We and others recently demonstrated that the electrical activity and calcium dynamics of corticotrophs are strikingly diverse, both spontaneously and in response to the agonists CRH and AVP. Here we demonstrate this diversity with electrophysiological measurements and use mathematical modeling to investigate its possible sources. We compare the known mechanisms of agonist-induced activity in the model, showing how the context of ionic conductances dictates the effects of agonists even when their target is fixed.
Keywords: corticotrophs, corticotropin-releasing hormone, vasopressin, action potentials, ion channels
Abstract
Pituitary corticotrophs fire action potentials spontaneously and in response to stimulation with corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), and such electrical activity is critical for calcium signaling and calcium-dependent adrenocorticotropic hormone secretion. These cells typically fire tall, sharp action potentials when spontaneously active, but a variety of other spontaneous patterns have also been reported, including various modes of bursting. There is variability in reports of the fraction of corticotrophs that are electrically active, as well as their patterns of activity, and the sources of this variation are not well understood. The ionic mechanisms responsible for CRH- and AVP-triggered electrical activity in corticotrophs are also poorly characterized. We use electrophysiological measurements and mathematical modeling to investigate possible sources of variability in patterns of spontaneous and agonist-induced corticotroph electrical activity. In the model, variation in as few as two parameters can give rise to many of the types of patterns observed in electrophysiological recordings of corticotrophs. We compare the known mechanisms for CRH, AVP, and glucocorticoid actions and find that different ionic mechanisms can contribute in different but complementary ways to generate the complex time courses of CRH and AVP responses. In summary, our modeling suggests that corticotrophs have several mechanisms at their disposal to achieve their primary function of pacemaking depolarization and increased electrical activity in response to CRH and AVP.
NEW & NOTEWORTHY We and others recently demonstrated that the electrical activity and calcium dynamics of corticotrophs are strikingly diverse, both spontaneously and in response to the agonists CRH and AVP. Here we demonstrate this diversity with electrophysiological measurements and use mathematical modeling to investigate its possible sources. We compare the known mechanisms of agonist-induced activity in the model, showing how the context of ionic conductances dictates the effects of agonists even when their target is fixed.
corticotrophs are a subpopulation of secretory anterior pituitary cells that produce adrenocorticotropic hormone (ACTH) under the control of two hypothalamic neurohormones, corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP). ACTH is secreted into the systemic circulation to stimulate the production and secretion of glucocorticoids (CORT; cortisol in humans or corticosterone in rodents) from the adrenal glands. CORT in turn provides negative feedback to the hypothalamus and pituitary to control ACTH release. The secretory function of corticotrophs is critically dependent on the patterns of electrical activity and the associated fluctuations of intracellular calcium concentration ([Ca2+]i) (Tse et al. 2012).
There have been a variety of reported levels and patterns of spontaneous corticotroph electrical activity, ranging from quiescence in all recorded male rat (Lee and Tse 1997) and mouse (Lee et al. 2011) cells to activity in nearly all female (Liang et al. 2011) and male (Duncan et al. 2015, 2016) mouse corticotrophs. We and others have reported ~60% active cells in mixed-sex mouse (Zemkova et al. 2016) and male rat (Kuryshev et al. 1995) corticotrophs, with quiescent cells significantly more hyperpolarized than active cells (Zemkova et al. 2016). Reported spontaneous electrical activity consists typically of low-frequency, high-amplitude single action potentials but may also include plateau-like bursting with tall spikes and pseudoplateau-like bursting with small-amplitude spikes superimposed on a plateau (Liang et al. 2011; Zemkova et al. 2016). The sources of this variation are not understood but may include intrinsic heterogeneity of corticotroph ion channel expression or differences in cell preparations and recording techniques.
CRH facilitates electrical activity and Ca2+ influx by activating the CRH receptor type 1-dependent PKA signaling pathway (Aguilera et al. 2004). CRH action in quiescent corticotrophs is characterized by a pacemaking depolarization, leading to electrical activity that includes spiking and bursting (Kuryshev et al. 1995; Lee and Tse 1997; Zemkova et al. 2016). Depolarization due to CRH has not always been reported for initially silent cells, such as AtT-20 cells and ACTH-secreting pituitary adenoma cells (Guérineau et al. 1991). Electrically active corticotrophs display a smaller depolarization along with increased firing frequency of spikes and bursts and may transition between spiking and bursting (Kuryshev et al. 1995, 1997; Liang et al. 2011; Zemkova et al. 2016). Several potential mechanisms for CRH action have been postulated based on activation of inward cationic currents and/or inhibition of outward K+ currents. The inability of CRH to depolarize corticotrophs bathed in Na+-deficient medium suggested the contribution of a background Na+ current (INab) in pacemaking depolarization (Liang et al. 2011). However, we recently reported that CRH could trigger depolarization and activity in either Na+- or Ca2+-deficient medium (Zemkova et al. 2016), suggesting involvement of a conductance such that these two cations could substitute for one another. Such a conductance has been shown in other rodent pituitary cell types (Tomić et al. 2011) and human pituitary adenoma cells (Takano et al. 1996). A major, but not exclusive, component of the CRH response was found to be mediated by the inwardly rectifying K+ current (IKir) in rat (Kuryshev et al. 1997) but not mouse (Liang et al. 2011) corticotrophs. A CRH-dependent reduction in current due to the TWIK-related K+ (TREK)-1 channel, which provides a background K+ leak current (IK,leak), has also been shown (Lee et al. 2011, 2015). The increase in a high-voltage-gated Ca2+ current (ICa) was observed in AtT-20 immortalized corticotrophs (Luini et al. 1985) and used as the primary mechanism of CRH action in several mathematical modeling studies (Duncan et al. 2015, 2016; LeBeau et al. 1997, 1998; Shorten et al. 2000). Another line of experiments showed that CRH inhibits large-conductance Ca2+- and voltage-activated K+ currents (IBK) in a PKA-dependent manner, an effect blocked by CORT in AtT-20 cells (Shipston et al. 1996). Furthermore, CRH-induced bursting activity, but not spike frequency increase, was reduced by blocking BK channels and in BK channel-knockout mice (Duncan et al. 2015, 2016). Whether, and to what degree, all these mechanisms overlap during the CRH response remains to be determined.
AVP stimulates corticotrophs through Gq/11-coupled AVP receptor type 1b, leading to an early inositol 1,4,5-trisphosphate-dependent Ca2+ release from the endoplasmic reticulum (ER) and sustained electrical activity-driven Ca2+ influx. These changes in [Ca2+]i are accompanied by a transient hyperpolarization of the membrane potential followed by an increase in spiking and bursting activity. Lee et al. (2015) showed that the transient hyperpolarization could be abolished by the small-conductance Ca2+-activated K+ channel blocker apamin. This unmasked a concomitant membrane depolarization, which was argued to be due to PKC-dependent inhibition of TREK-1 channels. In other tissues, AVP has been shown to stimulate cationic currents, such as TRPC6 (Mani et al. 2009). Duncan et al. (2015) reported that AVP increased frequency of single-spiking activity but did not trigger bursting, which they modeled by increasing a nonspecific cationic current. We recently showed that AVP could also trigger bursting in addition to increasing spike frequency (Zemkova et al. 2016). In both CRH and AVP responses, it is unclear whether the ionic mechanisms responsible for depolarization and event frequency changes are the same as or distinct from those responsible for transitions between spiking and bursting activity patterns.
Here we present electrophysiological recordings and a mathematical model of corticotroph electrical activity and Ca2+ dynamics with the aim of capturing the reported variability in spontaneous and agonist-induced activity patterns. We demonstrate how variation in the background currents INab, IKir, and IK,leak contributes to whether cells are active or silent. Because IBK has been reported to contribute to the patterns of spontaneous activity in corticotrophs (Duncan et al. 2015, 2016) and other pituitary cells (Van Goor et al. 2001a), we also examine the effect of variation of IBK in spontaneous and agonist-induced electrical activity patterns. Using the model, we test the hypothesis that while direct CRH-induced modulation of BK channels can cause transitions from spiking to bursting activity, this is not necessary; other CRH mechanisms can trigger bursting as well, depending on the amount of BK present. We compare the extent to which each of the proposed mechanisms of CRH, AVP, and CORT action can lead to observed agonist-induced changes in activity patterns and show that the different ionic mechanisms can contribute in different but complementary ways to generate the different phases of CRH- and AVP-induced responses. The dependence of CRH and AVP responses on the context of intrinsic corticotroph parameters is similar to state-dependent neuromodulation, which has been studied in neurons and neuronal networks (Goldman et al. 2001; Marder et al. 2014). We take advantage of the parallel computation capabilities of modern graphics processors to show how model parameters of interest affect electrical activity across full two-parameter planes in addition to time courses at single representative points in parameter space. Our parameter sampling technique, which aims to provide a more global perspective of model behavior, shares similarities with model database approaches (Günay 2015), in which multiple parameter combinations have been shown to give rise to similar neuronal activity (Goldman et al. 2001; Prinz et al. 2004; Taylor et al. 2009).
MATERIALS AND METHODS
Animals.
Experiments were performed on transgenic mice expressing the tdimer2 (12) engineered form of DsRed protein under the control of proopiomelanocortin regulatory elements (Hentges et al. 2009). Breeding pairs of congenic C57BL/6J homozygous mice were used to generate mice for pituitary harvest. Euthanasia was performed by decapitation after CO2 sedation. All animal procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee.
Cell cultures.
Pituitary glands from three to five mice of both sexes were collected in ice-cold Dulbecco’s phosphate-buffered saline. After removal of the neurointermediate lobe, anterior pituitary tissue was washed in Dulbecco’s phosphate-buffered saline containing 0.3% BSA, 2 mM glutamine, and MEM Vitamin Solution (Invitrogen, Carlsbad, CA) and treated with 0.4% trypsin for 15 min at 37°C. The cells were mechanically dispersed, filtered through a 40-µm nylon mesh, centrifuged at 300 g for 10 min, and resuspended in medium 199 containing Earle’s salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 U/ml), and streptomycin (100 µg/ml). For experiments, 0.1–0.2 million cells were plated onto 25-mm glass coverslips coated with 1% poly-l-lysine and cultured in a humidified 5% CO2 atmosphere at 37°C.
Electrophysiology.
Electrophysiological measurements were performed during the first 24 h after cell dispersion. Membrane potentials and CRH- or AVP- stimulated responses were measured in identified corticotrophs with the nystatin-perforated patch-clamp technique (Zemkova et al. 2016). Cells were continuously perfused with an extracellular solution containing (in mM) 150 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose; pH was adjusted to 7.3 with NaOH. The pipette solution contained (in mM) 70 K-aspartate, 70 KCl, 3 MgCl2, and 10 HEPES; pH was adjusted to 7.2 with KOH. Before measurement, Pluronic F-127, a dispersing agent, and then nystatin were added to the intracellular solution from stock solutions (nystatin, 50 mg/ml in dimethyl sulfoxide, always freshly prepared) to obtain a final concentration of 500 µg/ml Pluronic F-127 and 250 µg/ml nystatin. Recordings were performed with an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Data were captured and stored with the pCLAMP 10 software package in conjunction with a Digidata 1322A A/D converter (Axon Instruments). The recordings were not corrected for the liquid junction potential, which was +9.6 mV. Solutions containing CRH or AVP (from Bachem, Torrance, CA) were delivered to the recording chamber by a gravity-driven microperfusion system (RSC-200 Rapid Solution Changer, BioLogic). Electrophysiological experiments were performed at room temperature (21–23°C).
Mathematical model and numerical simulations.
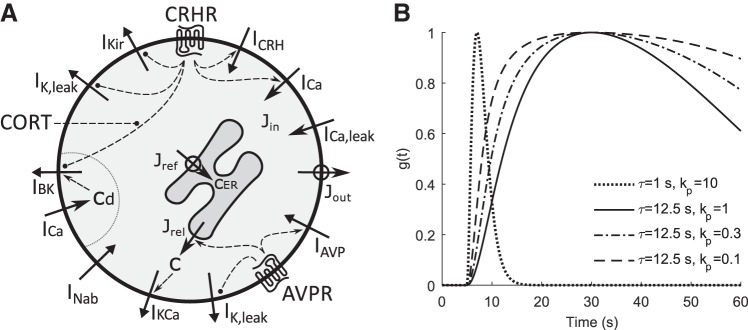
We use a biophysical model (Fig. 1A) based on in vitro data, similar in structure to a previously published model (Duncan et al. 2015, 2016). The model includes the several subthreshold currents that set the rest potential in silent cells. INab has been shown in corticotrophs (Liang et al. 2011; Zemkova et al. 2016), similar to that reported in other pituitary cell types (Tomić et al. 2011). We include IKir (Kuryshev et al. 1997) and IK,leak (Lee et al. 2011, 2015; Lee and Tse 1997) as potential targets of agonist-induced signaling. We also include a Ca2+ leak (ICa,leak) current to maintain store calcium levels in silent cells. Voltage- and Ca2+-dependent currents are also included for their contributions to patterns of electrical activity in corticotroph cells. These include voltage-dependent Na+ current (INaV), ICa, delayed-rectifier K+ current (IK), A-type transient K+ current (IA), Ca2+-activated K+ current (IKCa), and IBK. As putative targets of agonist action, CRH- and AVP-activated nonspecific cationic currents are also included as ICRH and IAVP, respectively. The membrane potential V is governed by the current balance equation
where Cm is the membrane capacitance. The ionic currents are defined as follows:
Fig. 1.
Mathematical model of pituitary corticotrophs. A: diagram showing agonist-modulated ion currents and Ca2+ fluxes. Variability in electrical activity patterns is modeled by variation in large-conductance Ca2+ activated K+ (IBK) and background Na+ (INab) currents. Bulk cytosolic and ER Ca2+ concentrations are denoted c and cER, respectively. The Ca2+ concentration in a microdomain near the mouth of open voltage-gated Ca2+ channels is denoted cd. CRH modulates five ionic currents: ICRH, inward cationic; IK,leak, K+ leak; IKir, inwardly rectifying K+; ICa, high voltage gated Ca2+; IBK (activated by cd). CORT is assumed to inhibit CRH action on BK channels (Shipston et al. 1996). The Ca2+ influx Jin depends on ICa and ICa,leak, a Ca2+ leak current. The Ca2+ fluxes into and out of the ER and out of the cell are Jref, Jrel, and Jout, respectively. AVP increases Jrel, releasing Ca2+ from the ER and activating a Ca2+ activated K+ current, IKCa. In parallel, AVP activates an inward leak current, IAVP, or inhibits IK,leak. ER Ca2+ dynamics are only included for simulations involving AVP. Activation and inhibition are denoted by dashed lines terminating in arrows or circles. CRHR, CRH receptor; AVPR, AVP receptor. B: time courses of pulses used to simulate CRH and AVP application. CRH application targeting gCRH, Vm, and kCaBK and AVP’s effect on gAVP were modeled with a pulse with τ = 12.5 s and kp = 1 (solid line). CRH effects on gKir and gK,leak were simulated with τ = 12.5 s and kp = 0.3 and 0.1, respectively (dash-dotted and dashed lines). AVP action on Ca2+ release from the ER was simulated with a pulse with τ = 1 s and kp = 10 (dotted line).
Gating variables are governed by equations of the form
where x ∈ {hNa, n, hA, nBK}. The steady-state activation and inactivation functions are of the form
for x ∈ {mNa, hNa, mCa, n, nKir, nA, hA, nBK}. We model a subset of BK similar to the stress-related exon splice variant (Shipston et al. 1999; Xie and McCobb 1998), as in prior theoretical work (Duncan et al. 2015, 2016). IBK is assumed to depend on the [Ca2+] in a microdomain near open calcium channels, cd. This is approximated by including Ca2+ domain dependence in the steady-state activation function for BK as follows:
where cd = −AICa and A converts ionic current to microdomain [Ca2+] (Tsaneva-Atanasova et al. 2007). With the default parameters, BK activation is a steep function of membrane potential with half-maximal activation at approximately −15.1 mV. When kCaBK is increased, the activation curve shifts to the right and becomes less steep preferentially at voltages above the half-maximal activation value. The activation of IKCa depends on the [Ca2+] in the cytosol, c, according to the activation function
Dynamics for c and [Ca2+] in the ER, cER, are governed by the equations
where σER is the ratio of cytosolic to ER volume and fcyt and fER are the fractions of free Ca2+ in the cytosol and ER, respectively. The calcium fluxes into the cell (Jin), out of the cell (Jout), into the ER (Jref), and out of the ER (Jrel) are given by
Here, α converts ionic current to [Ca2+]i, νc is the maximal calcium efflux rate across the plasma membrane, kc is the [Ca2+]i at which the plasma membrane pump is half-maximally active, prel is the rate of Ca2+ release from the ER, and kref is the rate of ER Ca2+ refilling. The terms involving ER Ca2+ are only included in simulations of AVP application, otherwise prel = kref = 0 and the cER equation is not included. Default parameter values are given in Table 1.
Table 1.
Default model parameter values
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| gNaV | 10 nS | Vn | 0 mV |
| gNab | 0.205 nS | sn | 5 mV |
| gCa | 1.4 nS | τn | 20 ms |
| gCa,leak | 0.025 nS | VnA | −20 mV |
| gK | 2.2 nS | snA | 10 mV |
| gKir | 1.3 nS | VhA | −60 mV |
| gA | 0.5 nS | shA | −10 mV |
| gKCa | 2 nS | τhA | 20 ms |
| gBK | 0.5 nS | kKCa | 0.4 µM |
| gK,leak | 0.1 nS | A | 0.11 µM·ms·fC−1 |
| gCRH | 0 nS | sBK | 5 mV |
| gAVP | 0 nS | kCaBK | 1.5 µM |
| ENa | 75 mV | τBK | 5 ms |
| ECa | 60 mV | VKir | −55 mV |
| EK | −75 mV | sKir | −10 mV |
| ENS | 0 mV | fcyt | 0.01 |
| cm | 6 pF | fER | 0.01 |
| VmNa | −15 mV | α | 0.0015 µM/fC |
| smNa | 5 mV | νc | 0.03 µM/ms |
| VhNa | −60 mV | kc | 0.1 µM |
| shNa | −10 mV | prel | 0.0001 ms−1 |
| τhNa | 2 ms | kref | 0.1 ms−1 |
| VmCa | −12 mV | σ | 31 |
| smCa | 10 mV |
To capture variability in activity patterns, we study model behavior across two-parameter planes in addition to single representative points in parameter space. The steady-state behavior at each point in 64 × 64 grids across two parameters of interest is simulated, and the number of peaks in a depolarization event is recorded. A depolarization event is an oscillatory simulation trajectory beginning at and returning to a small neighborhood of the point in state-space defined at the trajectory’s global minimum in membrane potential. The behavior of the model throughout the grid is summarized as a heat map indicating the number of peaks per event, where quiescent, spiking, and bursting patterns of activity are represented by zero, one, or greater than one peak per event, respectively.
We examine five different ways in which CRH can affect electrical activity (Fig. 1A): increasing gCRH, decreasing gKir, decreasing gK,leak, enhancing ICa by decreasing VmCa, and inhibiting IBK by increasing kCaBK. AVP response is modeled by increasing prel and in parallel increasing gAVP or decreasing gK,leak. The effects of CRH or AVP are explored in two ways. First, the steady-state behavior is studied with the parameter plane approach described above, where the parameter on the x-axis is the CRH- or AVP-modulated parameter of interest and the parameter on the y-axis represents a parameter for which intrinsic variability is being investigated. Second, the time courses of selected points in parameter space are displayed.
The second approach involves simulating a dynamic increase or decrease of a parameter according to a pulse profile (Fig. 1B) to approximate the application of agonists and intracellular signaling. This generates time courses for comparison to experimentally observed traces. The pulse function g(t) is designed to begin at 0 and peak at a value of 1. The quantityΔpg(t) is then added to the agonist-modulated parameter value to simulate agonist application, where Δp is the maximum change in the parameter value. To construct the pulse we start with the function
where t0 is the time of initiation of the pulse and the maximum value f(t) = 1 occurs at time t0 + 2τ. For some simulations it was necessary to better control the rate of increase and shape of the pulse, conveniently achieved by passing f(t) through a saturating Michaelis-Menten nonlinearity. The pulse equation is
where the positive parameter kp is the value of f(t) that yields g(t) = 0.5 and the factor (1 + kp) renormalizes the pulse so that g(t) = 1 when f(t) = 1. The new pulse function g(t) also peaks at t0 + 2τ. When kp is small, the pulse will rise to saturation rapidly and have a long duration, while as kp becomes large g(t)→f(t), with slower rise rate and a shorter duration.
Simulations were performed with the fourth-order Runge-Kutta method with a time step of 0.5 ms and custom software written in OpenCL (Fletcher et al. 2016). This software computes the simulations required for the two-parameter grids in parallel on a modern programmable graphics processing unit in a fraction of a second, allowing for a rapid exploration of the model’s parameter space. The model equations are defined in freely available files (http://lbm.niddk.nih.gov/sherman) that can also be directly simulated with XPPAUT software (Ermentrout 2002), which is freely available at http://www.math.pitt.edu/~bard/xpp/xpp.html.
RESULTS
Spontaneous and agonist-induced electrical activity of mouse corticotrophs in vitro.
Reports of spontaneous corticotroph activity patterns vary from silent (Lee et al. 2011, 2015; Lee and Tse 1997) to active (Duncan et al. 2015; Liang et al. 2011) to a mix of active and silent (Kuryshev et al. 1997; Zemkova et al. 2016), with a variety of activity patterns observed (Liang et al. 2011; Zemkova et al. 2016). In our perforated-patch recordings, essentially three patterns were observed in spontaneously active cells, plateau-like bursting, large-amplitude single-spiking activity, and pseudoplateau-like bursting, examples of which are shown in Fig. 2A. In agreement with previous reports (Liang et al. 2011), the majority of spontaneously active cells were spiking and a minority displayed bursting patterns (Zemkova et al. 2016).
Fig. 2.
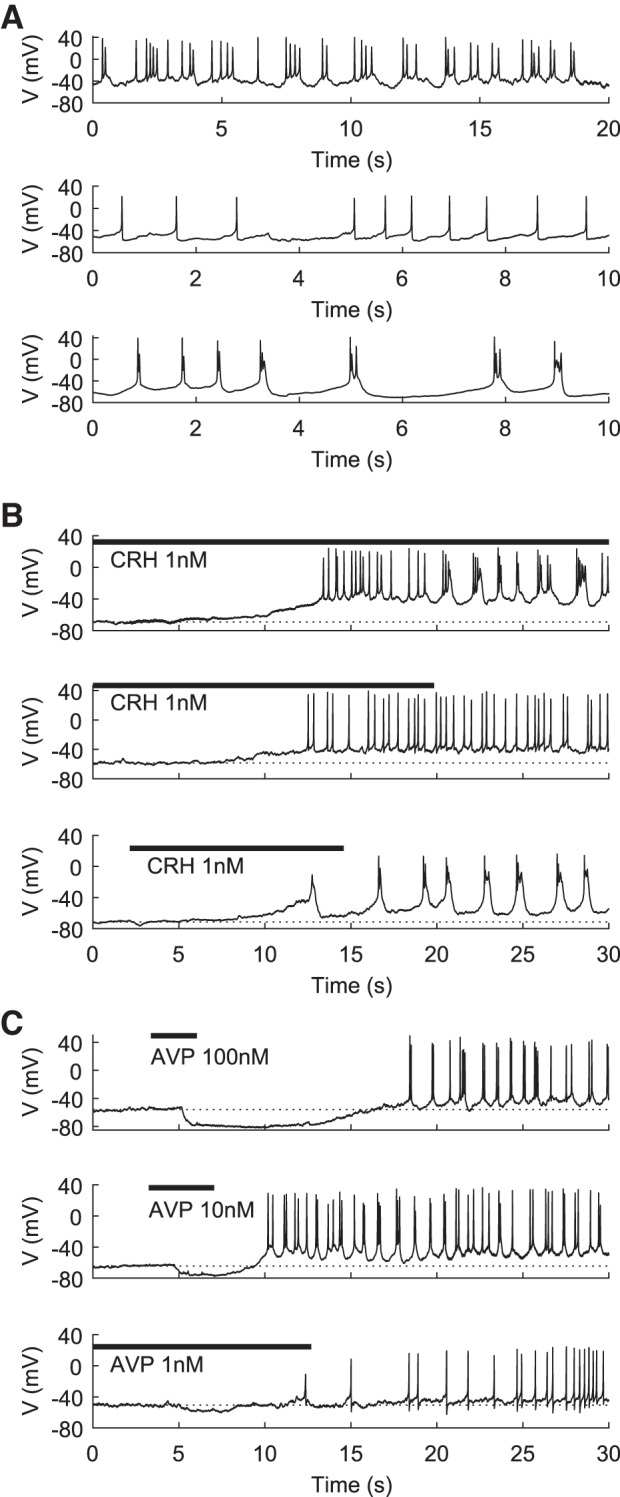
Representative patterns of spontaneous and agonist-induced electrical activity in mouse corticotrophs in vitro. A: examples of spontaneous plateau-like bursting (top), low-frequency spiking (middle), and pseudoplateau-like bursting (bottom). B: 3 cells that become gradually depolarized and then active in response to 1 nM CRH. Top: cell undergoes transitions from quiescence to spiking to plateau-like bursting. Middle: cell transitions from quiescence to spiking only. Bottom: cell transitions directly from quiescence to pseudoplateau-like bursting. C: 3 cells in which AVP causes a transient hyperpolarization and then a depolarization and transition to activity. Top: cell transitions directly to plateau-like bursting after a prolonged hyperpolarization in response to a 100 nM pulse of AVP. Middle: cell displays a brief hyperpolarization before a transition to irregular spikes and then plateau-like bursting in response to a 10 nM pulse of AVP. Bottom: cell shows a small hyperpolarization before transitioning to spiking only, in response to a 1 nM pulse of AVP. Horizontal bars in B and C indicate duration of CRH or AVP application.
CRH stimulation characteristically causes depolarization and increased electrical activity. Initially silent cells responded to CRH with a larger depolarization of ~10–30 mV (Kuryshev et al. 1995; Lee et al. 2011; Zemkova et al. 2016), while initially active cells were more modestly depolarized by 5–10 mV (Duncan et al. 2015; Kuryshev et al. 1995; Zemkova et al. 2016). It was previously reported that CRH only triggers pseudoplateau-like bursting in spiking cells (Duncan et al. 2015), but we found a greater diversity of transitions including transitions from silent or spiking to plateau or pseudoplateau-like bursting and silent to spiking (Zemkova et al. 2016). Figure 2B shows the responses of three cells to 1 nM CRH, demonstrating transitions from silent to spiking to plateau-like bursting, silent to spiking, and silent to pseudoplateau-like bursting, respectively.
Electrical responses to AVP in corticotrophs are characterized by an early transient hyperpolarization in response to release of Ca2+ from the ER and followed by depolarization and increased firing rate (Duncan et al. 2015; Lee et al. 2015; Zemkova et al. 2016). During the second phase, Duncan et al. (2015) observed only spiking with increased frequency, while we (Zemkova et al. 2016) observed spiking and/or plateau-like bursting. Figure 2C shows initially silent cells with three different AVP responses, which include varying degrees of first-phase hyperpolarization and subsequent transition to plateau bursts with tall spikes or spiking only.
Modeling spontaneous corticotroph electrical activity.
We study spontaneous activity by focusing on currents that can affect silent/active electrical status and currents that control the pattern of electrical activity. Kuryshev et al. (1997) suggested that IKir helps maintain the negative resting membrane potential (Vrest), and the TREK-1 has also been reported (Lee et al. 2011). INab has been shown to provide a depolarization (Liang et al. 2011; Zemkova et al. 2016) that counterbalances outward subthreshold currents, and we reported variability in the degree of expression of INab based on variability of hyperpolarization responses to Na+-deficient medium (Zemkova et al. 2016). Although not experimentally reported to our knowledge in corticotrophs, we find that a background Ca2+ conductance needs to be present in the model to maintain ER Ca2+ stores. Spontaneous activity occurs when there is a small net inward pacemaker current at hyperpolarized potentials, causing a depolarization and eventual crossing of the spike threshold. Variation in pacemaker or background conductances could therefore account for the presence or absence of spontaneous activity. For default values of model parameters, spontaneous activity emerges for approximately gNab > 0.2 nS (Fig. 3A), gKir < 1.3 nS (Fig. 4C), and gK,leak < 0.1 (Fig. 4E). Activity patterns near this transition have low frequencies of 1–3 Hz or less, compatible with previous reported observations (Liang et al. 2011; Zemkova et al. 2016).
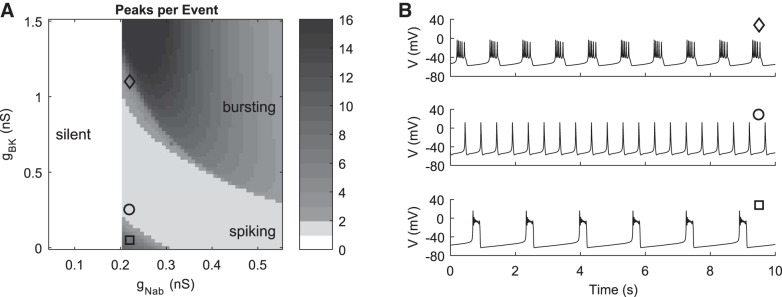
Fig. 3.
Spontaneous activity in the model as BK and background Na+ conductances are varied. A: heat map in this and subsequent figures shows the number of peaks per depolarization event as a function of 2 parameters, here gBK and gNab. Silent, spiking, and bursting time courses have 0 (white), 1 (light gray), or >1 (shades of dark gray) peak per event, respectively. There is a BK-independent transition from silent to active at gNab = 0.2 nS. The number of peaks per event was measured for each parameter combination of a 64 × 64 grid. B: 3 selected parameter combinations indicated by markers in A (each with gNab = 0.22 nS) that reproduce plateau-like bursting (◇; gBK = 1.1 nS), spiking (○; gBK = 0.25 nS), and pseudoplateau activity (□; gBK = 0.05 nS), similar to the time courses presented in Fig. 2.
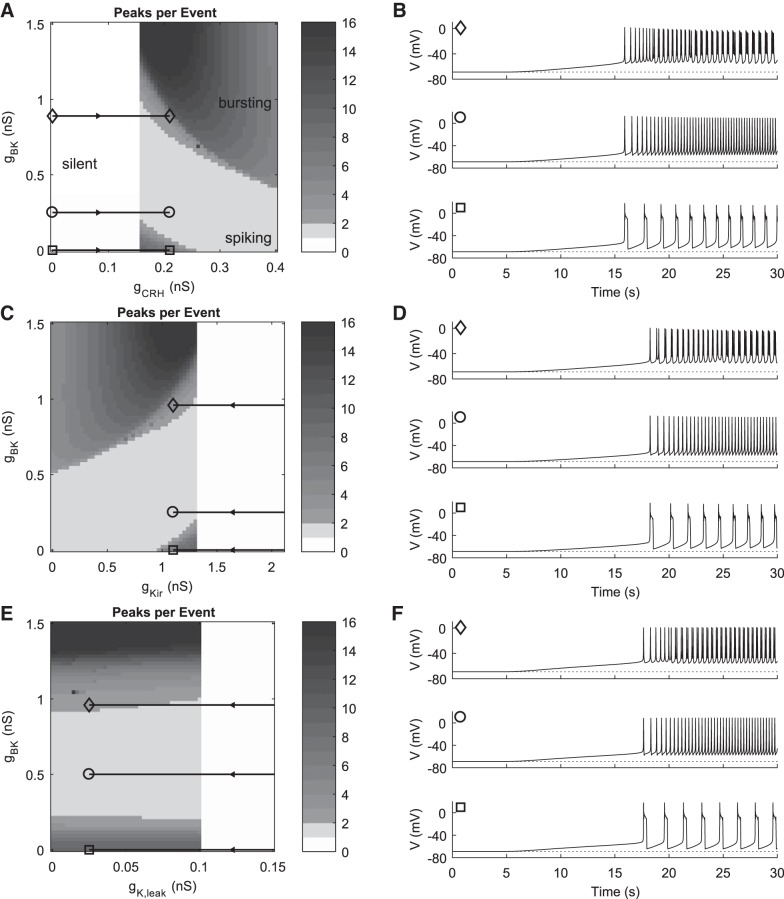
Fig. 4.
Simulation of CRH response by modulation of pacemaker currents. A, C, and E: peaks per event as a function of BK and CRH-induced inward, Kir, and K+ leak conductances, respectively. Horizontal black trajectories with markers indicate the gBK values and range of pacemaker conductances used in pulse simulations. B: time courses showing response to increasing gCRH from 0 nS to 0.22 nS at values of gBK indicated in A (gBK = 0.89 nS, gBK = 0.25 nS, and gBK = 0 nS). Default parameter values were used, except gNab = 0.05 nS, and pulse parameter kp = 1. D: time courses showing response to decreasing gKir from 3.5 nS to 1.1 nS at values of gBK indicated in C (gBK = 0.96 nS, gBK = 0.25 nS, and gBK = 0 nS). All other parameters had default values, and pulse parameter kp = 0.3. F: time courses showing response to decreasing gK,leak from 1.9 nS to 0.025 nS at values of gBK indicated in E (gBK = 0.96 nS, gBK = 0.5 nS, and gBK = 0 nS). All other parameters had default values, and pulse parameter kp = 0.1. In B, D, and F, the pulse of simulated CRH begins at 5 s and peaks at 30 s. Dotted lines indicate the initial Vrest of −69 mV, and top, middle, and bottom time courses show a depolarization of ~15 mV, 13 mV, and 5 mV, respectively.
In addition to background currents, the pattern of spontaneous activity depends on the combination of voltage- and Ca2+-dependent conductances. Because BK channels have been implicated in contributing to the patterns of bursting activity in corticotrophs (Duncan et al. 2015, 2016) and other pituitary cells (Van Goor et al. 2001a), we examined the effect of variation in gBK on spontaneous and agonist-induced activity. The dependence of activity patterns on gNab and gBK is summarized in a two-parameter heat map (Fig. 3A). Silent cells have zero peaks (white in the heat map), spiking cells have one peak (light gray), and bursting cells have more than one peak per event (shades of dark gray). The transition from silent to active at gNab = 0.2 nS is vertical, that is, BK independent. This is because the BK open probability at resting membrane potentials in silent models (less than −50 mV) is very near zero, which means that increasing gBK has no effect on whether the cell is active or silent. Within the active region (gNab > 0.2 nS) the levels of both gBK and gNab control whether the model spikes or bursts; the boundaries between spiking and bursting are sloped curves. There are two bursting regions. The first, smaller region comprises pseudoplateau-like bursting when both gBK and gNab are low, while for higher gBK there is a larger region with both plateau-like and pseudoplateau-like bursting. In both regions, bursting patterns tend to have taller spikes near the boundary with the spiking region. Including IA in the model helped to widen this boundary of plateau bursts consisting of few (2 or 3) tall spikes in the upper burst region. Representative time courses for which the model activity mimics the patterns observed in the data were generated at parameter combinations indicated by the circle, diamond, and square markers (Fig. 3B). Time courses with higher gBK have lower amplitude because fast-activating K+ currents such as IBK act to limit spike amplitude and thus compete with depolarizing currents such as INaV and ICa.
CRH-facilitated electrical activity.
Next we compare mechanisms of CRH-induced electrical activity in the model. All the proposed mechanisms except modulation of BK current involve increasing the net inward current at hyperpolarized potentials and are therefore hypothesized to be capable of causing a silent-to-active transition. It follows that at least one mechanism other than BK must be present to explain CRH’s effects on silent cells (Duncan et al. 2015). The first mechanism considered is the activation of a putative cationic current, modeled here as ICRH. To simulate the CRH effect on an initially silent cell, we set gNab = 0.05 nS so that the model is hyperpolarized with Vrest = −69 mV. The effect of increasing gCRH across a range of gBK values is then examined, summarized across a two-parameter grid in terms of the number of peaks per event (Fig. 4A). As in Fig. 3A, there is a BK-independent transition from silent to active, while in the active region both gBK and gCRH control the activity pattern. Choosing appropriate values of gBK and imposing a dynamic pulse increase [see the pulse equation g(t) in materials and methods] in gCRH from 0 to 0.22 nS (horizontal trajectories in Fig. 4A) reproduces time courses with the experimentally observed patterns (compare Fig. 4B to Fig. 2B). The degree of depolarization achieved depends on the degree of initial hyperpolarization and on the nature of the activity pattern (specifically its baseline potential) obtained by CRH stimulation. In this case, all three time courses have the same Vrest of −69 mV, and they achieve depolarization of ~15 mV, 13 mV, and 5 mV for high (diamond), medium (circle), and low (square) gBK, respectively. At the silent-spiking transition, increasing levels of gBK lead to increasingly depolarized baselines in the activity patterns, because activation of BK preempts activation of the delayed rectifier IK (Van Goor et al. 2001a). At silent-to-bursting transitions, the baseline potential is expected to be lower because during a burst there is more time during the burst active phase to activate IK and IKCa, leading to stronger repolarization.
CRH stimulation by reduction of IKir, modeled by reducing gKir, is also consistent with reported experimental data. The value of gNab was set to 0.205 nS, which makes the silent to active transition occur at the default value of gKir = 1.3 nS. With these parameters, it was necessary to set gKir = 3.5 nS to have Vrest = −69 mV. The boundary at gKir = 1.3 nS between silent and active is again vertical and gBK independent (Fig. 4C). Depending on the level of gBK, decreases in gKir from 3.5 to 1.1 nS yield transitions from silent to spiking to bursting, silent to spiking only, or silent to pseudoplateau-like bursting as experimentally observed (Fig. 4D). The same degree of depolarization as was seen using the gCRH mechanism is obtained here, for the same reasons. However, while the top 30% of the change in gCRH lies in the active region of the plane (Fig. 4A), only ~8% of the total change in gKir is in the active region. To cross the silent-active threshold at a comparable latency to the gCRH simulations therefore required a more rapidly rising pulse profile that saturates before peaking in the active region (obtained by decreasing kp from 1 to 0.3).
Inhibition of an outward leak is evaluated next. With gNab = 0.205 nS, the transition from silent to active occurs at the default value of gK,leak = 0.1 nS (Fig. 4E). To obtain Vrest = −69 mV without changing any other parameters, gK,leak had to be increased dramatically to 1.9 nS. Decreasing gK,leak can also cause activation of silent cells in a BK-independent manner. However, in this case there is a much narrower range of gBK values for which changes in gK,leak are able to cause the double transition of silent to spiking to bursting. In Fig. 4E, the bursting regions have relatively horizontal boundaries; changes in gK,leak are only weakly able to cause transitions between spiking and bursting. The same degree of depolarization is again possible with this mechanism (Fig. 4F). However, only the last 4% of the change in gK,leak lies in the active region, necessitating an even broader and more rapidly saturating pulse (kp = 0.1).
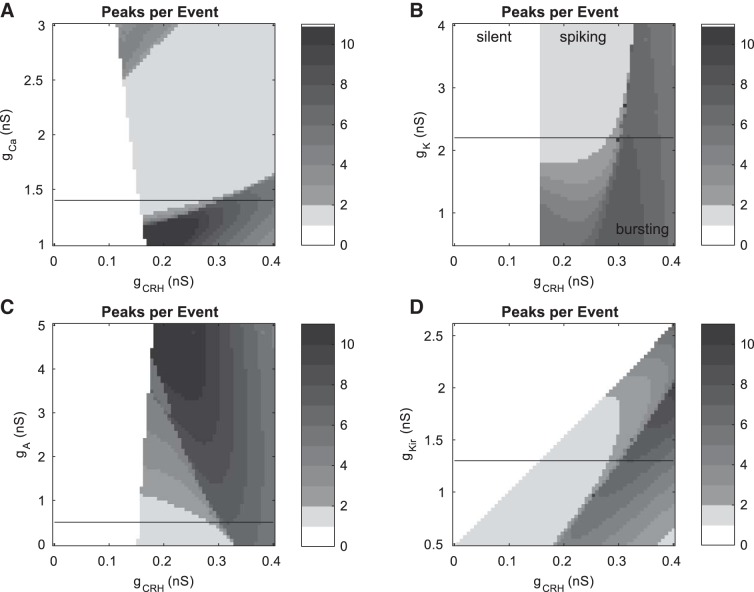
While variations in gBK support a wide range of experimentally observed spontaneous and agonist-induced electrical activity patterns, these can also be achieved by variation in other intrinsic model parameters. With gBK fixed at 0.6 nS, we demonstrate this with two-parameter grids featuring gCRH and each of gCa, gK, gA, and gKir (Fig. 5, A–D, respectively). The horizontal lines in Fig. 5 indicate the default value of the parameter on the y-axis. In each case, there is a range of gCa, gK, gA, or gKir values for which trajectories beginning in the silent region and moving rightward will undergo experimentally observed transitions patterns: silent to spiking, silent to bursting, and silent to spiking to bursting. In the case of gCa (Fig. 5A), there exist two burst regions; in this case the upper region has pseudoplateau-like bursting. The lower burst region in Fig. 5A shares commonalities with the burst region seen in the remaining panels. Namely, there is a region of plateau-like bursts with few tall spikes for lower gCRH values bordering the spiking region. For higher gCRH values, there is a transition to bursting with a higher number of peaks per event, which becomes progressively more pseudoplateau-like. The nature of the burst mechanisms involved in these two-parameter grids will be the subject of future investigation. The BK current is important for producing this bursting region (it is the same region as the upper burst region in Figs. 3 and 4). It has the correct shape relative to the silent and spiking regions to support the silent-to-spiking-to-bursting transition in response to CRH simulation using only one mechanism at a time. We note that it is also possible to achieve this compound transition when gBK = 0, but two or more CRH mechanisms must be invoked in tandem (not shown).
Fig. 5.
Variation in intrinsic model conductances with fixed gBK can give rise to variation in CRH-stimulated activity patterns. With gBK set to 0.6 nS, varying gCa (A), gK (B), gA (C), or gKir (D) allows the model to support the experimentally observed transitions from silent to spiking, silent to bursting, and silent to spiking to bursting as gCRH is increased. Horizontal black lines indicate the default parameter values for the 4 parameters.
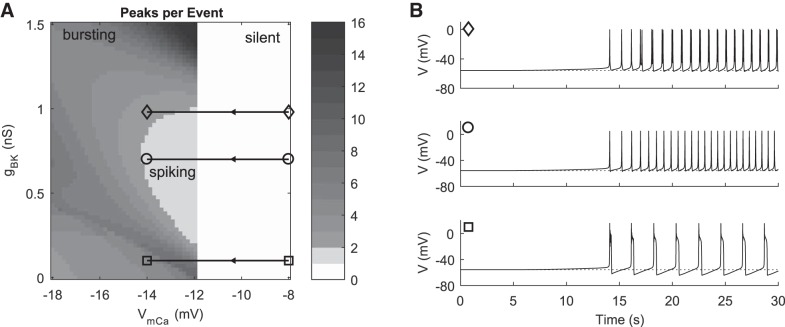
The model can also reproduce corticotroph-like pattern transitions in response to increases in ICa as was seen in earlier models (Duncan et al. 2015, 2016; LeBeau et al. 1997, 1998; Shorten et al. 2000). We increase ICa by left shifting the steady-state activation curve by decreasing VmCa. With gNab = 0.205 nS, a transition between silent and active occurs near the default value of VmCa = −12 mV (Fig. 6A). A hyperpolarized silent starting point was obtained by setting VmCa = −8 mV. This current is poorly activated at hyperpolarized potentials, so Vrest was relatively depolarized at −56 mV, with further increases in VmCa unable to appreciably hyperpolarize Vrest. This limits the potential for depolarization by this mechanism, consistent with previous models (LeBeau et al. 1997, 1998; Shorten et al. 2000). Decreasing VmCa from −8 mV to −14 mV (horizontal trajectories in Fig. 6A) at three representative values of gBK produces transitions from silent to active and between spiking and bursting activity patterns (Fig. 6B).
Fig. 6.
Simulation of CRH response by left shifting the steady-state activation curve of ICa. A: peaks per event as a function of BK conductance and VmCa. Horizontal black trajectories with markers indicate gBK values and range of VmCa used in pulse simulations. B: time courses showing response to decreasing VmCa from −8 mV to −14 mV at values of gBK indicated in A (gBK = 0.98 nS, gBK = 0.7 nS, and gBK = 0.1 nS). The pulse of simulated CRH begins at 5 s and peaks at 30 s with pulse parameter kp = 1. Dotted lines indicate the initial Vrest of −56 mV.
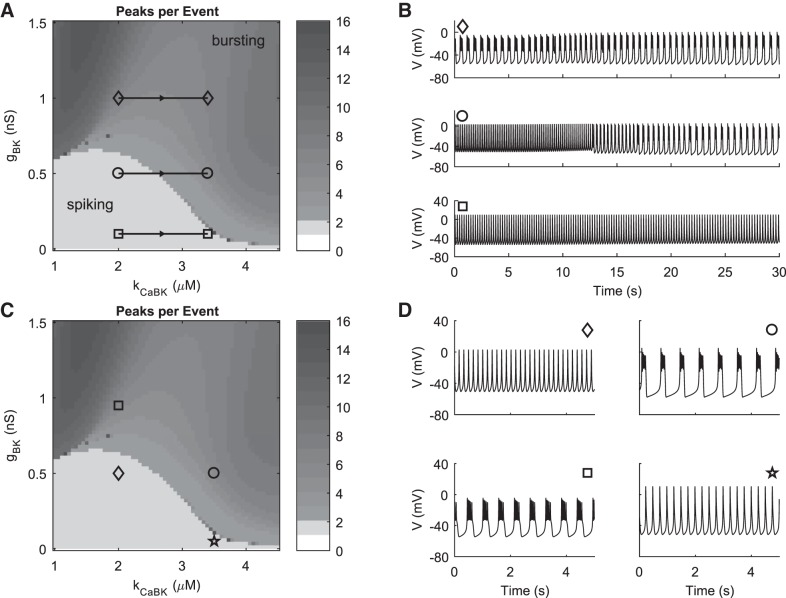
CRH-dependent suppression of BK current has been reported to be due to a right shift in its activation function and a reduction in its time constant (Duncan et al. 2015, 2016). We model the CRH effect by increasing the parameter kCaBK but maintain a fixed small time constant (τnBK = 5 ms) for BK activation. Because BK channels are inactive at hyperpolarized potentials, further BK inhibition cannot account for depolarization from a hyperpolarized Vrest and is only expected to be able to cause transitions between already active electrical patterns. Activity is ensured by setting gNab = 0.205 nS and gCRH = 0.105 nS. gCRH is not changed dynamically so that we can focus on the effect of BK modulation. The effect of increasing kCaBK is to right-shift the BK activation curve. Above kCaBK = 2 μM, there is also a significant reduction in the slope of the activation curve, preferentially for voltages above the half-maximal activation so that the activation curve saturates more gradually as voltage increases. The gBK-kCaBK parameter plane (Fig. 7A) shows that the model indeed predicts a transition from spiking to bursting for gBK values less than ~0.6 nS and for sufficient increase in kCaBK. The model also predicts such a transition if kCaBK is reduced, but only for a small range of gBK values. Time courses of responses to a dynamic increase of kCaBK for three representative values of gBK are shown in Fig. 7B, corresponding to the horizontal trajectories shown in Fig. 7A. Increasing kCaBK from 2 to 3.4 increases the half-maximal BK activation from −14.7 mV to −13.7 mV. The shift in BK activation does not appreciably increase the frequency of depolarization events, further supporting the hypothesis that one of the other mechanisms for CRH action should be present in addition to changes in BK activation.
Fig. 7.
Simulations of changes in BK conductance and CORT-sensitive CRH-induced bursting. A: peaks per event as a function of BK conductance and kCaBK, with gNab = 0.205 nS. A steady-state CRH-induced increase in frequency is also included by setting gCRH = 0.105 nS. Horizontal black trajectories with markers indicate gBK values and range of kCaBK used in pulse simulations. B: time courses showing the response to increasing kCaBK from 2 μM to 3.4 μM (corresponding to increasing the half-maximal BK activation from −14.7 mV to −13.7 mV) at values of gBK indicated in A (gBK = 1 nS, gBK = 0.5 nS, and gBK = 0.1 nS). The pulse of simulated CRH begins at 5 s and peaks at 30 s with pulse parameter kp = 1. C: peaks per event as in A but showing the parameter combinations used to simulate BK conductance changes and CORT effects on CRH-induced bursting. D: steady-state time courses for the points of interest in C. CRH action on kCaBK induces rightward motion in the plane, causing a spiking-to-bursting transition (◇ to ○). Subsequent block of BK conductance (or subtraction, as in a dynamic-clamp experiment) induces downward motion in the plane, causing a transition back to spiking (○ to ☆). CORT prevents CRH-induced rightward shift in BK activation, so CORT+CRH is modeled as remaining at the ◇ marker. Subsequently increasing BK conductance (for instance by dynamic clamp) causes upward motion in the plane (◇ to □), leading to a recovery of bursting behavior. (kCaBK, gBK) parameter combinations: ◇, (2, 0.5); ○, (3.5, 0.5); ☆, (3.5, 0.05); □, (2, 0.95).
The CRH-induced transition from spiking to bursting was shown to be reversed by subtraction of BK current with dynamic clamp (Duncan et al. 2016). In the same study, CORT-pretreated corticotrophs no longer burst in response to CRH, but bursting could be restored by addition of BK via dynamic clamp. These results can be reproduced in our model as follows. CRH induces rightward motion in the gBK-kCaBK plane and thus a transition from spiking to bursting (diamond to circle in Fig. 7C). Spiking is recovered by decreasing gBK (circle to star in Fig. 7C). CORT pretreatment is hypothesized to prevent the PKA-dependent change in BK channel activation via activation of a phosphatase (Tian et al. 1998), although it also has BK-independent effects (Duncan et al. 2016). We consider only the prevention of BK modulation by CORT, such that the CRH+CORT condition is simulated by simply remaining at the initial parameter point (diamond in Fig. 7C). Bursting is restored in this case by upward motion in the gBK-kCaBK plane (diamond to square in Fig. 7C). The time courses for each of the indicated parameter combinations are shown with matching markers in Fig. 7D.
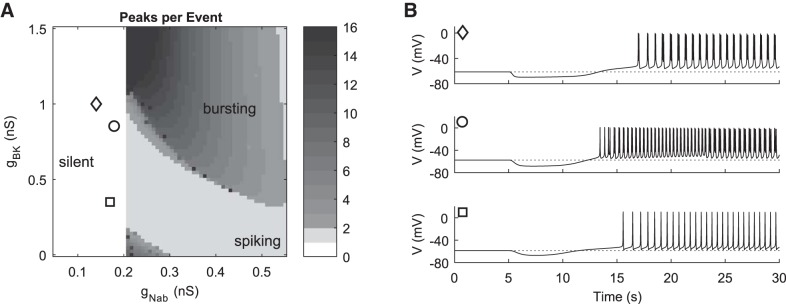
AVP-facilitated electrical activity.
To model AVP responses, we include an ER Ca2+ compartment, as described in materials and methods. The ohmic Ca2+ current ICa,leak is set to 0.05 nS to maintain ER Ca2+ store levels, and IKir is increased to 1.5 nS to compensate the extra background conductance. Variation in gBK and gNab leads to a similar pattern of silent, spiking, and bursting regions as before (compare Fig. 8A to Fig. 3A). We highlight three parameter combinations in the gBK-gNab plane for which the main features of the experimental time courses are reproduced when an AVP-like stimulus is applied (compare Fig. 8B to Fig. 2C). Note that gNab is not varied in the AVP stimulus, so only points and not trajectories are shown in Fig. 8A. AVP-induced hyperpolarization is reproduced with a dynamic increase in the ER Ca2+ release rate prel, following a rapid pulse profile. Increasing prel causes release of Ca2+ from the ER and a transient spike in cytosolic Ca2+, activating IKCa and thereby causing hyperpolarization. To reproduce the sustained depolarization with spiking- or bursting-driven Ca2+ influx, we tested two alternative mechanisms: the activation of an inward ohmic current, IAVP (Fig. 8), and the inhibition of IK,leak (not shown). These modulations in ionic currents were triggered at the same moment (time = 5 s) but followed a slower pulse profile identical to the above CRH manipulations. For the same reasons as in Fig. 4, the activation of IAVP or inhibition of IK,leak can both cause transitions from silent to active as well as transitions between spiking and bursting activity depending on the level of gBK. The three doses of AVP are modeled with different maximal changes in prel and gAVP, as indicated in Fig. 8. The duration of hyperpolarization and the sequence of transitions between activity patterns are all captured by the model. For strong ER Ca2+ release, the resulting high [Ca2+]i saturates the membrane calcium pumps, leading to a longer Ca2+ spike phase and hyperpolarization. The Ca2+ dynamics in the model also naturally provide a small depolarization as a consequence of store depletion, even without changes in IAVP or IK,leak. The release of store Ca2+ leaves the ER depleted and [Ca2+]i eventually drops below pre-AVP levels, in turn causing depolarization due to reduction of IKCa (Li et al. 1997). This effect is small, however, and can only trigger silent-to-active transitions if the pre-AVP model parameters are set close enough to the transition boundary. Depolarization is less dramatic in these cases compared with CRH responses mainly because the initial Vrest is more depolarized, similar to the traces shown in Fig. 2C.
Fig. 8.
Simulation of AVP responses in a model including ER Ca2+ dynamics. A: peaks per event as a function of variation in the 2 parameters gBK and gNab. Three representative parameter combinations are indicated by markers for which AVP stimulation reproduces the experimental time courses. ER Ca2+ dynamics are included, and the calcium leak and inward rectifier conductances were increased to gCa,leak = 0.05 nS and gKir = 1.5 nS, respectively. Parameter combinations (gNab, gBK): ◇, (0.14, 1); ○, (0.18, 0.85); □, (0.17, 0.35). All other parameter values had default values. B: time courses for AVP responses of the model at the 3 parameter combinations indicated by corresponding markers in A. AVP application is simulated beginning at time = 5 s by increasing the ER leak rate from default value prel = 0.0001 s−1 transiently following a fast pulse profile (τ = 1 s, kp = 10), and by simultaneously activating an inward ohmic conductance gAVP following a slower pulse profile (τ = 12.5 s, parameter kp = 1). To model the different doses of AVP in Fig. 2C, maximum increase in prel during the pulse was 0.01 s−1, 0.0025 s−1, and 0.001 s−1, and peak gAVP was 0.07 nS (top), 0.055 nS (middle), and 0.04 nS (bottom).
DISCUSSION
We have presented a mathematical model that captures many aspects of the diversity in spontaneous and agonist-stimulated electrical activity and Ca2+ dynamics of pituitary corticotrophs in vitro. Although it is likely that there is variation in several ionic conductances underlying this diversity, we show that variation in as few as two parameters is sufficient to capture this diversity: gNab to control activity status and gBK to determine activity pattern. We found at least two distinct bursting mechanisms giving plateau and pseudoplateau burst patterns in close proximity in the gBK-gNab parameter plane, along with silent and spiking states. We compared five potential mechanisms of action for CRH action and two for AVP action. Our comparative analysis of these mechanisms within a single mathematical model employed analysis of two-parameter planes to observe the totality of behaviors associated with each mechanism. We found that the different CRH mechanisms can each explain different subsets of the phases of the CRH response, including pacemaking depolarization and transitions between activity patterns. Specifically, the mechanisms involving ICRH, IKir, and IK,leak could account for all features of the CRH response, ICRH most efficiently. The ICa mechanism could account for transitions from silent to active and between activity patterns but not depolarization, while the BK mechanism could only account for transitions between spiking and bursting. The BK current is important for generating a bursting region in parameter space such that the silent-to-spiking-to-bursting transition could be obtained with a single one of these CRH mechanisms. By including ER Ca2+ dynamics and IAVP, we were also able to reproduce the phases of AVP responses. Taken together, these results suggest that several redundant mechanisms for agonist-induced electrical activity may coexist in corticotroph cells and that intrinsic variability or experimental perturbations may bias the cell toward a subset of these mechanisms.
To study the diversity in corticotroph electrical activity patterns in the model, we focused on the role of the ionic currents controlling whether the cell is active and currents that could generate diversity in the activity pattern. The background currents (INab, IKir, IK,leak, and ICa,leak) are the dominant factors controlling whether the cell is silent or active. Variations in these currents, either intrinsic or due to experimental perturbations during cell culture or electrophysiological recording, could influence Vrest and the percentage of cells observed to be spontaneously active. For instance, in whole cell recordings the concentrations of intracellular polyamine and other cations mediating the inward rectification of IKir are expected to run down, effectively converting IKir to IK,leak. In the model, such an increase in IK,leak would strongly suppress spontaneous activity but would not prevent depolarization due to the subsequent activation of ICRH or inhibition of IKir and/or IK,leak.
We found that variation of two parameters, gBK and gNab, was sufficient to observe spiking, plateau-like bursting, and pseudoplateau-like bursting, but questions remain as to the sources of diversity in corticotrophs. A diversity of activity patterns could also be achieved in the model with fixed nonzero gBK and variation in other parameters including one of gCa, gK, gA, or gKir. Fast-activating K+ conductances such as gBK limit action potential overshoot, because they directly compete with voltage-gated inward currents such as INaV. It remains to be seen whether tall-spiking corticotrophs indeed have lower BK currents or other fast-activating K+ currents and higher INaV. We did not include some other ionic currents reported in corticotrophs, such as the transient Ca2+ (Guérineau et al. 1991; Marchetti et al. 1987), hyperpolarization-activated cyclic nucleotide-gated (Tian and Shipston 2000), or other K+ channels. These other currents may be necessary for reproducing the full complement of diversity in activity patterns observed in corticotrophs (Liang et al. 2011; Zemkova et al. 2016). Further modeling could provide additional insight by incorporating recent reports of responses of corticotrophs to Na+- and Ca2+-deficient medium, inhibition of INaV by tetrodotoxin, and modulation of L-type Ca2+ channels by nifedipine and BAY K 8644 (Zemkova et al. 2016). Finally, we chose not to include noise in our simulated time courses, but this would help to reproduce experimentally observed features such as stochastic transitions between patterns (expected in our model for parameter sets near transition curves) and very low-frequency firing (expected for parameter sets in the silent region near silent-to-active transition).
Our comparative analysis shows that the different CRH mechanisms have different but complementary effects in generating the CRH response. Our results are largely consistent with previous theoretical results but extend them in several ways. Previous modeling studies found that reduction of IK,leak (LeBeau et al. 1997) or IKir (Shorten et al. 2000) alone could not trigger activity in silent cells. An increase in ICa was required, although a combination with one of the other two mechanisms was more efficient than a change in ICa alone. The CRH response by the ICa mechanism is comparable in our model to previous studies, showing activation of silent cells without depolarization. However, we show using two-parameter plane analysis that reduction of either of the K+ currents alone can trigger many features of the CRH response. This suggests that the context of model parameters may favor one or another mechanism; our model favored the mechanism of ICRH activation for reproducing all CRH response features, while still being permissive for the Kir and K+ leak mechanisms. Combinations of CRH mechanisms are also more efficient than individual CRH mechanisms alone in our model (not shown). The BK mechanism for converting spiking to bursting was coupled to an increase in ICa in a previous study to explain the CRH response (Duncan et al. 2015). We have clarified that using any of the other four mechanisms will allow silent cells to become activated, while any of the three pacemaker currents would be required to explain a gradual depolarization from rest to threshold. Once active, the cell’s activity pattern could be converted from spiking to bursting by CRH-induced changes in BK channel activation (or one of the other mechanisms in the case of lack of BK channel modulation).
The steady-state effects of AVP on corticotroph electrical and Ca2+ dynamics have been modeled previously (Duncan et al. 2015). We have extended this by including the full transient response to AVP. This required only simple Ca2+ dynamics in the cytosol and ER, as well as a parallel mechanism to directly affect ion channels similarly to CRH action. Our model could be further used to study how CRH and AVP synergize in their corticotroph-stimulating effects. The ability of AVP to trigger hyperpolarization in already silent cells required in the model a basal level of Ca2+ influx for filling the ER Ca2+ store while the membrane is at rest, accomplished here with an ohmic leak conductance. These components were sufficient to reproduce all phases of the AVP response. If present, a store-operated Ca2+ current would also promote both store refilling and AVP-induced depolarization and increased activity.
To compare model simulations of CRH and AVP to experimental time courses, we increased or decreased relevant parameters according to a time-dependent pulse profile. This was necessary to control transient features of the responses including latency to first spike and rate of depolarization. The latency to first spike reflects the distance from the initial parameter value of the silent-to-active transition point and the speed at which this distance is traversed. The first likely reflects variability in expression of pacemaker currents. The second depends on the properties of the intracellular signaling pathways linking receptors to ion channels. We did not model the biophysical details of these pathways, instead employing a phenomenological pulse function that approximated the latency in the CRH and AVP responses. In the cases of IKir and IK,leak mechanisms for CRH action, a large fraction of the initial conductance had to be blocked rapidly in order to reproduce the correct latency and depolarization rates, as reflected in the pulse properties used for those simulations. Whether differences exist in the kinetics controlling each of the different CRH- and AVP-modulated currents remains to be clarified experimentally.
The diversity of electrical activity patterns observed in corticotrophs includes patterns seen in other pituitary cell types, and they appear to be more diverse than other pituitary cell types. For example, corticotrophs often fire single action potentials with large overshoot and express INaV, much like gonadotrophs (Marchetti et al. 1987; Van Goor et al. 2001b). On the other hand, there is evidence that in at least some corticotrophs BK channels are involved in promotion of bursting patterns such as pseudoplateau-like bursting, which is characteristic of somatotroph and lactotroph activity (Tsaneva-Atanasova et al. 2007; Van Goor et al. 2001b). Tall-spiked bursting not typically seen in the other pituitary cell types is also observed (Liang et al. 2011; Zemkova et al. 2016). During embryonic development, corticotrophs are the earliest cell type to mature (Stojilkovic et al. 2010). It is tempting to speculate that the corticotroph is closest in its electrical signaling system to a common ancestor from which all pituitary cell types specialize. If this is the case, the anterior pituitary progenitor cell could have electrical activity, a property that has not yet been studied to our knowledge. From this perspective, one could imagine that expression of the components of the electrical signaling system of this common ancestor would be activated or repressed to give rise to the specific patterns of activity of the adult anterior pituitary cell types.
The dependence of hormone responses on the context of the cell’s intrinsic parameter values and initial activity status is similar to state-dependent neuromodulation, a ubiquitous phenomenon that has been well studied in neurons and neuronal networks (Goldman et al. 2001; Marder et al. 2014). We showed with two-parameter plane analysis that the pattern of activity triggered by a modulator depends on the initial context of intrinsic cell parameters. It remains unclear which subset of the intrinsic parameters of corticotroph cells, such as the maximal conductances, display variability in vivo or in vitro and which parameters are affected by physiological modulators or experimental procedures such as those required for the presently reported in vitro electrophysiological recording.
The organization of corticotrophs and other pituitary cell types into functional networks in the intact pituitary gland is likely to influence spontaneous and agonist-induced electrical activity in vivo. Recent studies have used three-dimensional imaging to reveal complex organization of several pituitary cell types (Le Tissier et al. 2012). In particular, proopiomelanocortin-positive cells in the anterior pituitary form homotypic strands of cells, which are in close proximity to homotypic networks of LH-positive cells and pituitary microvasculature (Budry et al. 2011). These reports suggest that a combination of intrinsic cell properties, network organization (including the effects of paracrine modulators and cell-cell coupling), and hormonal inputs from outside the gland will likely be needed to fully understand corticotroph function in vivo.
Advances in computing power have allowed large-scale explorations of the parameter space of models of neurons and neuronal networks, sometimes called the model database approach (Günay 2015; Prinz et al. 2003). Studies of this type have revealed the possibility that multiple parameter combinations can give rise to similar neuronal activity (Goldman et al. 2001; Prinz et al. 2004; Taylor et al. 2009). Conversely, the same modulation can have different effects depending on the starting point of the system in parameter space. Our use of parameter sampling on two-parameter grids demonstrates that in the corticotroph model several ionic mechanisms of agonist action give rise to similar responses.
GRANTS
This work was supported by the Grant Agency of the Czech Republic [Grant 16-12695S (H. Zemkova)] and by funding from the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (P. A. Fletcher and A. Sherman) and the National Institute of Child Health and Human Development (S. S. Stojilkovic).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of one of the authors, which at the time of publication they indicate is: http://lbm.niddk.nih.gov/sherman. These materials are not a part of this manuscript, and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
AUTHOR CONTRIBUTIONS
P.A.F., S.S.S., and A.S. conceived and designed research; P.A.F., H.Z., S.S.S., and A.S. interpreted results of experiments; P.A.F. prepared figures; P.A.F. drafted manuscript; P.A.F., H.Z., S.S.S., and A.S. edited and revised manuscript; P.A.F., H.Z., S.S.S., and A.S. approved final version of manuscript; H.Z. performed experiments; H.Z. analyzed data.
ACKNOWLEDGMENTS
We are thankful to Dr. Greti Aguilera for help with animals.
REFERENCES
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides 25: 319–329, 2004. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Budry L, Lafont C, El Yandouzi T, Chauvet N, Conéjero G, Drouin J, Mollard P. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA 108: 12515–12520, 2011. doi: 10.1073/pnas.1105929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PJ, Sengül S, Tabak J, Ruth P, Bertram R, Shipston MJ. Large conductance Ca2+-activated K+ (BK) channels promote secretagogue-induced transition from spiking to bursting in murine anterior pituitary corticotrophs. J Physiol 593: 1197–1211, 2015. doi: 10.1113/jphysiol.2015.284471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PJ, Tabak J, Ruth P, Bertram R, Shipston MJ. Glucocorticoids inhibit CRH/AVP-evoked bursting activity of male murine anterior pituitary corticotrophs. Endocrinology 157: 3108–3121, 2016. doi: 10.1210/en.2016-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout B. Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. Philadelphia, PA: SIAM, 2002. doi: 10.1137/1.9780898718195 [DOI] [Google Scholar]
- Fletcher P, Bertram R, Tabak J. From global to local: exploring the relationship between parameters and behaviors in models of electrical excitability. J Comput Neurosci 40: 331–345, 2016. doi: 10.1007/s10827-016-0600-1. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Golowasch J, Marder E, Abbott LF. Global structure, robustness, and modulation of neuronal models. J Neurosci 21: 5229–5238, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérineau N, Corcuff JB, Tabarin A, Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology 129: 409–420, 1991. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- Günay C. Neuronal model databases. In: Encyclopedia of Computational Neuroscience, edited by Jaeger D, Jung R. New York: Springer, 2015, p. 2024–2028. [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci 29: 13684–13690, 2009. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology 136: 3925–3935, 1995. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol 502: 265–279, 1997. doi: 10.1111/j.1469-7793.1997.265bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol 33: 252–266, 2012. doi: 10.1016/j.yfrne.2012.08.002. [DOI] [PubMed] [Google Scholar]
- LeBeau AP, Robson AB, McKinnon AE, Donald RA, Sneyd J. Generation of action potentials in a mathematical model of corticotrophs. Biophys J 73: 1263–1275, 1997. doi: 10.1016/S0006-3495(97)78159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau AP, Robson AB, McKinnon AE, Sneyd J. Analysis of a reduced model of corticotroph action potentials. J Theor Biol 192: 319–339, 1998. doi: 10.1006/jtbi.1998.0656. [DOI] [PubMed] [Google Scholar]
- Lee AK, Smart JL, Rubinstein M, Low MJ, Tse A. Reciprocal regulation of TREK-1 channels by arachidonic acid and CRH in mouse corticotropes. Endocrinology 152: 1901–1910, 2011. doi: 10.1210/en.2010-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol 504: 367–378, 1997. doi: 10.1111/j.1469-7793.1997.367be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Tse FW, Tse A. Arginine vasopressin potentiates the stimulatory action of CRH on pituitary corticotropes via a protein kinase C-dependent reduction of the background TREK-1 current. Endocrinology 156: 3661–3672, 2015. doi: 10.1210/en.2015-1293. [DOI] [PubMed] [Google Scholar]
- Li YX, Stojilković SS, Keizer J, Rinzel J. Sensing and refilling calcium stores in an excitable cell. Biophys J 72: 1080–1091, 1997. doi: 10.1016/S0006-3495(97)78758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Chen L, McClafferty H, Lukowski R, MacGregor D, King JT, Rizzi S, Sausbier M, McCobb DP, Knaus HG, Ruth P, Shipston MJ. Control of hypothalamic-pituitary-adrenal stress axis activity by the intermediate conductance calcium-activated potassium channel, SK4. J Physiol 589: 5965–5986, 2011. doi: 10.1113/jphysiol.2011.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A, Lewis D, Guild S, Corda D, Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci USA 82: 8034–8038, 1985. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Brueggemann LI, Cribbs LL, Byron KL. Opposite regulation of KCNQ5 and TRPC6 channels contributes to vasopressin-stimulated calcium spiking responses in A7r5 vascular smooth muscle cells. Cell Calcium 45: 400–411, 2009. doi: 10.1016/j.ceca.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Childs GV, Brown AM. Membrane currents of identified isolated rat corticotropes and gonadotropes. Am J Physiol Endocrinol Metab 252: E340–E346, 1987. [DOI] [PubMed] [Google Scholar]
- Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci 37: 329–346, 2014. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Billimoria CP, Marder E. Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol 90: 3998–4015, 2003. doi: 10.1152/jn.00641.2003. [DOI] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci 7: 1345–1352, 2004. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Shipston MJ, Duncan RR, Clark AG, Antoni FA, Tian L. Molecular components of large conductance calcium-activated potassium (BK) channels in mouse pituitary corticotropes. Mol Endocrinol 13: 1728–1737, 1999. doi: 10.1210/mend.13.10.0355. [DOI] [PubMed] [Google Scholar]
- Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem 271: 9197–9200, 1996. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- Shorten PR, Robson AB, McKinnon AE, Wall DJ. CRH-induced electrical activity and calcium signalling in pituitary corticotrophs. J Theor Biol 206: 395–405, 2000. doi: 10.1006/jtbi.2000.2135. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev 31: 845–915, 2010. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Yasufuku-Takano J, Teramoto A, Fujita T. Corticotropin-releasing hormone excites adrenocorticotropin-secreting human pituitary adenoma cells by activating a nonselective cation current. J Clin Invest 98: 2033–2041, 1996. doi: 10.1172/JCI119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci 29: 5573–5586, 2009. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Knaus HG, Shipston MJ. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J Biol Chem 273: 13531–13536, 1998. doi: 10.1074/jbc.273.22.13531. [DOI] [PubMed] [Google Scholar]
- Tian L, Shipston MJ. Characterization of hyperpolarization-activated cation currents in mouse anterior pituitary, AtT20 D16:16 corticotropes. Endocrinology 141: 2930–2937, 2000. doi: 10.1210/endo.141.8.7617 [DOI] [PubMed] [Google Scholar]
- Tomić M, Kucka M, Kretschmannova K, Li S, Nesterova M, Stratakis CA, Stojilkovic SS. Role of nonselective cation channels in spontaneous and protein kinase A-stimulated calcium signaling in pituitary cells. Am J Physiol Endocrinol Metab 301: E370–E379, 2011. doi: 10.1152/ajpendo.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol 98: 131–144, 2007. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- Tse A, Lee AK, Tse FW. Ca2+ signaling and exocytosis in pituitary corticotropes. Cell Calcium 51: 253–259, 2012. doi: 10.1016/j.ceca.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Van Goor F, Li YX, Stojilkovic SS. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J Neurosci 21: 5902–5915, 2001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Stojilkovic SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol 15: 1222–1236, 2001b. doi: 10.1210/mend.15.7.0668. [DOI] [PubMed] [Google Scholar]
- Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446, 1998. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- Zemkova H, Tomić M, Kucka M, Aguilera G, Stojilkovic SS. Spontaneous and CRH-induced excitability and calcium signaling in mice corticotrophs involves sodium, calcium, and cation-conducting channels. Endocrinology 157: 1576–1589, 2016. doi: 10.1210/en.2015-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]