed to the scalp according to the International 10–20 System by a certified EEG technologist. Electrodes were checked by both EEG technologist and the intensive care unit health personnel twice a day. Since MR-compatible electrodes were used, these electrodes were not removed when an MR-imaging control needed to be done. Prior to monitoring, a short EEG recording was done for 30 minutes.
Short-term EEG recording and cEEG monitoring were independently interpreted by two experienced electroencephalographers (one of them was blindfolded for clinical findings), according to the 2012 version of the American Clinical Neurophysiology Society’s standardized critical care terminology (1). Any disagreements between the electroencephalographers were resolved by a consensus meeting after reviews, and the interrater variability in the assessment of EEGs was excellent with a kappa index value of 0.81.
Posterior dominant rhythm, reactivity, variability, stage N2 sleep transients, epileptiform discharges, PD (PLEDs, GPEDs, bilateral independent periodic discharges [BIPLEDs], stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs), RDA and RSW were noted both at during initial 30-minutes screening baseline EEG and subsequent cEEG recordings. Seizures were documented as convulsive (CS), such as tonic-clonic, clonic, twitching, jerking, and other synonyms, or nonconvulsive. NCSs were considered even if subtle movements (facial twitching, eye deviation) were observed on video or noted clinically. Electrographic patterns were only considered ictal if they showed clear evolution in frequency, location, or morphology. Status epilepticus (SE) was reported as convulsive (CSE) for CS lasting more than five minutes or if two or more occurred without a return to baseline neurological function in between, or nonconvulsive SE for continuous ictal-appearing patterns lasting >30 minutes or ictal patterns present more than 50% of one hour of EEG recording.
According to the 2012 version of American Clinical Neurophysiology Society (ACNS), RPP was defined as follows:
Periodic discharge (PD)
This is a waveform with relatively uniform morphology and duration with a quantifiable regular interval between consecutive waves. A pattern was considered to be periodic and rhythmic if it continued at least throughout six cycles (1/sec for six seconds or 6/sec for one second).
Generalized periodic epileptiform discharge (GPED)
The term “generalized” refers to any bilateral, bisynchronous, and symmetric pattern.
Periodic lateralized epileptiform discharge (PLED)
Lateralized means that unilateral and bilateral synchronous but asymmetric; includes focal, regional, and hemispheric patterns.
Bilateral independent periodic lateralized epileptiform discharge (BiPLED)
“Bilateral independent” refers to the presence of two independent, asynchronous, lateralized patterns, one in each hemisphere.
Rhythmic delta activity (RDA)
This is a waveform with relatively uniform morphology and duration and without an interval between consecutive waveforms at delta frequency.
Repetitive spike-wave activity (RSW)
Polyspike, spike, or sharp wave consistently followed by a slow wave in a regularly repeating and alternating pattern, with a consistent relationship between the spike component and slow wave; and with no interval between one spike-complex and the next.
Triphasic wave (TW)
This is an EEG pattern consisting of medium or high-amplitude discharges (100–300 μV), with generally at 1.5–2.5 Hz frequency, mainly prominent on the anterior regions of the hemispheres that is characterized by a low-amplitude negative component at the outset, and afterwards, a high-amplitude positive component, and then, a final negative phase showing a low-amplitude gradual increase, morphologically.
After the electrographic patterns were identified, the prevalence and predictive factors of these patterns including clinical, radiological, and laboratory findings were investigated. The timing of CS and NCS during monitoring and RPP showing association with these seizures were also investigated.
Statistical Analysis
Data were analyzed using statistical software Statistical Package for the Social Sciences (version 20.0, IBM Corp.; Armonk, NY, USA). The patients were divided in two subgroups according to the presence of rhythmic periodic patterns. The comparison of the subgroups was done with the chi-square test for parametric data and with the Mann-Whitney U test for nonparametric data. p<0.05 were considered significant.
RESULTS
Clinical Findings
Fifty-seven patients (25 male, 32 female) followed up in the NICU due to a change of consciousness were enrolled in the study. The mean age of the patients was 68.2 ± 15.3 years. The mean cEEG monitoring period was 2532.6±2908.6 minutes (minimum: 62 minutes; maximum: 15.731 minutes). The simultaneous video recording could be performed in 33 patients (57.8%) with a mean duration of 2018.7±1876.1 minutes. The etiologies of the patients, clinical findings, and duration of hospitalization in the NICU are summarized in Table 1.
Table 1.
Comparison of the demographic and clinical findings of the patients according to presence of rhythmic periodic pattern (RPP), n
| Characteristics | RPP (+) patients n=25 |
RPP (−) patients n=32 |
Total n=57 |
p* |
|---|---|---|---|---|
| Age (mean±SD | 72.1±10.8 | 65.3±17.8 | 68.2±15.3 | NS |
| Gender (F, M) | 17 F, 8 M | 15 F, 17 M | 32 F, 25 M | NS |
| Past medical history | ||||
| Major medical problem** | 20 | 14 | 34 | p:0.003 |
| Stroke | 6 | 7 | 13 | NS |
| Previous brain surgery | 2 | 2 | 4 | NS |
| Epilepsy | 4 | 2 | 6 | NS |
| Diagnosis | ||||
| Acute brain damage | 13 | 17 | 30 | |
| Ischemic Stroke | 10 | 9 | 19 | NS |
| Intracerebral hematoma | 1*** | 3 | 4 | NS |
| Subarachnoid hemorrhage | - | 1 | 1 | NS |
| Traumatic brain damage | - | 4 | 4 | NS |
| MSS infection | 2 | - | 2 | NS |
| Acute systemic disease | 10 | 10 | 20 | NS |
| Hypoxic anoxic encephalopathy | 2 | 7 | 9 | NS |
| Toxic metabolic | 3 | 3 | 6 | NS |
| Sepsis | 1 | - | 1 | NS |
| Idiopathic | 4 | - | 4 | |
| Epilepsy | 2 | 5 | 7 | NS |
| Post-stroke | 2 | - | 2 | NS |
| Post-encephalopathic | - | 1 | 1 | NS |
| Brain tumor | - | 2 | 2 | NS |
| Idiopathic | - | 2 | 2 | NS |
| Duration of hospitalization in the NICU (days | 12.6±23 | 16.4±27.7 | 14.8±25.6 | NS |
F: female; M: male; CNS: central nervous system; NS: not significant; NICU: neurological intensive care unit; SD: standard deviation
p<0.05 was accepted as significant
The major medical problem was defined as the presence of at least two chronic systemic diseases, such as hospitalization and/or treatment-requiring diabetes mellitus, congestive heart failure, peripheral arterial disease, renal failure, or transplantation. It was found to be significantly high in the patient population in which RPP was detected
This patient, who presented with intracerebral hemorrhage, was diagnosed as amyloid angiopathy proven by pathologically
EEG Analysis
The EEG data of the patients was analyzed under two separate headings as “pre-monitoring” (the first 30-minute recording) and “cEEG monitoring.”
Analysis of pre-monitoring EEG
PD was detected most frequently (22.8%). The detection of any of the investigated electrographic patterns in EEG before monitoring was associated with the presence of any pattern in cEEG (59.3%, p<0.0001), but other findings of short EEG recording did not predict the presence of RPP during monitoring. The detailed evaluation of pre-monitoring EEG findings is summarized in Table 2.
Table 2.
Short EEG findings prior to cEEG monitoring
| Frequencies of RPP and NCS-NCSE | N | % | |
|---|---|---|---|
| PD | 13 | 22.8 | |
| RSW | 1 | 1.8 | |
| RDA | 1 | 1.8 | |
| NCS-NCSE | 4 | 7 | |
| Clinical seizures | 3 | 5.3 | |
| Findings of background activity | N | % | |
| Symmetry | Symmetrical | 38 | 66.7 |
| Asymmetrical | 19 | 33.3 | |
| Frequency | Theta | *47 | 82.5 |
| Delta | 9 | 15.8 | |
| Alpha | 8 | 14 | |
| Suppression | Symmetrical | 6 | 10.5 |
| Asymmetrical | 8 | 14 | |
| Rapid rhythms | 8 | 14 | |
| Nonspecific slow wave activity | 21 | 36.8 | |
| Focal epileptiform anomaly | 2 | 3.5 | |
NCS: nonconvulsive seizures; NCSE: nonconvulsive status epilepticus; PD: periodic discharge; RPP: rhythmic and periodic pattern; RDA: rhythmic delta activity; RSW: rhythmic spike waves
In this group, four patients had also alpha activities, and three patients had also delta activities
Analysis of cEEG monitoring
The frequency of electrographic patterns including PD, RSW, RDA, NCS, and NCSE was 47.4%. The most common electrographic patterns were PD (33%) and NCS-NCSE (26.3%). Furthermore, five patients had RDA (8.8%), and 4 patients had RSW (7%). The presence of NCS-NCSE was significantly associated with PD (57.9%, p<0.001). NCS and NCSE were also seen in 25% of the patients with RSW and in 60% of the patients with RDA without reaching statistically significance. According to the morphology, RPP, consisting of spike-wave activity, was seen most frequently (88.1%). The duration of monitoring was significantly longer in the group with PD and NCS-NCSE (p: 0.004, p: 0.014). The detailed evaluation of cEEG monitoring data is summarized in Table 3. Rapid rhythms (35.1%) were found as the most frequent nonperiodic paroxysmal activity and probably appeared due to medication. In 56.1% of the patients, some changes in the background activity were detected with treatment or during sleep. In the group with PD, the presence of focal epileptiform abnormality was significantly high (63.2%, p: 0.013), whereas the presence of RSW and RDA did not show any association with other EEG activities.
Table 3.
Findings of cEEG monitoring
| Frequencies of RPP and NCS-NCSE | N | % | ||
|---|---|---|---|---|
| PD | 19 | 33.3 | ||
| RSW | 4 | 7 | ||
| RDA | 5 | 8.8 | ||
| NCS-NCSE | 15 | 26.3 | ||
| Findings of background activity | N | % | ||
| Symmetry | Symmetrical | 35 | 61.4 | |
| Asymmetrical | 24 | 42.1 | ||
| Frequency | Theta | 41 | 71.9 | |
| Delta | 7 | 12.3 | ||
| Alpha | 11 | 19.3 | ||
| Suppression | Symmetrical | 11 | 19.3 | |
| Asymmetrical | 7 | 12.3 | ||
| Nonspecific slow wave activities | 44 | 77.2 | ||
| Focal epileptiform abnormality | 23 | 40.4 | ||
| Localization and morphological findings of RPP | N | % | ||
| PD | Localization | PLED* | 8 | 14 |
| GPED | 11 | 19.3 | ||
| BiPLED | 6 | 10.5 | ||
| Morphology | Sharp-waves | 12 | 63.1 | |
| Sharp waves with long duration | 3 | 15.8 | ||
| TWa | 4 | 21.1 | ||
| RSW | Morphology | Spike-waves | 1 | 25 |
| TWa | 3 | 75 | ||
| RDA | 5 | 8.8 | ||
BiPLED: bilateral PLED; GPED: generalized periodic epileptiform discharge; NCS: nonconvulsive seizures; NCSE: nonconvulsive status epilepticus; PD: periodic discharge; PLED: periodic lateralized epileptiform discharge; TW: triphasic wave
Six out of eight patients detected with PLED had also BIPLED activity
One patient had PD with TW had also RSW with triphasic morphology
Electrographic Patterns and Clinical, Laboratory, and Radiological Findings
PD and NCS-NCSE were the most frequently seen patterns in patients with acute stroke and hypoxic encephalopathy (Table 1). Any electrographic pattern (PD, RSW, RDA, and NCS-NCSE) was detected more frequently in women (70.4%, p: 0.040). Notably, the patients with PD had major medical problems (p: 0.057) whereas the patients with NCS-NCSE had hypoxia (p: 0.04) and the patients with RDD had hyperthermia (p: 0.007) more frequently.
In the evaluation of the RPP according to their morphology, TW morphology was seen in 10.5% of the patients. In this group, the presence of the electrolyte disorder was significantly high (hyperglycemia and hyponatremia in two patients, hypocalcemia in two patients, and hypomagnesemia in one patient) (83.3%; p:0.02).
It was also detected that any electrographic pattern was present in 63% of the patients who did not use sedatives and in 74.1% of the patients who did not use AED prior to the monitoring. RPP was detected in 52.4% of the patients with an acute ischemic or hemorrhagic lesion and in 33.3% of the patients with chronic ischemic or hemorrhagic lesion.
In the patients group with an electrographic pattern, 44.4% had fever, 66.7% had infection, and 63% had used antibiotic. Eighty percent of the patients with RPP had respiratory disease that required noninvasive ventilator support or intubation (p: 0.069), 44.4% had electrolyte disorder (Na, K, Ca, hypomagnesemia, hyperuricemia, hyperglycemia), and 41.2% had high levels of liver enzymes (AST-ALT and hyperammonemia). 51.9% of the patients had used AED, while 46.2% used sedatives during the monitoring. A treatment change in AED was applied in 44.4% of the patients due to the detection of an electrographic pattern, while a treatment change with antibiotics was performed in 11.1% of patients.
The presence of any electrographic pattern during monitoring had no impact on prognosis and mortality, but 22.2% of the patients with any electrographic pattern died.
Electrographic Patterns and Seizures
CS and NCS during monitoring was common in patients with electrographic patterns (p<0.0001). In this group, NCS-NCSE was seen in 44.4% of the patients, convulsive seizures in 18.5% of the patients, CS and NCS were seen in 11.1% of the patients. 66.7% of NCS-NCSE was seen within the first 12 hours and 26.7% of NCS-NCSE was seen within the 12–24 hours of the monitoring. Figure 1 shows the frequency and timing of the seizures during cEEG monitoring.
Figure 1.
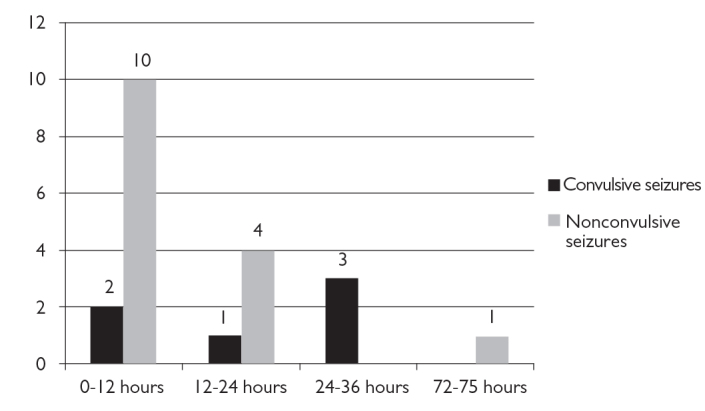
Frequency and timing of the convulsive and nonconvulsive seizures during cEEG monitoring. (Y-axis shows the number of patients)
DISCUSSION
Evaluating a cohort of NICU patients with a change of consciousness who underwent cEEG monitoring, we found that 1. The most common electroencephalographic pattern was PD, 2. The majority of seizures were NCS and NCSE occurred predominantly in patients with PD, 3. Pathologic EEG findings before monitoring indicated that these cases needed long-term EEG monitoring, and at least 24 hours of monitoring of these patients might be useful to detect clinical and/or electrographic seizures.
cEEG monitoring became a commonly used tool in assessing brain function. However, there was no uniformly accepted nomenclature for EEG patterns, frequently encountered in these patients such as RPP. Similarly, there was no consensus on which patterns were associated with ongoing neuronal injury, which patterns needed to be treated, or how aggressively to treat them. Defining the clinical significance of these patterns required standardized terminology with high interrater agreement. Firstly, the ACNS subcommittee on critical care monitoring published the first version of this standardized terminology for EEG patterns encountered in critically ill patients (4). Since 2005, the nomenclature has undergone extensive revisions on multiple occasions based on feedback from others and based on results of interrater reliability studies (5,6). Finally, the ACNS has published a new version of the guideline to standardize EEG terminology (1). Afterwards, an assessment study of agreement of the critical care EEG terminology (2012 version) showed a high interrater agreement between clinicians with the exception of triphasic morphology and evolution (7). This last version of critical care EEG terminology was used to interpret the EEG findings in our study by two experienced electroencephalographers with a kappa index of 0.81 (1).
Increasing use of cEEG revealed clinically undetected epileptiform activity in 10–67% of critically ill patients and resulted in higher detection rates than routine EEG because of the intermittent nature of the occult seizures (8). NCSE was observed in 1–10% of patients with stroke, in 8–14% of patients with traumatic brain injury, in 1–21% of patients with intracerebral hematoma (ICH), in 10–14% of patients with subarachnoid hemorrhage (SAH), and in 30% of patients with postanoxic coma. Using cEEG monitoring, we detected 56% of the seizures in the first hour and 88% of the seizures in the first 24 hours (9). In our study, NCS-NCSE was found in 26.3% of the critically ill patients with a change of consciousness, similar to the literature.
The most commonly observed RPP in our study was PD. The pathophysiology and clinical importance of PD in critically ill patients are still not clearly known. There are studies suggesting that PD is associated with seizures and poor outcome in patients with SE, ICH, and SAH (9,10). It is known that the presence of PD indicated the electrical activities caused by the acutely damaged brain tissue, with a high risk of clinical and/or electrographic seizures including NCS and NCSE (2). The presence of NCS-NCSE was significantly associated with PD (57.9%, p<0.001) in our study.
Periodic lateralized epileptiform discharge is the most common and well-known type of PD and have established associations with destructive focal lesions, usually acute-onset and related to seizures (11,12). It was reported that the PLED activity had been detected in an elderly patient with a confusional state, and the clinical and EEG findings of this patient were improved after diazepam treatment (13), suggesting NCSE is associated with PLED (13). BIPLED, though less commonly seen than PLED, was found more likely to be associated with the seizures in case of acute cerebral diseases, and related outcome could be worse than PLED (14). In our study, it was also determined that half of the cases with PLED had acute ischemic stroke and the presence of PLED significantly indicated association with NCS-NCSE. In addition, in six out of eight patients detected with PLED had also BIPLED activity, in our group.
Generalized periodic epileptiform discharge was reported first in patients with subacute sclerosing panencephalitis and Creutzfeldt-Jakob disease (15,16). GPED may result from thalamocortical pathway disruption associated with diffuse or multifocal cerebral dysfunction, or even in relation with systemic diseases (17). Gloor et al. (18) demonstrated that diffuse cortical and subcortical gray matter disease was required for development of this pattern. They also concluded that the electrophysiology reflects an abnormal system in which networks of damaged neurons discharge and become aberrantly synchronized, thereby appearing generalized (18). Subsequent to Gloor et al. (18), animal experiments have suggested that PD may represent the EEG correlates of the dying neurons (19).
In a study of 200 patients with GPED, it was ascertained that the most common etiologies found through cEEG monitoring were toxic-metabolic encephalopathy, septicemia, and stroke and that GPED was associated with the high incidence of NCS-NCSE (20). Similar to this study, the presence of NCS-NCSE showed significantly high association with GPED in our study.
The need to identify EEG patterns warranting emergent treatment was based on the assumption that certain types of sustained ictal activity might damage the brain. On the other hand, intensive use of AEDs, even the use of diazepam, was claimed to increase mortality (21). In the new SE classification, coma with epileptiform EEG is not included as definite SE but listed separately as a “boundary condition” to make it clear that there is a considerable degree of uncertainty, whether this represents true SE, or epiphenomenal activity of a severely damaged or dying brain (22). In our current understanding, the border between comatose NCSE and coma PD is difficult to draw (21). For example, one patient of our study who had severe postanoxic coma with a nonevolving GPED pattern could be diagnosed as coma GEDs and showed no improvement despite multiple combinations of AED.
It can be rather difficult to make a morphologic distinction between seizure-related GPED and metabolic encephalopathy-related GPED (originally called triphasic waves) (23). Some studies suggested that the use of wave morphology and/or response to intravenous benzodiazepine could help in diagnosis (20). In our study, the presence of metabolic disorder (specifically electrolyte disturbance) was significantly high in the patients with GPED having TW morphology. Out of three patients did not show any clinical response to IV diazepam despite suppression of electrophysiological activity, and clinical findings of these patients improved only after treatment of metabolic disorder.
The optimal time needed for cEEG monitoring is not known. Pandian et al. (24) found that electrographic seizures were detected in 11% of 105 patients in short EEG recording lasting 30 minutes prior to the long-term EEG monitoring, whereas 27% of them had electrographic seizures during long-term monitoring (mean: 2.9 days). It was also reported that because 88% of the electrographic seizures had been detected within the first 24 hours, at least 24 hours of monitoring is required to detect nonconvulsive seizures in patients with a coma (9). In our study, the duration of monitoring was significantly longer in the group with PD and NCS-NCSE (p=0.004, p=0.014). Moreover, similar to the literature, 66.7% of NCS-NCSE was seen within the first 12 hours and a further 26.7% was seen within 12–24 hours of monitoring.
Interestingly, RPP patterns were found to be more common among women (70.4%, p=0.040). Some retrospective studies speculated that this association could be due to hormonal, genetic, and epidemiological factors, but there is a need for further studies to clarify this issue (20).
The presence of any medical problem was found to be more common in the group with an electrographic pattern, which was thought to be due to the fact that our patient population mostly consisted of patients with acute ischemic stroke. It was seen that an electrographic pattern had been detected in 63% of the patients who did not use sedatives and in 74.1% of the patients who did not use AEDs. This result suggested that the treatment with sedatives and AED used for any reason before monitoring might reduce the incidence of an electrographic pattern (25).
The hypothesis that cEEG monitoring can provide information about prognosis is a useful and interesting point. Jaitly et al. (26) reported that NCS and NCSE, independent of etiology, were found to be associated with poor prognosis. In another study, cEEG monitoring performed on 164 patients after treatment of SE showed that 48% of patients had NCS; 14% of them also had NCSE. Besides, mortality and morbidity were high in the group with NCSE (51% mortality) or NCS (32% mortality) independent of age and etiology (27). On the other hand, the duration of seizure is an important factor for prognosis in NCSE apart from etiology. It was reported that the mortality rate proved to be 36% within the first half hour after the establishment of the diagnosis of NCSE in patients with coma, whereas this rate increased to 75% when the diagnosis was delayed to 24 hours (28). In our study, although the association with any RPP had no impact on prognosis and mortality, it was detected that, in this group, the disability was more severe and duration of hospitalization was longer.
Unexplained postural changes, rigidity, tremor, chewing activity, and agitation may be mistakenly evaluated as seizures in critically ill patients. cEEG monitoring without video by inexperienced staff may also lead to misdiagnosis of a seizure (29). For this reason, cEEG monitoring with video, allows for a continuous clinical and electrophysiological evaluation of the patients. In our study, video recording helped us to make a definite diagnosis of seizure or artefact in eight patients.
Because of the retrospective design of our study done in a moderate-sized patient population with a heterogeneous etiology, predictive clinical, laboratory, or radiologic factors associated with these electrographic patterns and impact of these patterns on outcome could not be identified. Therefore, further prospectively controlled studies are required in the future.
We conclude that the detection of an electrographic pattern (predominantly PD and NCSE) in EEG before monitoring was associated with the presence of any important pattern, in cEEG monitoring of patients with change in consciousness, showing a significant correlation with the duration of monitoring. Our findings suggest that at least 24 hours-monitoring of these patients could be useful for the diagnosis of clinical and/or electrographic seizures and PD.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of local ethics committee approved this study.
Informed Consent: Informed consent was not received due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.A., B.B.; Design - E.A., B.B.; Supervision - B.B., Y.K.; Resource - Z.V.O., S.T.Ö.; Materials - E.A., Z.V.O., S.T.Ö.; Data Collection and/or Processing - E.A., Z.V.O., S.T.Ö.; Analysis and/or Interpretation - E.A., Y.K., B.B.; Literature Search - Z.V.O., E.A.; Writing E.A., Z.V.O.; Critical Reviews - B.B., Y.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Hirsch LJ, Laroche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, Arif H, Jette N, Minazad Y, Kerrigan JF, Vespa P, Hantus S, Claassen J, Young GB, So E, Kaplan PW, Nuwer MR, Fountain NB, Drislane FW. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. https://doi.org/10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 2.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. https://doi.org/10.1097/01.WNP.0000158699.78529.AF. [DOI] [PubMed] [Google Scholar]
- 3.Altındağ E, Krespi Y. Nöroloji Yoğun Bakım Ünitesinde devamlı EEG monitorizasyonu. Epilepsi. 2009;15:69–76. [Google Scholar]
- 4.Hirsch LJ, Brenner RP, Drislane FW, So E, Kaplan PW, Jordan KG, Herman ST, LaRoche SM, Young B, Bleck TP, Scheuer ML, Emerson RG. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rythmic and periodic EEG patterns countered in critically patients. J Clin Neurophysiol. 2005;22:128–135. doi: 10.1097/01.wnp.0000158701.89576.4c. https://doi.org/10.1097/01.WNP.0000158701.89576.4C. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch LJ. Classification of EEG patterns in patients with impaired consciousness. Epilepsia. 2011;52:21–24. doi: 10.1111/j.1528-1167.2011.03228.x. https://doi.org/10.1111/j.1528-1167.2011.03228.x. [DOI] [PubMed] [Google Scholar]
- 6.Gerber PA, Chapman KE, Chung SS, Drees C, Maganti RK, Ng YT. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophysiol. 2008;25:241–249. doi: 10.1097/WNP.0b013e318182ed67. https://doi.org/10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 7.Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB Critical Care EEG Monitoring Research Consortium. Interrater agreement for critical care EEG terminology. Epilepsia. 2014;55:1–8. doi: 10.1111/epi.12653. https://doi.org/10.1111/epi.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter R, Stevens RD, Kaplan PW. Continous EEG monitoring in critically ill patients: indications, limitations, and strategies. Crit Care Med. 2013;41:1124–1132. doi: 10.1097/CCM.0b013e318275882f. https://doi.org/10.1097/CCM.0b013e318275882f. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. https://doi.org/10.1212/01.WNL.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 10.Claassen J, Jette N, Chum F, Green R, Schmidt M, Choi H, Hirsch J, Frontera JA, Connally ES, Emerson RG, Mayer SA, Hirsch LJ. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. https://doi.org/10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 11.Baykan B, Kinay D, Gökyigit A, Gürses C. Periodic lateralized discharges: association with seizures. Seizure. 2000;9:402–406. doi: 10.1053/seiz.2000.0435. https://doi.org/10.1053/seiz.2000.0435. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Morales I, Garcia MT, Galan-Davila L, Gomez-Escalonilla C, Saiz-Diaz R, Martinez-Salio A, de la Pena P, Tejerina JA. Periodic lateralized epileptiform discharges: etiology, clinical aspects, seizures, and evolution in 130 patients. J Clin Neurophysiol. 2002;19:172–177. doi: 10.1097/00004691-200203000-00009. https://doi.org/10.1097/00004691-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Terzano MG, Parrino L, Mazzucchi A, Moretti G. Confusional states with periodic lateralized epileptiform discharges (PLEDs): a peculiar epileptic syndrome in the elderly. Epilepsia. 1986;27:446–457. doi: 10.1111/j.1528-1157.1986.tb03566.x. https://doi.org/10.1111/j.1528-1157.1986.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 14.Brenner RP, Schaul N. Periodic EEG patterns: classification, clinical correlation and pathophysiology. J Clin Neurophysiol. 1990;7:249–267. https://doi.org/10.1097/00004691-199004000-00007. [PubMed] [Google Scholar]
- 15.Cobb W, Hill D. Electroencephalogram in subacute progressive encephalitis. Brain. 1950;73:392–404. doi: 10.1093/brain/73.3.392. https://doi.org/10.1093/brain/73.3.392. [DOI] [PubMed] [Google Scholar]
- 16.Jones DP, Nevin S. Rapidly progressive cerebral degeneration (subacute vascular encephalopathy) with mental disorder, focal disturbances, and myoclonic epilepsy. J Neurol Neurosurg Psychiatry. 1954;17:148–159. doi: 10.1136/jnnp.17.2.148. https://doi.org/10.1136/jnnp.17.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner RP. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11:271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. https://doi.org/10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]
- 18.Gloor P, Kalabay O, Giard N. The electroencephalogram in diffuse encephalopaties: electroencephalographic correlates of grey and white matter lesions. Brain. 1968;91:779–802. https://doi.org/10.1093/brain/91.4.779. [Google Scholar]
- 19.Putten MJAM, Hofmeijer J. Generalized periodic discharges: pathophysiology and clinical considerations. Epilepsy Behav. 2015;49:228–233. doi: 10.1016/j.yebeh.2015.04.007. https://doi.org/10.1016/j.yebeh.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Foreman B, Claassen J, Abou-Khaled KJ, Jırsch J, Alschuer DM, Wittman J, Emerson RG, Hirch LJ. Generalize periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012;79:1951–1960. doi: 10.1212/WNL.0b013e3182735cd7. https://doi.org/10.1212/WNL.0b013e3182735cd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinka E, Leitinger M. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy&Behavior. 2015;49:203–222. doi: 10.1016/j.yebeh.2015.05.005. https://doi.org/10.1016/j.yebeh.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH. A definition and classification of status epilepticus-report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. https://doi.org/10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan PW, Sutter R. Affair with triphasic waves-their striking presence, mysterios significance, and cryptic origins: what are they? J Clin Neurophysiol. 2015;32:401–405. doi: 10.1097/WNP.0000000000000151. https://doi.org/10.1097/WNP.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 24.Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital Video-Electroencephalographic Monitoring in the Neurological-Neurosurgical Intensive Care Unit: Clinical Features and Outcomes. Arch Neurol. 2004;61:1090–1094. doi: 10.1001/archneur.61.7.1090. https://doi.org/10.1001/archneur.61.7.1090. [DOI] [PubMed] [Google Scholar]
- 25.Ponten SC, Ronner HE, Strijers RLM, Visser MC, Peederman SM, Vandertop WP, Beishuzen A, Girbes ARJ, Stam CJ. Feasbility of online seizure detection with continuous EEG monitoring in the intensive care unit. Seizure. 2010;19:580–586. doi: 10.1016/j.seizure.2010.09.007. https://doi.org/10.1016/j.seizure.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Jaitly R, Sgro JA, Towne AR, Ko D, DeLorenzo RJ. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol. 1997;14:326–334. doi: 10.1097/00004691-199707000-00005. https://doi.org/10.1097/00004691-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 27.DeLorenzo RJ, Waterhouse EJ, Towne AR, Boggs JG, Ko D, DeLorenzo GA, Brown A, Garnett L. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–840. doi: 10.1111/j.1528-1157.1998.tb01177.x. https://doi.org/10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 28.Young GB, Jordan K, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–89. doi: 10.1212/wnl.47.1.83. https://doi.org/10.1212/WNL.47.1.83. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch LJ. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol. 2004;21:332–340. [PubMed] [Google Scholar]


