Abstract
Non-small cell lung cancer adenocarcinoma in the past decade has targeted therapies as the cornerstone for therapy. In specific patients with epidermal growth factor receptor mutation have three different therapy approaches with the tyrosine kinase inhibitors: erlotinib, gefitinib and afatinib. Nowadays we can use tyrosine kinase inhibitors as second line treatment for squamous cell carcinoma. We present a case with a patient with squamous cell carcinoma receiving afatinib tyrosine kinase inhibitor who presented elbow bursitis or olecranon bursitis in both elbows.
Keywords: Tyrosine kinase inhibitors, Afatinib, Elbow bursitis, Olecranon bursitis
1. Introduction
In the past ten years new treatment options were introduced for non-small cell lung cancer [1], [2], [3]. Regarding adenocarcinoma the novel tyrosine kinase inhibitor (TKI) afatinib was introduced to the market for epidermal growth factor receptor positive (EGFR) patients [2]. Recently osimertinib was introduced for EGFR patients who relapsed and the mutation T790M was observed either from re-biopsy or liquid biopsy [4], [5]. Moreover; recently pembrolizumab was introduced as first line treatment for both adenocarcinoma and squamous cell carcinoma in patients with programmed death-ligand 1 (PD-L1) > 50% investigated with DAKO technique as indicated by the respective pharmaceutical company that produces the drug. Pembrolizumab can also be used as second line therapy for both adenocarcinoma and squamous cell if the expression of PD-L1 is >1% [6]. Recently afatinib has also indication as second line treatment for squamous cell carcinoma [7], [8]. Each drug has its own adverse effects. Tyrosine kinase inhibitors have usually skin-related (rash, xerosis and paronychia) and gastrointestinal-related (diarrhea and stomatitis) adverse events (AEs), these effects are usually mild. But severe cases can occur [9]. We present a rare case of elbow bursitis or olecranon bursitis due to afatinib administration.
2. Case presentation
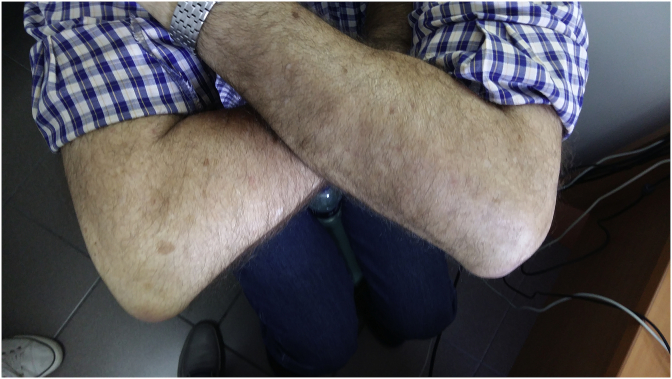
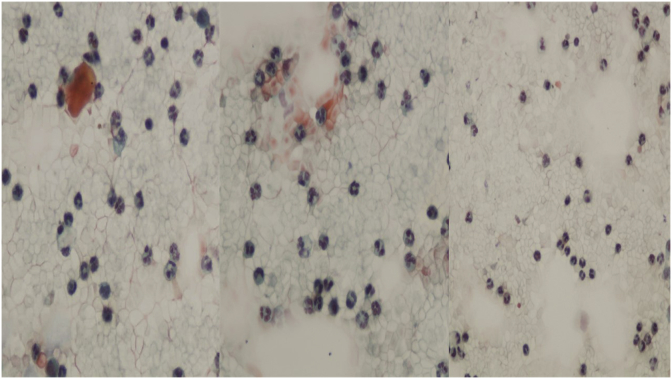
A sixty five year old patient was diagnosed with squamous cell carcinoma in 1999 with bronchoscopy and he had disease relapse in 5/11/14, diagnosed again with bronchoscopy. He initiated chemotherapy in 11/12/14 and received four cycles of carboplatin plus paclitaxel as first line treatment. He had complete response and remained under observation until 18/1/17 where disease relapse was observed with PET-CT and EBUS biopsy. (Fig. 1, Fig. 2). We investigated with DAKO technique programmed death-ligand 1 (PD-L1) but the tissue had negative expression, and it was decided to initiate afatinib 40 mg as second line therapy. Due to severe grade 4 adverse effects with mucositis, skin rash and infected pimples for which he received antibiotics. Immediately a dose reduction was done with to 30mg/daily. Again, a grade 4 toxicity remained and again a dose reduction was performed to 20mg/daily. The symptoms were reduced to grade 2, however; elbow bursitis or olecranon bursitis was observed on the left elbow and the liquid was removed with surgery as we wanted to take tissue samples and liquid in order to investigate for metastasis. The samples were negative for malignancy (Fig. 3). Again after almost a month elbow bursitis or olecranon bursitis was observed on the right elbow and again the same therapeutic approach was performed with negative results (Fig. 4, Fig. 5). Nowadays the patient is on the fifth month of his second line therapy (see Fig. 6).
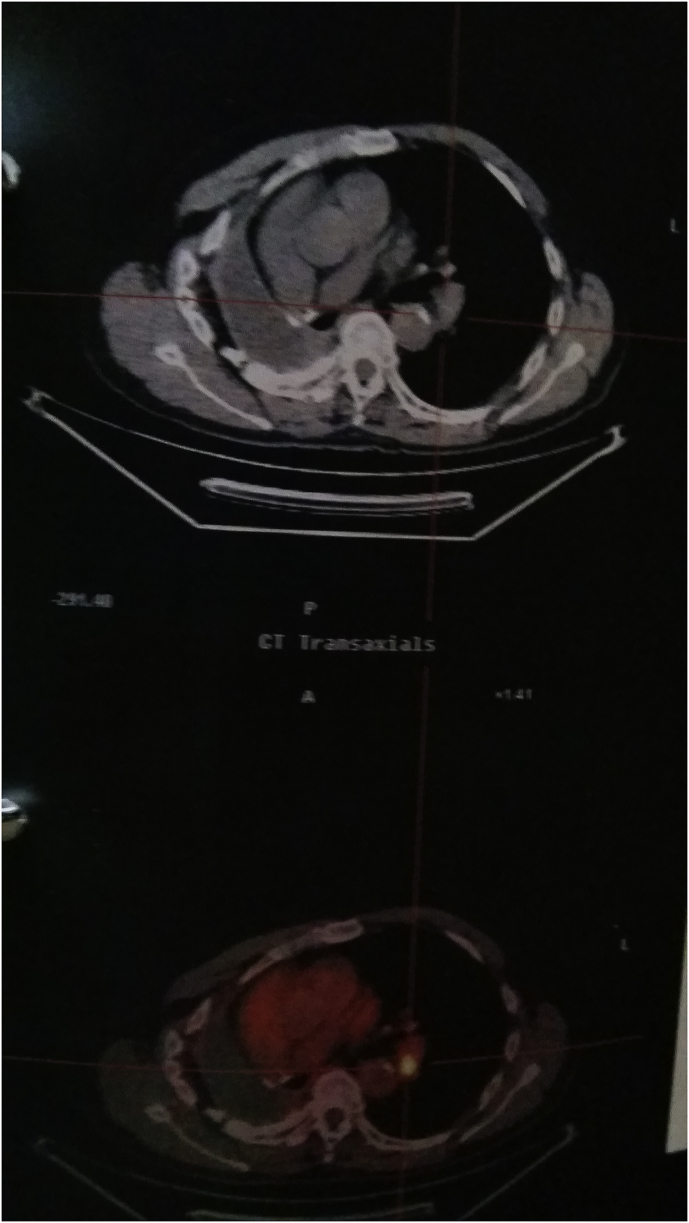
Fig. 1.
Pet-CT upon disease relapse.
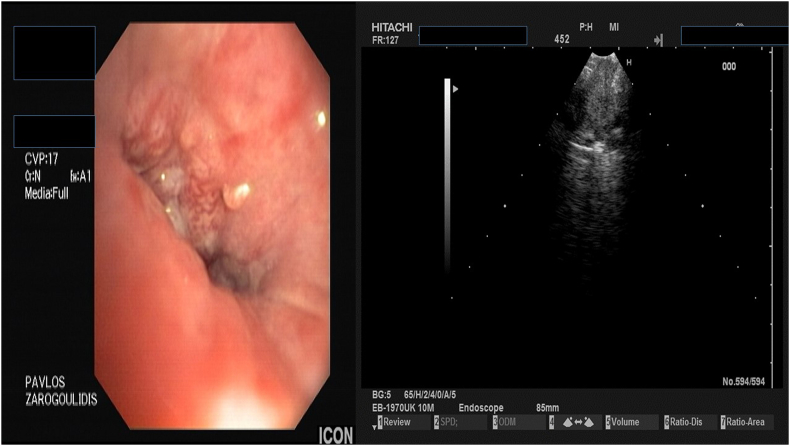
Fig. 2.
Endoscopy performed by Paul Zarogoulidis with a Pentax EB-1970UK EBUS system after Pet-CT.
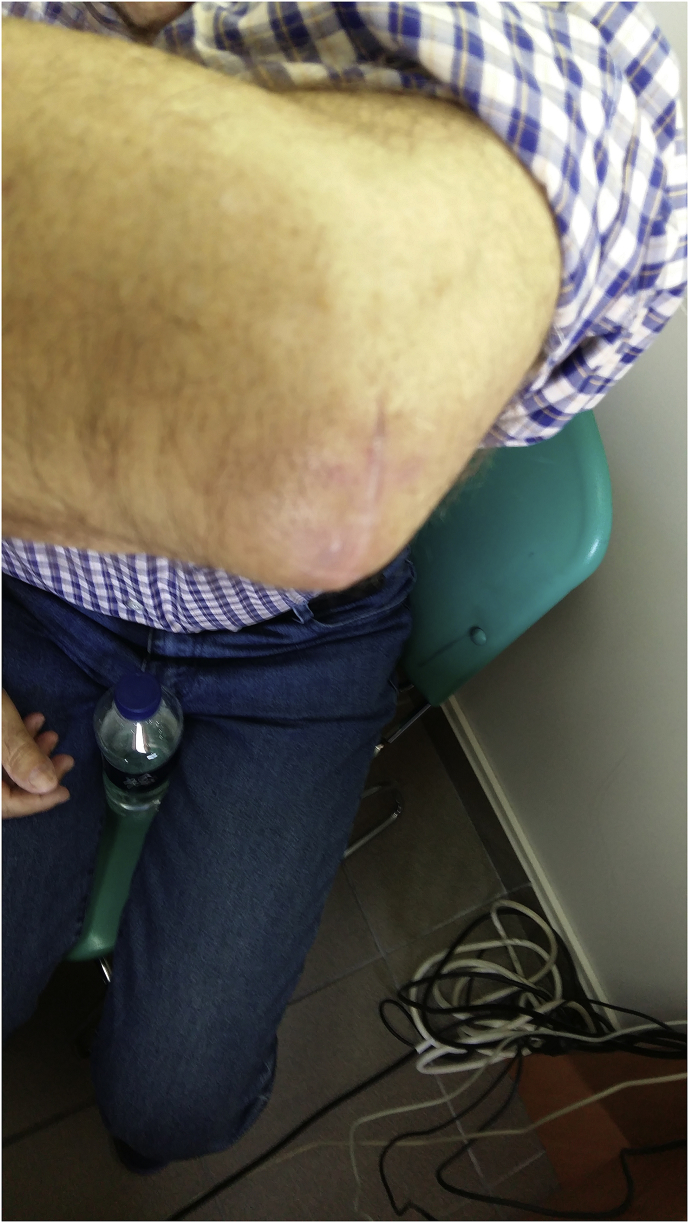
Fig. 3.
Left elbow after surgery for elbow bursitis or olecranon bursitis.
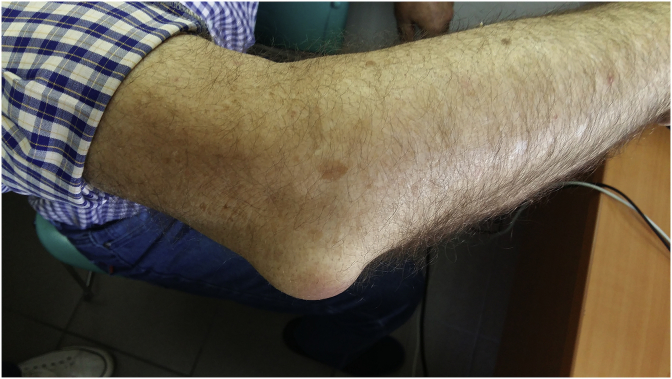
Fig. 4.
Elbow bursitis or olecranon bursitis of the right hand.
Fig. 5.
Both hands.
Fig. 6.
Presence of inflammatory cells (lymphocytes, plasma cells, neutrophils) and foci of hemorrhage.
3. Discussion
The EGFR TKIs has changed the treatment paradigm for advanced NSCLC, providing patients with better efficacy and quality of life than chemotherapy. The EGFR TKIs also have favorable toxicity profile. A “third-generation” EGFR TKI group known as wild-type EGFR sparing inhibitors may provide an alternative option in the future [10]. Until now we can change the dose of in patients receiving erlotinib from 150mg/daily to 100mg/daily in the case of severe adverse effects. Afatinib has the unique advantage of dose reduction from 40mg/daily to 20mg/daily if necessary. Firstly we try to increase the dose from 40mg/daily to 50mg/daily, however; unfortunately an increased dose >40mg/daily usually has increased side effects. Mucositis has been previously observed as adverse effect [9], in our case we attribute elbow bursitis or olecranon bursitis to afatinib administration and surgical approach was firstly performed in the left elbow and after 1 month in the right hand as a 1 month past since the appearance of the symptom from hand to another. The patient is under close follow-up for other adverse effects as the fifth month from the initiation is passing. To our knowledge this is the first case of such adverse effect presentation.
Conflict of interest
None to declare.
References
- 1.Zarogoulidis K., Zarogoulidis P., Darwiche K., Boutsikou E., Machairiotis N., Tsakiridis K., Katsikogiannis N., Kougioumtzi I., Karapantzos I., Huang H., Spyratos D. Treatment of non-small cell lung cancer (NSCLC) J. Thorac. Dis. 2013;5(Suppl 4):S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domvri K., Zarogoulidis P., Darwiche K., Browning R.F., Li Q., Turner J.F., Kioumis I., Spyratos D., Porpodis K., Papaiwannou A., Tsiouda T., Freitag L., Zarogoulidis K. Molecular targeted drugs and biomarkers in NSCLC, the evolving role of individualized therapy. J. Cancer. 2013;4(9):736–754. doi: 10.7150/jca.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domvri K., Darwiche K., Zarogoulidis P., Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Transl. Lung Cancer Res. 2013;2(4):256–258. doi: 10.3978/j.issn.2218-6751.2012.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarogoulidis P., Gaga M., Huang H., Darwiche K., Rapti A., Hohenforst-Schmidt W. Tissue is the issue and tissue competition. Re-biopsy for mutation T790 where and why? Clin. Transl. Med. 2017;6(1):6. doi: 10.1186/s40169-017-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricciuti B., Baglivo S., Paglialunga L., De Giglio A., Bellezza G., Chiari R., Crino L., Metro G. Osimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: rationale, evidence and place in therapy. Ther. Adv. Med. Oncol. 2017;9(6):387–404. doi: 10.1177/1758834017702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller M., Schouten R.D., De Gooijer C.J., Baas P. Pembrolizumab for the treatment of non-small cell lung cancer. Expert Rev. Anticancer Ther. 2017;17(5):399–409. doi: 10.1080/14737140.2017.1311791. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh V. New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. OncoTargets Ther. 2017;10:2513–2526. doi: 10.2147/OTT.S104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohenforst-Schmidt W., Zarogoulidis P., Steinheimer M., Benhassen N., Sardeli C., Stalikas N., Toitou M., Huang H. Second-line afatinib administration in an elderly patient with squamous cell carcinoma. Ther. Clin. Risk Manag. 2017;13:341–343. doi: 10.2147/TCRM.S130816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aw D.C., Tan E.H., Chin T.M., Lim H.L., Lee H.Y., Soo R.A. Management of epidermal growth factor receptor tyrosine kinase inhibitor-related cutaneous and gastrointestinal toxicities. Asia-Pacific J. Clin. Oncol. 2017 doi: 10.1111/ajco.12687. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Tan C.S., Cho B.C., Soo R.A. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor -mutant non-small cell lung cancer. Lung Cancer. 2016;93:59–68. doi: 10.1016/j.lungcan.2016.01.003. [DOI] [PubMed] [Google Scholar]