In vestibular calyx terminals of mature cristae we find that the majority of excitatory postsynaptic currents (EPSCs) are rapid monophasic events mediated by AMPA receptors. Spontaneous EPSCs are reduced by an L-type Ca2+ channel blocker and notably enhanced in extracellular Sr2+. EPSC frequency is greater in central areas of the crista compared with peripheral areas and may be associated with more numerous presynaptic ribbons in central hair cells.
Keywords: type I hair cell, crista, AMPA receptor, glutamate, cyclothiazide
Abstract
In the vestibular periphery neurotransmission between hair cells and primary afferent nerves occurs via specialized ribbon synapses. Type I vestibular hair cells (HCIs) make synaptic contacts with calyx terminals, which enclose most of the HCI basolateral surface. To probe synaptic transmission, whole cell patch-clamp recordings were made from calyx afferent terminals isolated together with their mature HCIs from gerbil crista. Neurotransmitter release was measured as excitatory postsynaptic currents (EPSCs) in voltage clamp. Spontaneous EPSCs were classified as simple or complex. Simple events exhibited a rapid rise time and a fast monoexponential decay (time constant < 1 ms). The remaining events, constituting ~40% of EPSCs, showed more complex characteristics. Extracellular Sr2+ greatly increased EPSC frequency, and EPSCs were blocked by the AMPA receptor blocker NBQX. The role of presynaptic Ca2+ channels was assessed by application of the L-type Ca2+ channel blocker nifedipine (20 µM), which reduced EPSC frequency. In contrast, the L-type Ca2+ channel opener BAY K 8644 increased EPSC frequency. Cyclothiazide increased the decay time constant of averaged simple EPSCs by approximately twofold. The low-affinity AMPA receptor antagonist γ-d-glutamylglycine (2 mM) reduced the proportion of simple EPSCs relative to complex events, indicating glutamate accumulation in the restricted cleft between HCI and calyx. In crista slices EPSC frequency was greater in central compared with peripheral calyces, which may be due to greater numbers of presynaptic ribbons in central hair cells. Our data support a role for L-type Ca2+ channels in spontaneous release and demonstrate regional variations in AMPA-mediated quantal transmission at the calyx synapse.
NEW & NOTEWORTHY In vestibular calyx terminals of mature cristae we find that the majority of excitatory postsynaptic currents (EPSCs) are rapid monophasic events mediated by AMPA receptors. Spontaneous EPSCs are reduced by an L-type Ca2+ channel blocker and notably enhanced in extracellular Sr2+. EPSC frequency is greater in central areas of the crista compared with peripheral areas and may be associated with more numerous presynaptic ribbons in central hair cells.
vestibular neuroepithelia in amniotes contain two sensory hair cell types called type I (HCI) and type II (HCII) hair cells. These hair cells transduce head movement information into receptor potentials. Receptor potential information is relayed across the synapse to vestibular afferent fibers, and different hair cells make synapses with different types of afferent fibers. Each HCI is enshrouded by a large afferent calyx nerve ending (Fig. 1A), whereas HCIIs have much smaller afferent bouton synapses. Pure calyx afferents contact only HCIs, bouton afferents contact only HCIIs, and branched dimorphic afferents contact both hair cell types (Eatock and Songer 2011; Goldberg 2000). Vestibular afferent fibers fire spontaneously at rest, with hair bundle deflection modulating the frequency of firing (Goldberg 2000). At the first auditory and vestibular synapses, chemical transmission occurs via elaborate ribbon-type synapses, which allow the rapid and efficient transfer of sensory signals. Each ribbon synapse consists of a presynaptic dense body surrounded by numerous vesicles containing neurotransmitter. In the vestibular peripheral end organs, the large calyx terminals contacting HCIs are more amenable to patch-clamp recordings than bouton terminals. Neurotransmitter release from the presynaptic hair cell can be detected as transient changes in postsynaptic receptor conductances. Previous recordings of excitatory postsynaptic currents (EPSCs) from vestibular calyx terminals in turtle (Contini et al. 2017; Highstein et al. 2014, 2015; Holt et al. 2007) and rodent (Dulon et al. 2009; Rennie and Streeter 2006; Sadeghi et al. 2014; Songer and Eatock 2013) preparations support the notion that quantal release of neurotransmitter is an important mechanism for conveying sensory information across the HCI-calyx synapse. However, unresolved questions remain concerning how activation of postsynaptic receptors impacts action potential firing, how responses might vary with ribbon numbers across vestibular epithelia, and whether the neurotransmitter glutamate accumulates in the restricted intercellular synaptic cleft.
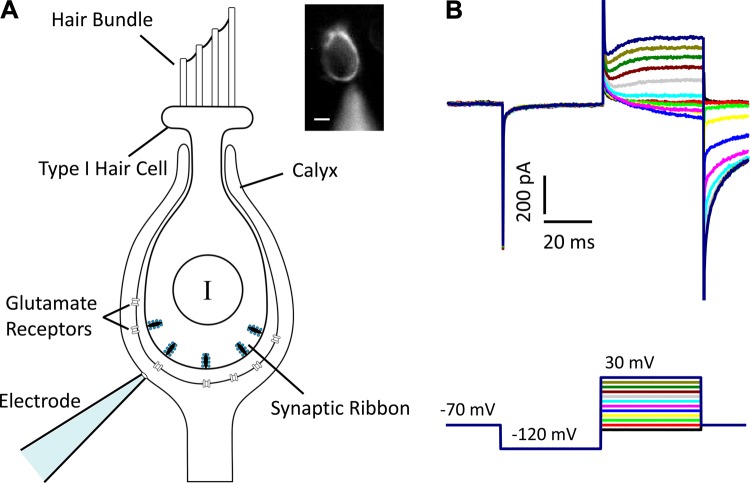
Fig. 1.
The isolated calyx preparation. A: schematic of an isolated type I hair cell (HCI) and calyx terminal. The calyx envelops the basolateral regions of the HCI, where several presynaptic ribbons are present. Glutamate receptors are located on the inner face of the postsynaptic calyx in opposition to the presynaptic ribbons. A patch electrode is shown on the outer face of the calyx membrane. Inset: a calyx filled with Alexa 488 introduced via the patch electrode. Scale bar, 4 µm. B: typical whole cell currents from an isolated calyx terminal in the presence of ion channel blockers. In voltage clamp, cells were held at −70 mV and 10-mV steps were applied in increments from −80 to 30 mV after a −120-mV prepulse (voltage protocol shown at bottom). A small, slowly developing inward current preceded small residual outward currents. QX-314 and Cs2+ were present in the electrode solution to block Na+ and K+ channels, respectively.
Hair cell Ca2+ influx through voltage-gated Ca2+ channels is closely linked to exocytosis of transmitter (Dulon et al. 2009; Vincent et al. 2014), but the precise interactions between Ca2+ domains, presynaptic ribbons, and transmitter release from vesicles is unclear (Kim et al. 2013). Hair cell afferent synapses must faithfully transmit both phasic and tonic aspects of incoming stimuli, and vestibular-driven reflexes are among the fastest in the body, indicating a need for signals to be transmitted rapidly and accurately. Vestibular HCIs in mouse utricle were estimated to have ~160 Ca2+ channels per ribbon (Vincent et al. 2014), but studies in bullfrog hair cells suggest that even the local opening of a single Ca2+ channel may be sufficient to drive simultaneous release of several presynaptic vesicles (Kim et al. 2013; Li et al. 2009). At the HCI-calyx synapse there is evidence for both quantal (Bonsacquet et al. 2006; Dulon et al. 2009; Highstein et al. 2015; Rennie and Streeter 2006; Sadeghi et al. 2014) and nonquantal (Highstein et al. 2014; Holt et al. 2007; Songer and Eatock 2013; Yamashita and Ohmori 1990) forms of synaptic transmission, but neither mechanism is well understood. Previous mammalian studies have largely focused on the utricle (Dulon et al. 2009; Vincent et al. 2014) or saccule (Songer and Eatock 2013) at early postnatal ages. The goal of this study was to characterize the quantal-mediated component of transmission between HCI and calyx to better understand the characteristics of postsynaptic responses and function of this unusual synapse in the mature mammalian crista. Most of the data were collected in calyces isolated together with their presynaptic HCI, but we also used slices to compare EPSCs in different zones of the crista for the first time. We found rapid simple and complex EPSCs in both types of preparation and higher rates of release in calyces in central compared with peripheral regions.
MATERIALS AND METHODS
Tissue preparation.
Mongolian gerbils (Meriones unguiculatus) of both sexes aged between postnatal day (P)19 and P29 were obtained from an in-house breeding colony. An intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) mixed in sterile saline was used to induce anesthesia. The cristae were removed from the semicircular canals of the vestibular system after decapitation. Procedures adhered to protocols approved by the University of Colorado’s Institutional Animal Care and Use Committee.
Gerbils are useful models for vestibular research, having larger cristae than rats and mice and more than twice the number of hair cells compared with mice (Desai et al. 2005). Also, afferent and efferent innervation patterns have been extensively described in gerbils (Kevetter et al. 2004; Purcell and Perachio 1997).
Isolated cells.
Cristae were incubated in a high-Mg2+, low-Ca2+ solution containing (in mM) 135 NaCl, 5 KCl, 10 MgCl2, 0.02 CaCl2, 10 HEPES, and 3 d-glucose, pH 7.4 with NaOH and osmolality 300–305 mosmol/kgH2O, at 37°C for between 15 and 25 min. Tissue was then transferred to Leibovitz’s L-15 medium (pH 7.4–7.45, osmolality 300–305 mosmol/kgH2O) with bovine serum albumin (0.5 mg/ml) for a minimum of 50 min at room temperature (21–24°C). Cells were then mechanically dissociated from each crista in a recording dish containing a small volume of L-15 by drawing a fine probe across the epithelial surface and allowing cells to settle for 5–10 min (Rennie and Streeter 2006). The volume of the bath was subsequently increased to ~2 ml before recording.
Slice preparation.
To compare EPSCs in calyx terminals terminating in peripheral and central locations of the crista, thin slices were made as described previously (Meredith and Rennie 2015). Cristae were trimmed, embedded in a solution of 4% low-gelling-temperature agarose (2-hydroxyethylagarose, type VII, Sigma-Aldrich, St. Louis, MO) and sliced transversely at a thickness of 100–120 µm with a Vibratome 3000 EP (St. Louis, MO), yielding a maximum of six slices per crista. Each slice, secured with a small weight, was immersed in L-15 for electrophysiological recordings. In a few cases, instead of slicing microdissection scissors were used to cut the two peripheral ends of the crista, which were held down with a minuten pin in the recording chamber (Meredith and Rennie 2015).
Electrophysiological recordings.
Cells were viewed under an Olympus upright microscope (BX50WI or BX51WI) with water-immersion objectives (×40 or ×60) and differential interference contrast optics. Calyces remained attached to their HCIs and could be visually identified as encompassing the basolateral region and neck of the hair cell as described previously (Rennie and Streeter 2006). Micropipettes were made from electrode glass (PG165T, Warner Instruments, Hamden, CT) on a micropipette puller (P-97, Sutter Instruments, San Rafael, CA), and tips were heat polished on a Narishige MF 830 microforge (Narishige International USA, East Meadow, NY). Silicone elastomer (Sylgard 184, Dow Corning, Midland, MI) was applied close to the electrode tip to reduce stray capacitance. Electrode solution contained (in mM) 115 CsF, 10 CsCl, 2 NaCl, 10 HEPES, 3 d-glucose, 2 MgCl2, 10 EGTA, and 4 QX-314, pH 7.4 adjusted with CsOH, osmolality 300–305 mosmol/kgH2O (adjusted with mannitol). Electrode open-tip resistance was 2–7 MΩ. A patch amplifier (Axopatch-1D or Axopatch 200B, Molecular Devices, Sunnyvale, CA), connected to a PC through an A/D converter (Digidata 1320A or 1440A, Molecular Devices), was used to obtain whole cell recordings at room temperature (21–24°C). Patch electrodes were placed on calyces near the base of the HCI as depicted in Fig. 1A. In some cases Alexa 488 (50 µM; Life Technologies) was included in the electrode solution to visualize afferent terminals (Fig. 1A). pCLAMP software (v8 or 10) was used for data acquisition. Data were sampled at 10–20 kHz and low-pass filtered online at 5 or 10 kHz. After the whole cell recording configuration was achieved, calyces were held at −70 mV in voltage clamp and spontaneous EPSC data were collected in successive periods of 5 or 20 s. Recordings from each calyx typically lasted for 10–30 min. The holding current was less than −250 pA, and cells were excluded if EPSCs were very infrequent or showed significant rundown with time. To suppress known large Na+ and K+ conductances in the calyx (Dhawan et al. 2010; Meredith et al. 2011, 2012) and improve space clamp, the Na+ channel blocker QX-314 and the K+ channel blocker Cs+ were included in the patch electrode solution. It was necessary to block Na+ channels since they would be active at the holding potentials used in our recordings (Rennie and Streeter 2006). In claw-shaped afferents of turtle auditory papilla, smaller EPSC peak amplitudes occurred when Na+ channels were blocked with tetrodotoxin, indicating that Na+ channel activity can influence EPSC shape (Schnee et al. 2013). After membrane breakthrough into a calyx, transient inward Na+ currents were initially observed in response to a series of voltage steps but were blocked within seconds as QX-314 from the electrode solution entered the cell. The liquid junction potential was <2 mV and was not corrected during data analysis. Fast capacitance transients were minimized before the whole cell configuration was achieved, allowing estimates of cell capacitance in a subset of isolated cells and calyces in slices as described previously (Meredith and Rennie 2015). Mean capacitance was 6.2 ± 0.8 pF and uncompensated series resistance 16.1 ± 2.3 MΩ (n = 8) in isolated calyces, compared with values of 8.7 ± 1.6 pF and 11.2 ± 1.0 MΩ (n = 8) in slice calyces.
Pharmacological agents.
Most reagents including nifedipine and SrCl2 were obtained from Sigma-Aldrich. 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), cyclothiazide (CTZ), BAY K 8644, γ-d-glutamylglycine (γ-DGG), and QX-314 were purchased from Tocris Bioscience (Ellisville, MO). Externally applied drugs were dissolved in L-15 and were applied by rapid manual bath replacement or with a peristaltic pump (Gilson), which perfused the recording chamber with solution at a flow rate of 0.5–1.0 ml/min.
For high-Ca2+ experiments a HEPES-based solution was used containing (in mM) 120 NaCl, 5 KCl, 10 HEPES, 1.8 MgCl2, 8 CaCl2, and 3 d-glucose, pH 7.4 adjusted with NaOH.
Data collection and analysis.
pCLAMP and Clampfit 8 and 10 (Molecular Devices) and Sigmaplot 11 (Systat Software, San Jose, CA) were used to analyze voltage- and current-clamp data. Mini Analysis software (Synaptosoft, Decatur, GA) was used to collect and analyze EPSCs. EPSC threshold was set at three times the value of the root mean square of the baseline noise. Events were automatically detected by the program and subsequently confirmed by eye. Detection parameters were adjusted from automatic selection to better represent EPSC form. Events were categorized by event morphology. Simple events were visually identified as having a rapid rise time and a single, slower exponential decay, and averages were created by aligning event rise time. All other morphologies were categorized as complex events. Statistical significance was determined with the paired t-test (same population; before and after), the Mann-Whitney rank sum test (when data were not normally distributed), and the Kolmogorov-Smirnov test (for 1-dimensional probability distributions). In box plots the lines indicate median values and the whiskers represent 10th and 90th percentiles. Values are presented as means ± SE or medians. For all tests of statistically significant change, a result was deemed significant when P < 0.05. For individual events in Mini Analysis the decay time was the time elapsed from the event peak to 37% of the peak amplitude. For averaged events, EPSC rise and decays were fit with single exponents to estimate values for rise tau and decay tau.
RESULTS
Whole cell recordings (Fig. 1B) were made from unmyelinated calyx terminals isolated from gerbil semicircular canal cristae as depicted in Fig. 1A. The calyx terminal surrounds a HCI, forming a large area of synaptic apposition. Several presynaptic ribbons are present in each HCI, and postsynaptic NMDA and AMPA receptors (Bonsacquet et al. 2006; Ishiyama et al. 2002; Matsubara et al. 1999) have been localized to the inner face of the calyx (Fig. 1A). Recordings in Fig. 1B and Figs. 2–5 were from single isolated calyces innervating one HCI unless otherwise noted. Recordings in Fig. 6 were from single calyces in slice preparations. With Cs+ as the main cation in the electrode solution, a small current persisted at voltage steps to potentials above approximately −60 mV (Fig. 1B). This current, presumably residual current through unblocked K+ channels, was first seen as an inward current and became outward at potentials above about −20 mV (Fig. 1B). This small remaining current did not interfere with EPSC recordings, which were made at a holding potential of −70 mV or more negative. We interpreted rapid downward deflections on the current trace (Fig. 2A) as EPSCs occurring in response to glutamate release from presynaptic hair cells, and we characterized the underlying kinetics and responses to pharmacological agents.
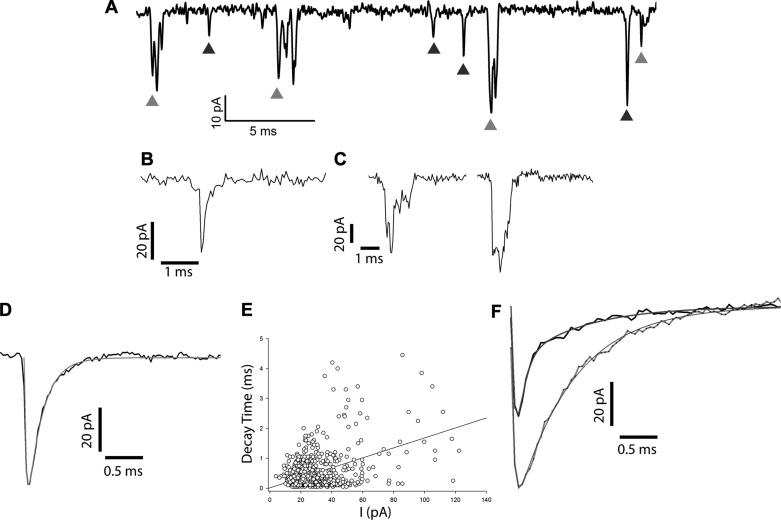
Fig. 2.
Characterization of EPSC event types. A: spontaneous recording of EPSCs showing a wide range of amplitudes from an isolated calyx. Cell was held at −70 mV in voltage clamp, and events (downward deflections) were identified as simple (black arrowheads) or complex (gray arrowheads). B: example of a simple/monophasic event type with a simple rise time and single exponential decay. C: examples of complex events demonstrating a high degree of variability with staggered rise times, overlapping events, and multiphasic decay. D: averaged monophasic events from a control cell (n = 119, simple events). The sum of 2 single exponential fits to the rise and decay is shown in gray, giving an activation time constant of 0.04 ms and a decay time constant of 0.3 ms. E: decay times for simple events as determined by Mini Analysis. I, current. F: addition of cyclothiazide (CTZ, 40 µM) produced an increase in amplitude and slowing of averaged monophasic events consistent with AMPA receptor involvement. For the calyx shown, averages were constructed from 71 (control, black trace) and 131 (CTZ, gray trace) events. Exponential fits to the rise and decay of averages for control and CTZ are indicated by smooth lines. Decay tau was 0.42 ms in control and 0.75 ms in CTZ.
Fig. 5.
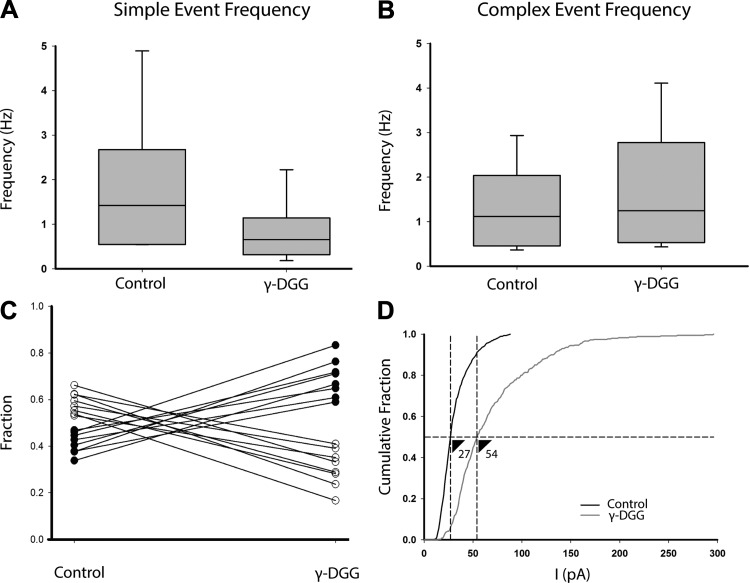
Effect of γ-DGG on EPSCs. A and B: occurrence of simple (A) and complex (B) EPSCs in control cells and after exposure to γ-DGG, a low-affinity AMPA-type glutamate receptor antagonist (n = 8, 6 single and 2 double calyces). There was an overall decrease in simple events and increase in complex events in 2 mM γ-DGG, but the difference was not statistically significant. C: the fraction changed from a majority of simple events to preferentially complex events. Open symbols represent simple events and filled symbols represent complex events for 8 individual cells. D: γ-DGG increased the average amplitude of EPSCs and shifted the cumulative amplitude histogram rightward, changing the median from 27 pA (control shown in black) to 54 pA (γ-DGG shown in gray; n = 8 cells, Kolmogorov-Smirnov test P = 0.001).
Fig. 6.
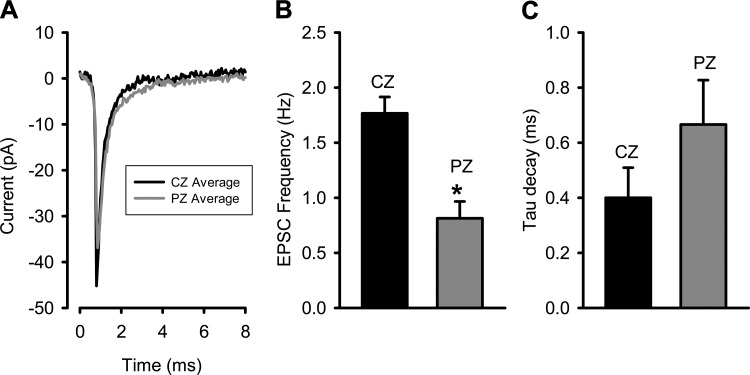
A comparison of EPSCs in central and peripheral calyces from intact crista slices. A: average of monophasic events from central zone (CZ) calyces (n = 4 cells, 468 events, black trace) and peripheral zone (PZ) calyces (n = 5 cells, 545 events, gray trace) recorded from crista slice preparations. B: EPSC frequency averaged 1.77 ± 0.15 Hz in CZ cells (n = 4) and 0.81 ± 0.15 Hz in PZ cells (n = 5); the difference between zones was statistically significant (*P = 0.003, t-test). C: the decay time for each cell EPSC average was fit with a single exponential, and mean values of 0.40 ± 0.11 for CZ calyces (n = 4) and 0.67 ± 0.16 for PZ calyces (n = 5) were obtained. There was no significant difference in decay tau between zones (P = 0.237, t-test).
Approximately one-third of isolated calyces studied (22/67) showed no or minimal spontaneous EPSCs and were not analyzed further. Previous results from isolated gerbil HCIs and embedded mouse HCIs indicate average resting potentials of −67 mV and −62 mV, respectively (Lim et al. 2011; Rennie et al. 1996). A lack of EPSC activity may reflect HCIs with more hyperpolarized resting potentials, at which voltage-dependent Ca2+ channel openings would be minimal (Almanza et al. 2007; Bao et al. 2003; Dou et al. 2004; Dulon et al. 2009).
EPSC properties.
Figure 2A shows several EPSCs representing spontaneous activity in a calyx terminal held at −70 mV. In normal extracellular L-15 solution, the average EPSC frequency was 1.24 ± 0.22 Hz (mean ± SE; n = 45 calyces, 16–1,892 events/cell). Within recordings from a single calyx, individual EPSCs varied greatly in their size and shape. Many events showed a simple rapid and smooth rise to a peak followed by a slower monoexponential decay to baseline (Fig. 2B), whereas other events consisted of more complex and variable shapes as shown in Fig. 2C. Therefore we divided our data sets into simple (monophasic) and complex (multiphasic) events as described for EPSCs recorded from auditory afferents (Grant et al. 2010; Schnee et al. 2013). In control conditions the majority of events were rapid simple monophasic events, making up on average 58.7 ± 1.6% (n = 8 cells) of the total number of events. An example of averaged simple monophasic events (n = 119) from one calyx recording is shown in Fig. 2D. When fit with two single exponentials to the rise and decay phases of the average EPSC, the activation time constant for this cell was 0.04 ms and the time constant of decay was 0.3 ms (Fig. 2D). The decay times as determined by Mini Analysis for 1,535 simple events from 11 calyces are shown in Fig. 2E. EPSCs for this population varied considerably in their peak amplitude and ranged from <15 pA to >120 pA. Complex events typically had larger amplitudes than simple events (see Fig. 3F). Many ribbon-containing synapses show large variations in the amplitudes of their postsynaptic EPSCs, which in auditory afferents has been interpreted as evidence for coordinated multivesicular release (Glowatzki and Fuchs 2002; Grant et al. 2010; Li et al. 2009; Schnee et al. 2013). Since a single calyx envelops a HCI with many presynaptic ribbons, multivesicular release is a likely mechanism underlying the broad range of EPSC amplitudes in vestibular calyx afferents.
Fig. 3.
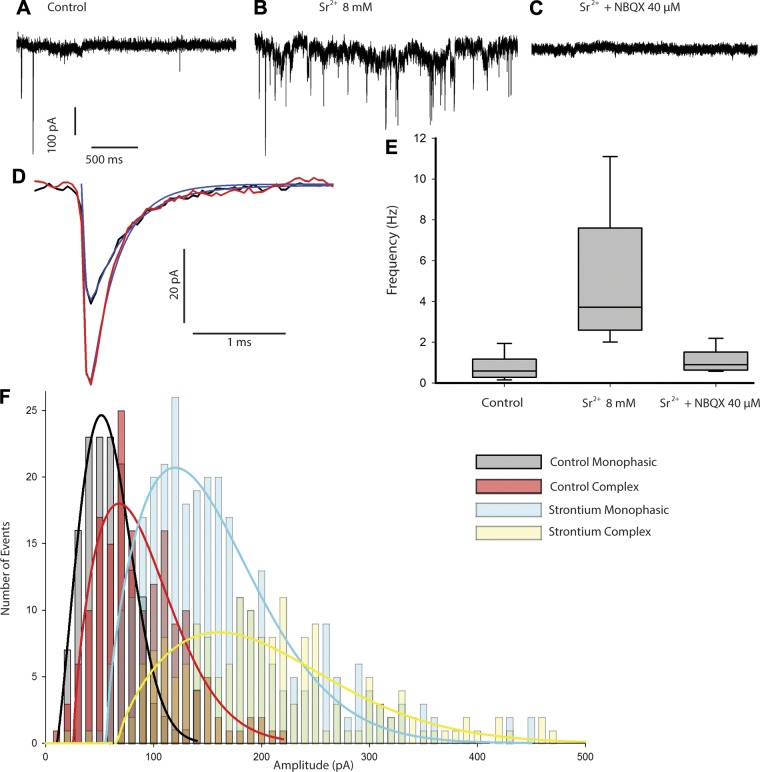
Effect of Sr2+ on EPSCs. A and B: EPSCs in control (A) and in the presence of 8 mM Sr2+ (B), where the rate of spontaneous events increased markedly. C: EPSCs in Sr2+ were blocked by NBQX (40 µM), confirming that events were mediated by AMPA receptors. D: averages of monophasic events in control (n = 117 events) and Sr2+ (n = 603 events) are shown for a single calyx. Exponential fits to the rise and decay phases are shown for control (blue trace, decay tau 0.41 ms) and Sr2+ (red trace, decay tau 0.29 ms). E: the increase in frequency of spontaneous events in Sr2+ was statistically significant (n = 9, P < 0.001, Mann-Whitney test). F: amplitude histograms for events in control and 8 mM Sr2+ for 1 calyx. EPSCs were separated into monophasic (simple) and complex for both groups. The area under the curves (fit with Weibull distribution functions) demonstrates an increase in both frequency and amplitude of EPSCs in Sr2+. Complex events (yellow bars, median 231.2 pA) in Sr2+ were typically larger than monophasic events (blue bars, median 151.2 pA), and the difference was significant (Kolmogorov-Smirnov test, D = 0.3229, P < 0.001).
Previous recordings from vestibular calyces of turtles and rodents have shown that spontaneous EPSCs and excitatory postsynaptic potentials (EPSPs) are blocked by the competitive antagonists CNQX and NBQX and are therefore mediated by AMPA receptors (Dulon et al. 2009; Highstein et al. 2014; Holt et al. 2007; Meredith and Rennie 2015; Sadeghi et al. 2014). To determine whether desensitization of postsynaptic AMPA receptors plays a role in shaping EPSCs, CTZ, which removes desensitization, was applied and the effects on simple events analyzed (Fig. 2F). CTZ (100 µM) increased the mean peak amplitude and markedly increased the EPSC decay time of averaged events from 0.42 to 0.75 ms in the cell shown. In a group of five cells, peak amplitude increased to a mean value of 246.3 ± 60.0% in CTZ and the decay time constant increased from 0.35 ± 0.07 to 0.67 ± 0.08 (n = 5 cells, P = 0.017, t-test). The frequency of spontaneous events in isolated gerbil calyces was low at ~1 EPSC/s, which is similar to rates described for other mammalian inner ear preparations (Glowatzki and Fuchs 2002; Sadeghi et al. 2014). In contrast, release rates were reported to be much higher in nonmammalian inner ear preparations such as calyces in the turtle vestibular lagena (Highstein et al. 2015) and afferents in bullfrog auditory papilla (Keen and Hudspeth 2006; Li et al. 2009) and turtle papilla (Schnee et al. 2013), where spontaneous rates ranged from 22 to 60 Hz. We therefore explored methods to increase the frequency of EPSCs in isolated mammalian calyces.
External Sr2+ increases EPSC frequency and amplitude.
Presynaptic Ca2+ channels in hair cells are clustered at active zones beneath or adjacent to synaptic ribbons (Issa and Hudspeth 1994; Roberts et al. 1990; Rutherford and Pangršič 2012), and a clear linear relationship has been described for Ca2+ influx and exocytosis of synaptic vesicles in auditory (Beurg et al. 2008; Goutman and Glowatzki 2007; Johnson et al. 2005; Keen and Hudspeth 2006) and more recently in vestibular (Dulon et al. 2009) hair cells. Depolarizations above ~-60 mV activate L-type Ca2+ currents in rodent vestibular hair cells (Almanza et al. 2007; Dou et al. 2004; Vincent et al. 2014), which increased in magnitude when extracellular Ca2+ was raised from 1.3 to 5 mM and would be expected to increase neurotransmitter release (Bao et al. 2003; Dulon et al. 2009). However, we observed no overall change in the rate of spontaneous events in the presence of raised extracellular Ca2+, where mean EPSC frequency was 2.5 ± 2.6 Hz in controls and 2.4 ± 3.5 Hz (mean ± SE, n = 9 calyces; data not shown) in 8 mM Ca2+. To further investigate synaptic release mechanisms at the HCI-calyx synapse, we tested the effects of external Sr2+ ions on EPSCs. Sr2+ is permeable through Ca2+ channels, supports transmitter release, and has been used as a tool to study release at various synapses including central (Xu-Friedman and Regehr 1999, 2000) and peripheral (Schnee et al. 2013) auditory synapses. However, because of its slower kinetic properties, Sr2+ desynchronizes vesicle release and enhances delayed release (Xu-Friedman and Regehr 1999). Sr2+ might be expected to lower efficiency and reduce the rate of release from synaptic ribbons, but we observed a pronounced increase in calyx EPSC frequency when Sr2+ was added to the normal extracellular solution (Fig. 3). Median EPSC frequency increased significantly from 0.59 Hz to 3.72 Hz in Sr2+ (Mann-Whitney test, P < 0.001, n = 9 cells), and the average amplitude of simple events also increased (Fig. 3D). To confirm that the events in high Sr2+ were mediated by AMPA receptors, we tested the effect of NBQX, a selective blocker of AMPA receptors (Fig. 3C). NBQX (40 µM) significantly reduced the median frequency of events in Sr2+ from 3.72 Hz to 0.91 Hz (n = 9 cells, Mann-Whitney test, P < 0.001), (Fig. 3, C and E). Both simple and complex EPSCs were increased in the presence of 8 mM Sr2+ (Fig. 3F); the increase in complex events likely indicates an increase in summation of single events. The marked enhancement of EPSC frequency by Sr2+ has not been reported at other ribbon-containing hair cell-afferent synapses. To rule out a possible presynaptic effect of Sr2+ on HCI K+ currents, we made whole cell recordings from isolated HCIs lacking calyces. There was no significant effect of 8 mM Sr2+ on outward K+ currents recorded from isolated HCIs (peak current 3.4 ± 1.2 nA in control vs. 2.9 ± 1.2 nA in Sr2+, n = 5 cells, t-test P > 0.05). In addition, application of Sr2+ did not alter outward K+ conductances in two isolated calyces patched with high-K+ electrode solution (data not shown).
Effects of L-type Ca2+ channel modulators on EPSCs.
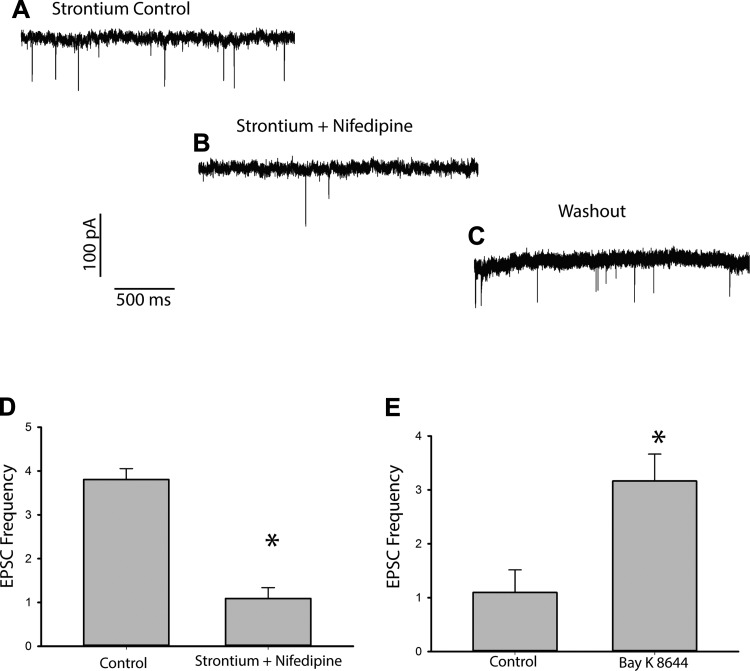
In early postnatal utricles, depolarization of the presynaptic hair cell opens voltage-dependent Ca2+ channels and the resulting influx of Ca2+ ions enhances neurotransmitter release (Dulon et al. 2009; Vincent et al. 2014). The dihydropyridine nifedipine was reported to block ~70% of the whole cell Ca2+ current in hair cells of mouse utricle (Dulon et al. 2009). A reduction in Ca2+ influx is expected to reduce evoked activity, but the effect on spontaneous release of transmitter is unknown. We recorded EPSCs in four calyces in normal extracellular solution followed by solution containing 20 µM nifedipine and saw no significant difference in release rates (mean EPSC rate in control was 1.35 ± 0.92 and in nifedipine was 2.13 ± 2.85, t-test not significant, P = 0.530). However, when calyces were exposed to high Sr2+ to enhance release, followed by a combination of high Sr2+ and 20 µM nifedipine (Fig. 4, A and B), a dramatic decrease in the frequency of events was observed from a mean value of 3.81 ± 0.20 Hz to 1.09 ± 0.25 Hz (Fig. 4D; n = 6 calyces). The effect of nifedipine was reversible, with EPSC frequency increasing again after return to Sr2+-only solution (Fig. 4C). Since nifedipine is a selective antagonist of L-type Ca2+ channels, this strongly suggests that the increase in event frequency seen when Ca2+ is replaced by Sr2+ involves permeation of Sr2+ through L-type Ca2+ channels and interaction with vesicle release machinery. The L-type Ca2+ channel dihydropyridine agonist BAY K 8644 resulted in a significant increase in mean EPSC frequency when calyces were exposed to normal extracellular solution containing 25 µM BAYK 8644, also confirming involvement of L-type Ca2+ channels (Fig. 4E).
Fig. 4.
Effects of calcium channel modulators on EPSCs. A and B: EPSCs in the presence of 8 mM Sr2+ (A) followed by the application of nifedipine (20 µM) and 8 mM Sr2+ (B), which resulted in a reduction in EPSC frequency. C: frequency of events increased after nifedipine washout. D: frequency of EPSCs in cells bathed in 8 mM Sr2+ (control) followed by a combination of Sr2+ and 20 µM nifedipine decreased from 3.81 ± 0.20 Hz to 1.09 ± 0.25 Hz (n = 6 cells, t-test, *P < 0.005). E: treatment of cells (control) with the L-type Ca2+ channel activator BAY K 8644 (25 µM) resulted in an increase in mean frequency from 1.10 ± 0.84 Hz to 3.16 ± 1.00 Hz (n = 4 cells, t-test, *P < 0.05).
Effect of γ-DGG on EPSCs.
Near-simultaneous release of multiple vesicles from one or more adjacent presynaptic ribbons might elevate the concentration of glutamate in the cleft between HCI and calyx, which could impact the temporal characteristics of EPSCs. We therefore investigated the effect of γ-DGG, a broad-spectrum low-affinity AMPA receptor antagonist. γ-DGG rapidly dissociates from receptor binding sites and can be used to test the hypothesis that larger EPSCs occur because of glutamate accumulation in the synaptic cleft. At central synapses, small EPSCs were blocked by γ-DGG to a greater extent than large EPSCs when higher concentrations of cleft glutamate were present (Christie and Jahr 2006; Singer et al. 2004; Wadiche and Jahr 2001). In control conditions we observed that simple EPSCs in calyces tended to have smaller amplitudes than complex events (Fig. 3F). We applied 2 mM γ-DGG to calyces bathed in normal external solution (L-15) and analyzed the effects on simple and complex EPSCs. Mean total EPSC frequency was 2.78 ± 0.86 in control conditions and 2.40 ± 0.56 in γ-DGG (n = 8 calyces, means ± SE). We found that the median frequency of simple events decreased in 2 mM γ-DGG and the median frequency of complex events was relatively unchanged (data not statistically significant; Fig. 5, A and B). However in each cell tested, the relative number of simple events decreased in γ-DGG as the number of complex events increased (Fig. 5C; n = 8 cells), indicating that released glutamate competes with γ-DGG for binding sites on postsynaptic AMPA receptors. This impacts smaller simple events generated by release from single vesicles more than larger events, which are presumably generated by release of several quanta. When the cumulative amplitude plots for control events and those in γ-DGG were compared (data pooled from all cells, n = 8), there was a clear shift to a greater proportion of larger-amplitude events in γ-DGG (Fig. 5D). Therefore during spontaneous release from the hair cell, sufficient glutamate accumulation occurred to impact EPSC shape and produce larger events.
EPSCs in crista slice calyces.
We recently made recordings from calyx terminals in slices of crista and utricle and showed differences in K+ currents between peripheral zone (PZ) and central zone (CZ) calyces within the neuroepithelia (Meredith et al. 2015; Meredith and Rennie 2015). Regional differences in afferent firing pattern are also known, but underlying mechanisms are not understood and could involve differences in synaptic properties (Eatock and Songer 2011). To determine whether regional differences in EPSCs exist between zones, we recorded EPSCs from calyces in crista slices (Fig. 6). The averaged combined monophasic events are shown for CZ and PZ cells (Fig. 6A) in crista slice recordings. Mean EPSC frequency was on average approximately twice the value in CZ cells compared with PZ cells (Fig. 6B). The decay time for averaged monophasic events was 0.40 ± 0.11 for CZ calyces (n = 4 cells) and 0.67 ± 0.16 for PZ calyces (n = 5 cells) and was not significantly different between zones (Fig. 6C).
DISCUSSION
We examined the temporal characteristics of EPSCs recorded from calyx terminals isolated along with their presynaptic HCI and investigated their responses to pharmacological agents to gain insight into the function of this atypical synapse. We also recorded EPSCs in calyces in crista slices for zonal comparison. The majority (~60%) of EPSCs are simple events with very fast onset and decay kinetics and properties consistent with rapidly desensitizing AMPA receptors. Presynaptic L-type Ca2+ channels are involved in spontaneous transmitter release, and extracellular application of Sr2+ surprisingly enhances transmitter release. At the ages studied here (P19–P29), the voltage-gated basolateral conductances of gerbil crista hair cells are mature (Li et al. 2010). In contrast, previous synaptic studies were carried out within the first 2 postnatal weeks, during which time calyx endings are still forming cup-shaped synaptic terminals around HCIs and hair cells undergo electrophysiological maturation (Eatock and Songer 2011; Rüsch et al. 1998; Songer and Eatock 2013; Vincent et al. 2014). Early postnatal hair cells transiently express Na+ currents, which enable evoked firing of action potentials (Wooltorton et al. 2007). Adult mammalian vestibular hair cells do not fire action potentials but instead respond to sensory input with graded changes in their receptor potential. Adult HCIs have a low input resistance reflecting the expression of a low-voltage-activated K+ conductance (Li et al. 2010; Rüsch et al. 1998). With the developmental appearance of this K+ conductance, HCIs garner the ability to respond rapidly, but with a lower gain to hair bundle mechanical stimuli. The impact of maturation of pre- and postsynaptic properties on vestibular afferent firing patterns and the functional properties of adult calyx synapses are not well understood.
Postsynaptic responses in different vestibular epithelia and species.
There is substantial evidence for both quantal and nonquantal forms of synaptic transmission at vestibular hair cell afferent synapses (Bonsacquet et al. 2006; Contini et al. 2017; Dulon et al. 2009; Highstein et al. 2014, 2015; Holt et al. 2007; Rennie and Streeter 2006; Sadeghi et al. 2014; Songer and Eatock 2013; Yamashita and Ohmori 1990), but their specific roles in the transmission of vestibular signals are unclear. Recent reports have documented quantal responses from calyces in various vestibular end organs, with some notable differences between preparations. The frequency of spontaneous EPSCs in mature gerbil calyces from isolated and slice preparations measured here was ~1–2 Hz, as described previously for calyx recordings from young rat vestibular epithelia (Sadeghi et al. 2014; Songer and Eatock 2013). In the striola of the turtle lagena, average spontaneous EPSC activity was reported to be much higher at 52 Hz (Highstein et al. 2015). Almost all of our recordings were from isolated calyces containing a single HCI, whereas multicalyces, enveloping up to five HCIs, are frequently found in turtle otolith organs (Highstein et al. 2015; Huwe et al. 2015). Transmitter release from a greater number of presynaptic ribbons could contribute to the enhanced activity in turtle calyces, but in general recorded EPSC rates are much higher in amphibian auditory and vestibular epithelia (Highstein et al. 2015; Li et al. 2009; Schnee et al. 2013) compared with mammals (Glowatzki and Fuchs 2002; Sadeghi et al. 2014; Songer and Eatock 2013). However, the fact that each calyx receives input from multiple presynaptic ribbons suggests that this synapse is capable of sustaining prolonged neurotransmitter release.
A wide range of kinetic properties of vestibular calyx EPSCs is also evident. In recordings from calyx afferents in rat crista explants, the average exponential decay time for spontaneous EPSCs was 10.7 ms (Sadeghi et al. 2014). In a single P6 calyx from rat saccule, the mean decay time constant for EPSCs evoked by hair bundle movement was 3.1 ms (Songer and Eatock 2013). In contrast, we found an average time constant of decay of <1 ms for both isolated and slice crista calyces, similar to data from turtle lagena (Highstein et al. 2015). In turtle calyces, EPSC size increased during hair cell depolarization but the decay time constant was virtually unchanged compared with spontaneous events. However, stimulation of different HCIs converging on calyces evoked marked differences in EPSC size and kinetics (Highstein et al. 2015). Our data suggest that, even for spontaneous release, individual HCIs may produce appreciably different EPSCs within a single calyx, perhaps because of differences in coordinated release of vesicles from presynaptic ribbons.
Impact of afferent morphology.
Vestibular calyx endings have a highly unusual microarchitecture, and several anchoring proteins and ion channels have been localized to domains within both the inner and outer faces of calyx terminals (Lysakowski et al. 2011; Sousa et al. 2009). Although there are regions of close apposition between HCI and calyx, fluorescent dye introduced via the patch electrode (Fig. 1) does not pass across the synapse (Dhawan et al. 2010; Eatock and Songer 2011; Highstein et al. 2014; Yamashita and Ohmori 1990), consistent with earlier freeze-fracture electron microscopy work that found no evidence for gap junctions (Gulley and Bagger-Sjöbäck 1979). Calyx terminals, especially multicalyces, have large surface membrane areas with complex morphology (Desai et al. 2005; Huwe et al. 2015). Given that pure calyx terminals are compact and receive input from ribbons that are relatively close to one another, large and rapid EPSPs may occur at the spike initiation zone to generate action potentials. Dimorphic afferents will receive input from ribbons from a much more extensive collecting area through smaller-diameter bouton fibers (Huwe et al. 2015). Therefore EPSPs in dimorphic afferent fibers contacting both HCIs and HCIIs may be smaller and slower than those in pure calyx afferents. Space clamp was found to be compromised in whole mount preparations, when collateral calyces, boutons, and axons were part of the recording configuration (Contini et al. 2017; Highstein et al. 2015), and poor space clamp could filter the EPSC waveform, producing recordings with slower kinetics. An advantage of the isolated calyx preparation is that the calyx axon, collateral processes, and efferent fibers are removed, allowing voltage control of the electrically compact calyx terminal, which has a cell capacitance of ~6 pF. Synaptic input from HCIIs, which make synapses with the outer face of calyx terminals (Holt et al. 2007; Lysakowski and Goldberg 1997), is also absent. Capacitance values were slightly higher for calyces in slices (6–9 pF) as measured here and reported previously (Meredith and Rennie 2015), suggesting a more intact axon joining the calyx terminal. However, capacitance values were much higher for calyx recordings in whole mount organ preparations, averaging 28 pF in rat crista with attached ganglion (Sadeghi et al. 2014) and 35 pF in the turtle lagena (Highstein et al. 2015).
AMPA receptors with rapid kinetics mediate spontaneous EPSCs.
Fast synaptic excitatory transmission is typically mediated by glutamate receptors. AMPA receptors show fast activation and deactivation rates and often strong desensitization, whereas NMDA receptors have much slower activation and deactivation kinetics with little or no desensitization (Traynelis et al. 2010). We confirmed that the postsynaptic receptors mediating spontaneous EPSCs in calyces are AMPA receptors with unusually fast kinetic properties. Spontaneous EPSCs are almost completely blocked by AMPA receptor antagonists (Highstein et al. 2014; Meredith and Rennie 2015; Sadeghi et al. 2014). Here we show that Sr2+-evoked EPSCs are also blocked by the AMPA receptor antagonist NBQX. How might fast AMPA-receptor mediated quantal transmission contribute to signaling at the HCI-calyx synapse? In calyx recordings from immature rat saccule, membrane responses demonstrated phase locking to hair bundle stimuli up to ~20 Hz and individual EPSCs and EPSPs did not summate, suggesting that rapid receptor kinetics are advantageous at this synapse (Songer and Eatock 2013). Tonic hair bundle displacement in turtle lagena HCIs initially increased calyx EPSC rates to >1,000/s, quickly followed by a decline in rate and magnitude during the maintained stimulus (Highstein et al. 2015). The decrease in magnitude over time suggested that desensitization could be an important property of calyceal postsynaptic receptors, and we show here that removal of desensitization of AMPA receptors with CTZ increased the magnitude and decay time of EPSCs (Fig. 2F).
HCIs are unique to amniote vestibular neuroepithelia and appear optimized to detect and rapidly convey head motion signals in several ways. First, striola HCIs in adult mice utricles may have stiffer hair bundles than extrastriolar hair cells, which could aid detection of large, high-frequency head accelerations (Li et al. 2008). Second, larger transduction currents in HCIs compared with HCIIs could enable faster presynaptic membrane depolarizations (Rennie et al. 2004). Third, the very low input resistance of mature HCIs reduces the membrane time constant and allows a rapid response to incoming stimuli (Rennie et al. 1996; Rüsch and Eatock 1996). Pure calyx afferents also have the largest diameters and most irregular firing patterns, features that are aptly suited to encode higher-frequency motion signals (Eatock and Songer 2011; Sadeghi et al. 2007). The fast onset and rapid decay of AMPA receptor-mediated EPSCs demonstrated here, coupled with the relatively high input resistance of calyx terminals (Dhawan et al. 2010), could further facilitate the rapid transmission of sensory information across the synapse with high fidelity. At peripheral and central auditory synapses EPSC kinetics speed up with postnatal development and may enable high-frequency firing of action potentials without loss of spike amplitude and preservation of timing information (Grant et al. 2010; Joshi et al. 2004). To achieve this, GluA1 receptors with slow decay kinetics are replaced during maturation with fast-gating GluA3/4 receptors (Joshi et al. 2004). The AMPA receptor subunit composition underlying the rapidly gating EPSCs in mature calyx terminals is unknown, but since GluA4-containing receptors have the very fastest kinetic properties, including deactivation time constants < 1 ms (Traynelis et al. 2010), they may be important participants. Immunostaining for GluA2/3 and GluA4 receptors has been reported at rat vestibular synapses (Matsubara et al. 1999; Sadeghi et al. 2014).
Significance of simple and complex EPSCs.
At mammalian auditory and vestibular synapses EPSCs assume a variety of shapes and amplitudes (Dulon et al. 2009; Glowatzki and Fuchs 2002; Rutherford et al. 2012; Sadeghi et al. 2014) and can be categorized into simple monophasic and complex multiphasic events (Grant et al. 2010). Small EPSCs are thought to occur with single vesicle release, whereas larger and more complex EPSCs are likely multivesicular. In amphibian auditory and vestibular epithelia multiphasic EPSCs are rare (Highstein et al. 2015; Keen and Hudspeth 2006; Li et al. 2009; Schnee et al. 2013). At the inner hair cell afferent synapse, complex events were frequent, representing ~44% of events in prehearing (P8–P11) and 29% of events in posthearing (P19–P21) rats (Grant et al. 2010). We found that ~40% of spontaneous EPSCs recorded from young adult gerbil calyces were complex. Several potential release mechanisms could account for EPSC variability (Kim et al. 2013). Larger EPSCs may occur with coordinated release of primed vesicles (Graydon et al. 2011; Singer et al. 2004) or from synaptic vesicles that have joined together to form a larger vesicle before membrane fusion (Matthews and Sterling 2008). Alternatively, fusion pore dynamics could modulate transmitter release and the size and kinetics of the postsynaptic response (Chapochnikov et al. 2014). Our data indicate a mean size for simple EPSCs of ~40–50 pA, which may represent the release of one or more vesicles and is similar to mean values of 42 pA and 35 pA reported for calyces in turtle lagena and rat saccule, respectively (Highstein et al. 2015; Songer and Eatock 2013). Larger events may represent overlapping release of quanta from vesicles. Since several presynaptic ribbons are present at the HCI-calyx synapse, multivesicular events may originate from a single presynaptic zone or from multiple ribbons.
The role of multiquantal release in driving vestibular calyx afferents to spike threshold is not known. At auditory hair cell synapses, complex EPSCs are more likely to generate action potentials than simple EPSCs (Rutherford et al. 2012; Schnee et al. 2013), and this is likely the case in calyx afferents. Although it is generally held that spontaneous activity in inner ear afferents is due to transmitter release from ribbons, recent data from rodent utricle and crista suggest that calyx afferents continue to fire action potentials in the absence of mechanosensory or synaptic input, perhaps because of voltage-gated ion channel activity within the calyx itself (Horwitz et al. 2014; Meredith and Rennie 2015). There are approximately seven ribbons per HCI in young postnatal mouse utricle (Vincent et al. 2014). In chinchilla crista there was an average of 15–20 ribbons per hair cell, and CZ HCIs contained more ribbons and more invaginations of the calyx membrane into the HCI than PZ cells (Lysakowski and Goldberg 1997). Differences in ribbon number and morphology could influence the amount of transmitter released and impact firing in vestibular afferents. In photoreceptors smaller ribbons generated smaller and less frequent EPSCs (Mehta et al. 2013). In cochlea, variability in the number of presynaptic hair cell Ca2+ channels, ribbon size, and density of postsynaptic glutamate receptor patches impacts EPSC heterogeneity (Frank et al. 2009; Liberman et al. 2011). Although we found that averaged monophasic EPSCs had similar kinetics between zones, EPSC frequency was approximately double in CZ compared with PZ calyces and could reflect greater ribbon numbers in presynaptic HCIs in central regions of the crista as reported in chinchilla crista (Lysakowski and Goldberg 1997).
Ca2+ dependence of EPSCs.
Hair cells contain voltage-gated Ca2+ channels that allow Ca2+ influx and couple neurotransmitter release to membrane depolarization. Mice deficient in the subunit Cav1.3 were deaf, and their inner hair cells lacked L-type Ca2+ currents (Platzer et al. 2000). However, vestibular deficits were not noted and Cav1.3 channels accounted for about half of the total Ca2+ current in vestibular hair cells, suggesting that other Ca2+ channel types could mediate presynaptic Ca2+ influx (Dou et al. 2004; Dulon et al. 2009). Ca2+ current activation and exocytosis of neurotransmitter occurred at more negative potentials in early postnatal HCIs compared with inner hair cells (Vincent et al. 2014). Although we saw a reduction of EPSC frequency in nifedipine, EPSCs were not abolished, suggesting a role for non-L-type Ca2+ channels in spontaneous transmitter release. Candidates include T-type (Nie et al. 2008) and R-type (Martini et al. 2000) Ca2+ channels, and Ca2+ release from hair cell intracellular stores may also contribute to exocytosis (Castellano-Muñoz et al. 2016). Sr2+ has a greater permeability through Ca2+ channels than Ca2+ and desynchronizes transmitter release at central synapses that lack ribbons (Xu-Friedman and Regehr 1999, 2000). Endogenous hair cell buffers may have a lower affinity for Sr2+, slowing its cytoplasmic removal and potentiating exocytosis. At the goldish retinal bipolar cell ribbon synapse, the slow phase of exocytosis was faster in Sr2+ compared with Ca2+ and Ba2+ (Neves et al. 2001). We observed a robust enhancement of EPSC frequency in Sr2+, and both simple and complex EPSCs were increased.
Glutamate receptor saturation at HCI/calyx cleft.
We found that the low-affinity AMPA receptor antagonist γ-DGG reduced the relative frequency of simple events compared with complex events, supporting a direct role for glutamate accumulation in the restricted cleft between HCI and calyx. At bullfrog auditory synapses the glutamate transporter blocker TBOA did not significantly alter afferent EPSCs, suggesting less glutamate buildup (Graydon et al. 2014). Unlike the vestibular calyx, the bullfrog afferent terminal is a clawlike structure and extracellular spaces between the claws may enhance diffusion away from the synaptic sites (Graydon et al. 2014). TBOA also inhibited transporter currents in HCIs (Dalet et al. 2012) and increased mean EPSC decay time constant from 4.7 to 6.6 ms in rat calyces (Sadeghi et al. 2014). During prolonged hair cell depolarization, as might occur during various vestibular stimuli, large amounts of glutamate could be released from hair cells and overwhelm clearance mechanisms. Resulting accumulation in the HCI/calyx cleft could play a significant role at the postsynaptic calyx and impact EPSC shape.
GRANTS
Funding was provided by the American Hearing Research Foundation, National Institutes of Health National Center for Advancing Translational Sciences Colorado CTSI Grant Number UL1 TR-001082, Departments of Otolaryngology and Physiology (K. J. Rennie) at the University of Colorado, and the Children’s Hospital Colorado Research Institute (T. A. Benke).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.E.K., F.L.M., and K.J.R. performed experiments; M.E.K., F.L.M., T.A.B., and K.J.R. analyzed data; M.E.K., F.L.M., and K.J.R. interpreted results of experiments; M.E.K. and F.L.M. prepared figures; M.E.K., F.L.M., T.A.B., and K.J.R. edited and revised manuscript; M.E.K., F.L.M., and K.J.R. approved final version of manuscript; K.J.R. conceived and designed research; K.J.R. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dylan Ray for excellent technical assistance.
REFERENCES
- Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. J Neurophysiol 97: 1188–1195, 2007. doi: 10.1152/jn.00849.2006. [DOI] [PubMed] [Google Scholar]
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol 90: 155–164, 2003. doi: 10.1152/jn.00244.2003. [DOI] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci 28: 1798–1803, 2008. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsacquet J, Brugeaud A, Compan V, Desmadryl G, Chabbert C. AMPA type glutamate receptor mediates neurotransmission at turtle vestibular calyx synapse. J Physiol 576: 63–71, 2006. doi: 10.1113/jphysiol.2006.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Muñoz M, Schnee ME, Ricci AJ. Calcium-induced calcium release supports recruitment of synaptic vesicles in auditory hair cells. J Neurophysiol 115: 226–239, 2016. doi: 10.1152/jn.00559.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapochnikov NM, Takago H, Huang CH, Pangršič T, Khimich D, Neef J, Auge E, Göttfert F, Hell SW, Wichmann C, Wolf F, Moser T. Uniquantal release through a dynamic fusion pore is a candidate mechanism of hair cell exocytosis. Neuron 83: 1389–1403, 2014. doi: 10.1016/j.neuron.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Christie JM, Jahr CE. Multivesicular release at Schaffer collateral-CA1 hippocampal synapses. J Neurosci 26: 210–216, 2006. doi: 10.1523/JNEUROSCI.4307-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D, Price SD, Art JJ. Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. J Physiol 595: 777–803, 2017. doi: 10.1113/JP273060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalet A, Bonsacquet J, Gaboyard-Niay S, Calin-Jageman I, Chidavaenzi RL, Venteo S, Desmadryl G, Goldberg JM, Lysakowski A, Chabbert C. Glutamate transporters EAAT4 and EAAT5 are expressed in vestibular hair cells and calyx endings. PLoS One 7: e46261, 2012. doi: 10.1371/journal.pone.0046261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol 93: 267–280, 2005. doi: 10.1152/jn.00747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan R, Mann SE, Meredith FL, Rennie KJ. K+ currents in isolated vestibular afferent calyx terminals. J Assoc Res Otolaryngol 11: 463–476, 2010. doi: 10.1007/s10162-010-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Vazquez AE, Namkung Y, Chu H, Cardell EL, Nie L, Parson S, Shin HS, Yamoah EN. Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice. J Assoc Res Otolaryngol 5: 215–226, 2004. doi: 10.1007/s10162-003-4020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci 29: 10474–10487, 2009. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011. doi: 10.1146/annurev-neuro-061010-113710. [DOI] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci USA 106: 4483–4488, 2009. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5: 147–154, 2002. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci USA 104: 16341–16346, 2007. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Yi E, Glowatzki E. Two modes of release shape the postsynaptic response at the inner hair cell ribbon synapse. J Neurosci 30: 4210–4220, 2010. doi: 10.1523/JNEUROSCI.4439-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon CW, Cho S, Diamond JS, Kachar B, von Gersdorff H, Grimes WN. Specialized postsynaptic morphology enhances neurotransmitter dilution and high-frequency signaling at an auditory synapse. J Neurosci 34: 8358–8372, 2014. doi: 10.1523/JNEUROSCI.4493-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon CW, Cho S, Li GL, Kachar B, von Gersdorff H. Sharp Ca2+ nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J Neurosci 31: 16637–16650, 2011. doi: 10.1523/JNEUROSCI.1866-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley RL, Bagger-Sjöbäck D. Freeze-fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea-pig vestibular system. J Neurocytol 8: 591–603, 1979. doi: 10.1007/BF01208511. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR, Mann MA, Rabbitt RD. Evidence that protons act as neurotransmitters at vestibular hair cell-calyx afferent synapses. Proc Natl Acad Sci USA 111: 5421–5426, 2014. doi: 10.1073/pnas.1319561111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highstein SM, Mann MA, Holstein GR, Rabbitt RD. The quantal component of synaptic transmission from sensory hair cells to the vestibular calyx. J Neurophysiol 113: 3827–3835, 2015. doi: 10.1152/jn.00055.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol 98: 1083–1101, 2007. doi: 10.1152/jn.00332.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Holt JR. Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons. J Gen Physiol 143: 481–497, 2014. doi: 10.1085/jgp.201311126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwe JA, Logan GJ, Williams B, Rowe MH, Peterson EH. Utricular afferents: morphology of peripheral terminals. J Neurophysiol 113: 2420–2433, 2015. doi: 10.1152/jn.00481.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama G, Lopez I, Williamson R, Acuna D, Ishiyama A. Subcellular immunolocalization of NMDA receptor subunit NR1, 2A, 2B in the rat vestibular periphery. Brain Res 935: 16–23, 2002. doi: 10.1016/S0006-8993(02)02419-8. [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA 91: 7578–7582, 1994. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol 563: 177–191, 2005. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Shokralla S, Titis P, Wang LY. The role of AMPA receptor gating in the development of high-fidelity neurotransmission at the calyx of Held synapse. J Neurosci 24: 183–196, 2004. doi: 10.1523/JNEUROSCI.1074-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci USA 103: 5537–5542, 2006. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Leonard RB, Newlands SD, Perachio AA. Central distribution of vestibular afferents that innervate the anterior or lateral semicircular canal in the mongolian gerbil. J Vestib Res 14: 1–15, 2004. [PubMed] [Google Scholar]
- Kim MH, Li GL, von Gersdorff H. Single Ca2+ channels and exocytosis at sensory synapses. J Physiol 591: 3167–3178, 2013. doi: 10.1113/jphysiol.2012.249482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99: 718–733, 2008. doi: 10.1152/jn.00831.2007. [DOI] [PubMed] [Google Scholar]
- Li GL, Keen E, Andor-Ardó D, Hudspeth AJ, von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell’s ribbon synapse. J Neurosci 29: 7558–7568, 2009. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Meredith FL, Rennie KJ. Development of K+ and Na+ conductances in rodent postnatal semicircular canal type I hair cells. Am J Physiol Regul Integr Comp Physiol 298: R351–R358, 2010. doi: 10.1152/ajpregu.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci 31: 801–808, 2011. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Kindig AE, Donne SW, Callister RJ, Brichta AM. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp Brain Res 210: 607–621, 2011. doi: 10.1007/s00221-011-2592-4. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Gaboyard-Niay S, Calin-Jageman I, Chatlani S, Price SD, Eatock RA. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J Neurosci 31: 10101–10114, 2011. doi: 10.1523/JNEUROSCI.0521-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol 389: 419–443, 1997. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Rossi ML, Rubbini G, Rispoli G. Calcium currents in hair cells isolated from semicircular canals of the frog. Biophys J 78: 1240–1254, 2000. doi: 10.1016/S0006-3495(00)76681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Takumi Y, Nakagawa T, Usami S, Shinkawa H, Ottersen OP. Immunoelectron microscopy of AMPA receptor subunits reveals three types of putative glutamatergic synapse in the rat vestibular end organs. Brain Res 819: 58–64, 1999. doi: 10.1016/S0006-8993(98)01345-6. [DOI] [PubMed] [Google Scholar]
- Matthews G, Sterling P. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J Neurosci 28: 5403–5411, 2008. doi: 10.1523/JNEUROSCI.0935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta B, Snellman J, Chen S, Li W, Zenisek D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron 77: 516–527, 2013. doi: 10.1016/j.neuron.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Benke TA, Rennie KJ. Hyperpolarization-activated current (Ih) in vestibular calyx terminals: characterization and role in shaping postsynaptic events. J Assoc Res Otolaryngol 13: 745–758, 2012. doi: 10.1007/s10162-012-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Kirk ME, Rennie KJ. Kv1 channels and neural processing in vestibular calyx afferents. Front Syst Neurosci 9: 85, 2015. doi: 10.3389/fnsys.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Li GQ, Rennie KJ. Postnatal expression of an apamin-sensitive k(ca) current in vestibular calyx terminals. J Membr Biol 244: 81–91, 2011. doi: 10.1007/s00232-011-9400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Rennie KJ. Zonal variations in K+ currents in vestibular crista calyx terminals. J Neurophysiol 113: 264–276, 2015. doi: 10.1152/jn.00399.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Neef A, Lagnado L. The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J Physiol 535: 809–824, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Zhu J, Gratton MA, Liao A, Mu KJ, Nonner W, Richardson GP, Yamoah EN. Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear. J Neurophysiol 100: 2287–2299, 2008. doi: 10.1152/jn.90707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97, 2000. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Manning KC, Ricci AJ. Mechano-electrical transduction in the turtle utricle. Biomed Sci Instrum 40: 441–446, 2004. [PubMed] [Google Scholar]
- Rennie KJ, Ricci AJ, Correia MJ. Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol 75: 2117–2123, 1996. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Streeter MA. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol 95: 26–32, 2006. doi: 10.1152/jn.00641.2005. [DOI] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci 10: 3664–3684, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A, Eatock RA. A delayed rectifier conductance in type I hair cells of the mouse utricle. J Neurophysiol 76: 995–1004, 1996. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Chapochnikov NM, Moser T. Spike encoding of neurotransmitter release timing by spiral ganglion neurons of the cochlea. J Neurosci 32: 4773–4789, 2012. doi: 10.1523/JNEUROSCI.4511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Pangršič T. Molecular anatomy and physiology of exocytosis in sensory hair cells. Cell Calcium 52: 327–337, 2012. doi: 10.1016/j.ceca.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E. Glutamatergic signaling at the vestibular hair cell calyx synapse. J Neurosci 34: 14536–14550, 2014. doi: 10.1523/JNEUROSCI.0369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee ME, Castellano-Muñoz M, Ricci AJ. Response properties from turtle auditory hair cell afferent fibers suggest spike generation is driven by synchronized release both between and within synapses. J Neurophysiol 110: 204–220, 2013. doi: 10.1152/jn.00121.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassová L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7: 826–833, 2004. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. doi: 10.1523/JNEUROSCI.4067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AD, Andrade LR, Salles FT, Pillai AM, Buttermore ED, Bhat MA, Kachar B. The septate junction protein caspr is required for structural support and retention of KCNQ4 at calyceal synapses of vestibular hair cells. J Neurosci 29: 3103–3108, 2009. doi: 10.1523/JNEUROSCI.4868-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496, 2010. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent PF, Bouleau Y, Safieddine S, Petit C, Dulon D. Exocytotic machineries of vestibular type I and cochlear ribbon synapses display similar intrinsic otoferlin-dependent Ca2+ sensitivity but a different coupling to Ca2+ channels. J Neurosci 34: 10853–10869, 2014. doi: 10.1523/JNEUROSCI.0947-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron 32: 301–313, 2001. doi: 10.1016/S0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, Lysakowski A, Eatock RA. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol 97: 1684–1704, 2007. doi: 10.1152/jn.00649.2006. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Presynaptic strontium dynamics and synaptic transmission. Biophys J 76: 2029–2042, 1999. doi: 10.1016/S0006-3495(99)77360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci 20: 4414–4422, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Ohmori H. Synaptic responses to mechanical stimulation in calyceal and bouton type vestibular afferents studied in an isolated preparation of semicircular canal ampullae of chicken. Exp Brain Res 80: 475–488, 1990. doi: 10.1007/BF00227989. [DOI] [PubMed] [Google Scholar]