Abstract
Radiation therapy has emerged as a useful alternative therapy for patients with early-stage, non-resectable lung cancer. In patients whose malignancies are difficult to localize on computed tomography imaging, such therapy becomes difficult. Fiducial markers are frequently placed in peripheral pulmonary lesions to assist radiation therapy. Although placement of markers under linear endobronchial ultrasonography within mediastinal and hilar lymph nodes has been reported, no strategy has been described to assist radiotherapy of purely endobronchial tumors. We present a case of bilateral, unresectable, radiographically occult endobronchial squamous cell carcinoma treated with radiotherapy guided by fiducial markers placed under linear endobronchial ultrasonographic guidance. The patient subsequently underwent intensity-modified radiation therapy to both lesions with pathologic complete response. Linear endobronchial ultrasound is a promising tool for placement of markers to guide radiation therapy of these difficult-to-treat lesions.
Keywords: Endobronchial ultrasound, Lung cancer, Bronchoscopy, Radiation therapy
1. Introduction
When radiography alone is insufficient for targeting radiation therapy, placement of small, radiographically opaque devices known as fiducial markers has proven to be a safe and effective means of marking the position of neoplasms for radiation therapy [1], [2]. Case reports and series describe the successful use of linear endobronchial ultrasonography (EBUS) to place markers in mediastinal lymph nodes [3] and central masses [4], [5] with no complications or episodes of migration [3]; however, the literature provides no guidance on how to place fiducial markers in endobronchial lesions. We describe a case where linear EBUS was successfully used for this purpose.
2. Case report
A 66-year-old gentleman with a history of rectal cancer, treated with chemoradiation, was referred to the interventional pulmonary clinic at the Robley Rex VA Medical Center. He had originally undergone chest CT 3 months prior for progressive dyspnea on exertion; this showed multiple pulmonary nodules. 3-month follow-up CT scan demonstrated stability of these nodules; however, there was progressive soft tissue thickening at the right lower lobe bronchus concerning for malignancy (See Fig. 1). Social history was significant for a 100–150 pack-year smoking history and previous work in construction with known exposure to asbestos.
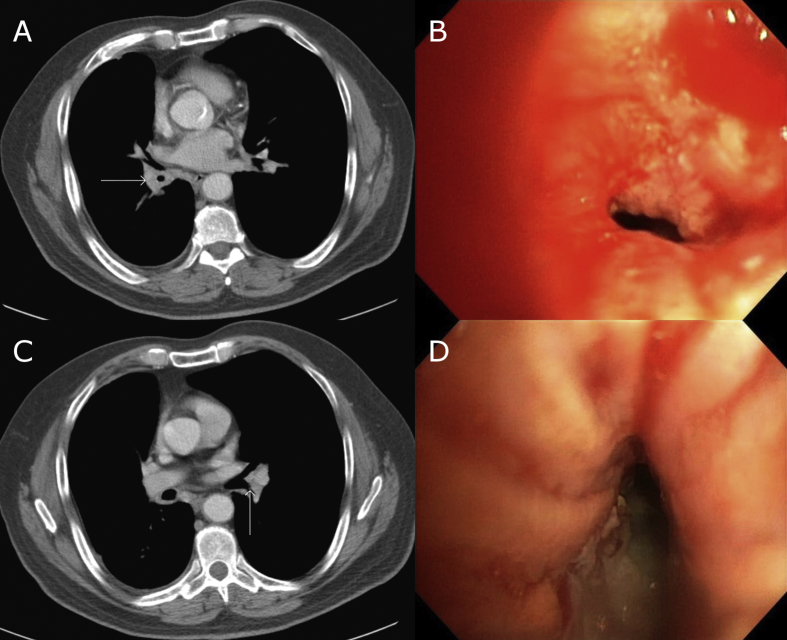
Fig. 1.
A. CT chest at the level of the right lower lobe lesion. Arrow points to lesion. B. Bronchoscopic view of right lower lobe lesion. C. CT chest at the level of the left lower lobe lesion. Arrow points to lesion, visible as subtle soft tissue density in bronchus. D. Bronchoscopic view of left lower lobe lesion.
Fiberoptic bronchoscopy revealed a circumferential, sessile, verrucous lesion at the right lower lobe bronchus with narrowing of the bronchial lumen (See Fig. 1). A similar-appearing lesion was also found overlying the carina at the ostium of the left lower lobe superior segment bronchus which did not correlate to any reported radiographic abnormality. Endobronchial biopsies of both lesions revealed invasive squamous cell carcinoma. After two cycles of cisplatin/etoposide, repeat bronchoscopy showed marked endoscopic improvement. Based on this clinical response, our multidisciplinary team decided to proceed with radiotherapy. Because the lesions could not be localized on CT with sufficient precision for simulation, fiducial marker placement was required.
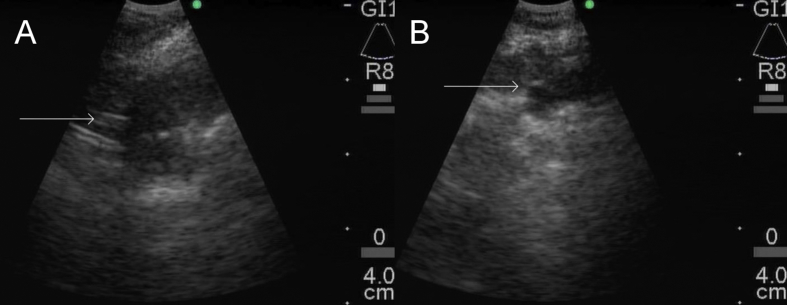
Under general anesthesia, we performed repeat airway exam, identifying the previously-known malignant lesions. We then examined the lesions under linear EBUS, revealing about 1cm tumor invasion in the left lower lobe and 1.5 cm tumor invasion in the right lower lobe. We slightly withdrew the stylet of an Olympus 21-gauge EBUS aspiration needle (Olympus Medical Systems, Tokyo, Japan) and back-loaded a 0.018 inch in diameter, 5mm length Cook Medical Hilal microembolization coil (Cook Medical, Bloomington, IN), securing with bone wax. The needle was inserted into the working channel, and the EBUS scope was positioned with the probe overlying the left lower lobe lesion, providing a clear ultrasonographic view of the lesion and confirming absence of nearby vascular structures. The needle was deployed under real-time ultrasonographic guidance, leaving sufficient space within the tumor for the fiducial marker. The stylet was then advanced to force the coil into the tumor (See Fig. 2). A second coil was placed into the right lower lobe lesion in a similar fashion. We estimated the tumor extent to be about 2 cm proximal and distal to each marker by narrow-band imaging. There were no procedural complications.
Fig. 2.
A. EBUS view of fiducial marker placement in right lower lobe lesion. Arrow points to marker. B. EBUS view of fiducial marker placement in left lower lobe lesion. Arrow points to marker.
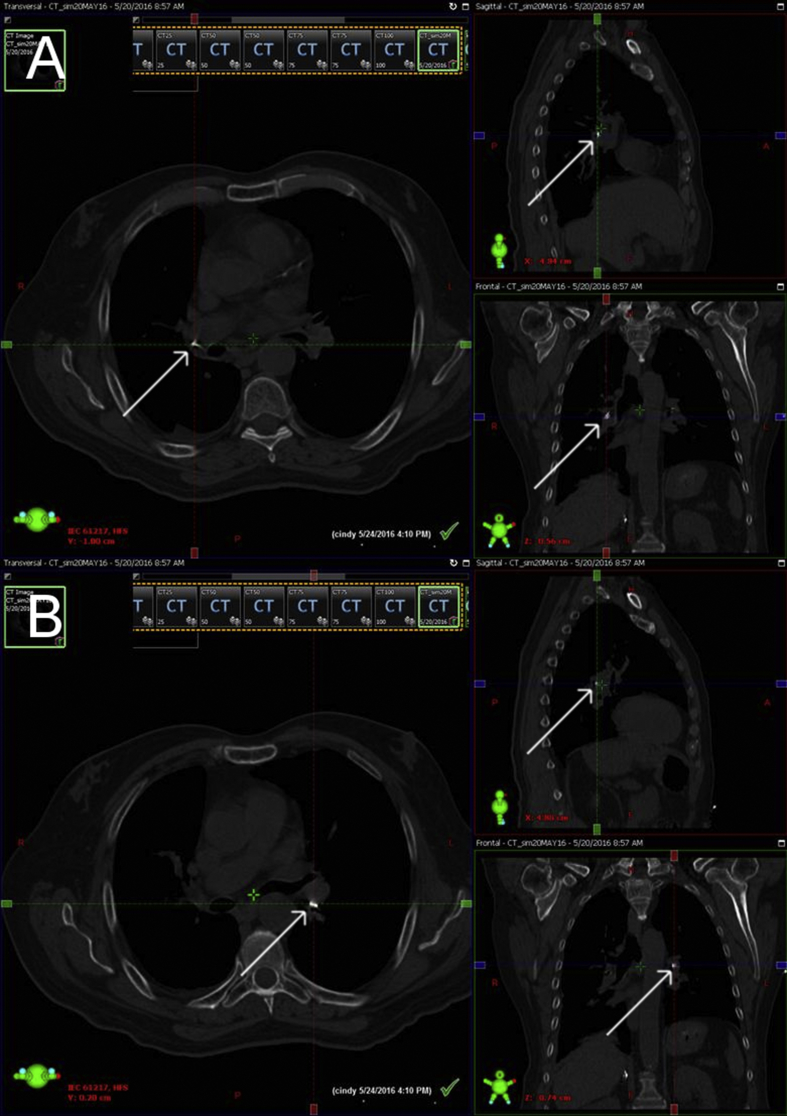
CT scan performed at simulation demonstrated satisfactory position of the fiducial markers (See Fig. 3). Simulation was performed with a target area encompassing 2 cm around each fiducial marker. Our patient received intensity-modulated radiation therapy at a dose of 7,020cGy in 26 fractions. Post-radiation bronchoscopy showed bilateral lower lobe bronchial stenosis and friable mucosa consistent with post-radiation effects; repeat endobronchial biopsy showed no evidence of residual malignancy.
Fig. 3.
A. 3-view CT simulation of right lower lobe. Arrows point to fiducial marker in each view. B. 3-view CT simulation of left lower lobe. Arrows point to fiducial marker in each view.
3. Discussion
Multiple synchronous primary lung cancers, defined by Martini and Melamed [6] as synchronous tumors with either different histologies or similar histologies but no metastases to extrathoracic sites or common lymphatics, present a noteworthy challenge for the treating physician. In surgical series, multiple synchronous primary cancers have been identified in 5.1–11.5% of patients presenting with non-small cell lung cancer [7]. Although data from Adebonojo et al. showed no patients surviving to five years after resection of synchronous lesions [7], subsequent investigators have achieved 74% and 63% 3-year survival in those with synchronous pathologic stage 1A and 1B disease, respectively [8].
For inoperable early-stage cancer, stereotactic body radiotherapy (SBRT) has shown a 55.8% 3-year overall survival with a 27% rate of therapy-related adverse events [9]. Patients with multiple primary tumors have not been as rigorously studied, but SBRT has shown one-year progression-free survival of 91.9%, albeit with about 50% acute and late toxicity [10]. Furthermore, a 5-year survival rate of 72.3% has been achieved with a combination of radiotherapy and endoluminal brachytherapy in radiographically occult endobronchial carcinoma; however, this was at the cost of greater than 90% incidence of radiation fibrosis [11].
Our case demonstrates an unusual and challenging clinical scenario. Curative-intent resection was not an option as the central location of both malignancies in the lobar bronchi would have necessitated bilateral lower lobectomy, carrying an unacceptable risk of poor post-operative pulmonary function. Although photodynamic therapy has shown promise in early-stage endobronchial disease with a 5-year survival rate of 61% [12], we abandoned this strategy because of cost concerns, unavailability at our institution, and lack of validation as definitive therapy. This left concurrent chemotherapy and radiation as the only feasible curative-intent option.
Radiotherapy without fiducial markers is feasible when a tumor can be localized radiographically. In our patient's case, however, the right-sided tumor appeared only as a subtle thickening of the lobar bronchus, and the left-sided tumor was nearly invisible. In both cases, it was impossible to radiographically determine the extent of infiltration. Tumor location presented difficulty in placing fiducial markers. Neither percutaneous nor endovascular approach was considered suitable due to tumor location. Bronchoscopic placement presented significant challenges; because the tumor was in the wall of a large bronchus, the marker needed to be accurately placed and fully embedded within the tumor to avoid placement through the bronchial wall and migration.
Linear EBUS solved these problems. Prior to placement, we were able to visualize the depth of tumor invasion and select a site where the markers could be best secured within the tumor. The needle fully embedded the markers within the tumor; the ultrasound then guided deployment of the marker and confirmed intralesional placement. Previous authors have used a 0.35 mm diameter dedicated fiducial marker [3], [4]; alternatively, case series have shown effectiveness of microembolization coils as when placed by the endovascular route [2]. Reports of fiducial marker infection after placement in prostate tumors via transrectal ultrasound do raise concern for the same possibility in the lung [13]. Fortunately, infection has never been reported as a complication of pulmonary fiducial marker placement, and the markers can remain in place indefinitely.
4. Conclusion
To our knowledge, this is the first reported case where linear EBUS allowed placement of fiducial markers in two radiographically occult, purely endobronchial malignant lesions. Our case shows the feasibility of using markers to guide radiotherapy of inoperable endobronchial lesions.
Conflicts of interest
None of the authors have any conflicts of interest to disclose.
References
- 1.Harada T., Shirato H., Ogura S. Real-time tumor-tracking radiation therapy for lung carcinoma by the aid of insertion of a gold marker using bronchofiberscopy. Cancer. 2002;95:1720–1727. doi: 10.1002/cncr.10856. [DOI] [PubMed] [Google Scholar]
- 2.Nuyttens J.J., Prevost J.B., Praag J. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: marker placement and early results. Acta Oncol. 2006;45:961–965. doi: 10.1080/02841860600902205. [DOI] [PubMed] [Google Scholar]
- 3.McGuire F.R., Liming J., Ochran T. Real-time endobronchial ultrasound-guided implantation of radiotherapy monitoring devices. J. Bronchol. 2007;14:59–62. [Google Scholar]
- 4.Argento A.C., Decker R., Puchalski J. Fiducial marker placement via convex-probe EBUS. J. Bronchol. Intervent. Pulmonol. 2016;23:181–185. doi: 10.1097/LBR.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 5.Casutt A, Koutsokera A, Peguret N, et al. Linear-endobronchial ultrasound-guided insertion of a fiducial marker: a new tool for tracking central lesions. J. Bronchol. Intervent. Pulmonol. [epub ahead of print]. http://dx.doi.org/10.1097/LBR.0000000000000258. (Accessed August 16, 2016). [DOI] [PubMed]
- 6.Martini N., Melamed M.R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975;70:607–612. [PubMed] [Google Scholar]
- 7.Adebonojo S.A., Moritz D.M., Danby C.A. The results of modern surgical therapy for multiple primary lung cancers. Chest. 1997;112:693–701. doi: 10.1378/chest.112.3.693. [DOI] [PubMed] [Google Scholar]
- 8.Finley D.J., Yoshizawa A., Travis W. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J. Thorac. Oncol. 2010;5:197–205. doi: 10.1097/JTO.0b013e3181c814c5. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early-stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen D., Olivier K.R., Mayo C.S. Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat. Oncol. February 2015;10(43):1–8. doi: 10.1186/s13014-015-0340-9. [serial online] Available from: BioMed Central, London, United Kingdom. Accessed May 23, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito M., Yokoyama A., Kurita Y. Treatment of roentgenographically occult endobronchial carcinoma with external beam radiotherapy and intraluminal low-dose-rate brachytherapy: second report. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:673–680. doi: 10.1016/s0360-3016(00)00489-2. [DOI] [PubMed] [Google Scholar]
- 12.Moghissi K., Dixon K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC) Photodiagnosis Photodyn. Ther. 2008;5:10–18. doi: 10.1016/j.pdpdt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Loh J., Baker K., Sridharan S. Infections after fiducial marker implantation for prostate radiotherapy: are we underestimating the risk? Radiat. Oncol. February 2015;10(38):1–5. doi: 10.1186/s13014-015-0347-2. [serial online] Available from: BioMed Central, London, United Kingdom. Accessed June 7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]