Abstract
In 95% of patients with pancreatic ductal adenocarcinoma, recurrence is observed following chemotherapy. Findings from several studies have indicated that cancer stem cells (CSCs) are resistant to anticancer agents and may be involved in cancer recurrence and metastasis. The CD44 protein is a major CSC marker, and CD44 also plays an indispensable role in the CSC properties in several cancers, including pancreatic cancer; however, no clinical approach exists to inhibit CD44 activity. Here, we have performed knock-in/knockdown experiments, and we demonstrate that the forkhead box O3 (FOXO3)/liver kinase B1 (LKB1)/AMP-activated protein kinase/peroxisome proliferator-activated receptor-γ co-activator-1β (PGC-1β)/pyruvate dehydrogenase-A1 pathway is essential for CD44 expression and CSC properties. We observed that patients exhibiting high pyruvate dehydrogenase-A1 expression have a poor prognosis. Systemic PGC-1β knock-out mice are fertile and viable and do not exhibit an overt phenotype under normal conditions. This suggests that cGMP induction and PGC-1β inhibition represent potential strategies for treating patients with pancreatic ductal adenocarcinoma.
Keywords: CD44, FOXO, liver kinase B1 (LKB1), pancreatic cancer, stem cells, PGC-1β
Introduction
Pancreatic ductal adenocarcinoma (PDAC)3 is a cancer associated with a poor prognosis and has become the fourth most common cause of cancer-related mortality in developed countries (1). Due to the lack of specific symptoms and the absence of an established screening approach for PDAC, most patients already exhibit the distant spread of cancer, and only a portion of patients are diagnosed with localized disease (2). Moreover, even the patients who are diagnosed with only localized disease often experience a metastatic recurrence (2). Therefore, systemic chemotherapy plays a central role in PDAC therapy.
Several strategies have been performed to improve chemotherapy for patients with the PDAC; however, they have achieved little success. Gemcitabine is a difluorinated nucleoside analog that has exhibited significant improvement in the median overall survival (5.7 versus 4.4 months) (3). The phase III PRODIGE 4/ACCORD 11 trial demonstrated that FOLFIRINOX consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin could be a potentially novel treatment for pancreatic cancer. In that report, the median overall survival was 11.1 months in the FOLFIRINOX group compared with 6.8 months in the gemcitabine group; however, the 5-year survival period did not improve (4). Collectively, the conventional therapeutic strategy has experienced difficulty in extending the long-term survival of patients with PDAC, and there is currently no potent therapeutic measure for the eradication of pancreatic cancer. Thus, the establishment of a novel therapeutic strategy is in high demand to improve the therapeutic situation of patients with PDAC.
Emerging evidence suggests that the capacity of a tumor to grow and propagate is dependent on a small subset of cells, known as cancer stem cells (CSCs). Moreover, a recent study suggested that CSCs are thought to play a pivotal role in cancer recurrence following chemotherapy and metastasis (5–7). In addition, several studies have demonstrated that treatment with the anticancer agent gemcitabine enriched cancer cells with a significantly enhanced expression of the CSC markers CD44 (17-fold change) and CD24 (47-fold change) in PDAC (5). Using an in vivo model, gemcitabine (one of the standard anticancer agents for PDAC treatment) was found to significantly inhibit the tumor growth of PDAC cells with low CSC marker expression; however, the same dose of gemcitabine failed to inhibit the increase in tumor volume of PDAC cells with high CSC marker expression (6). Data from our previous study showed that the anticancer agents oxaliplatin and erlotinib increased CD44 expression in PDAC cells (8). Consistent with these findings, a recent pathological study of 101 patients with PDAC also found that there was a correlation between the frequency of CD44+/CD24+ double-positive cells and a poor prognosis (9). In that study, patients with low CD44+/CD24+ expression were associated with approximately a 30% 5-year median survival rate, which is a significantly better prognosis compared with patients exhibiting high CD44+/CD24+ expression whose 5-year median survival rate is less than 10%. There is no currently established clinical approach to eliminate CSCs in patients with PDAC. Collectively, establishing an applicable approach for targeting CSCs could be a novel therapeutic strategy against PDAC.
The maintenance of pancreatic CSCs has been reported to involve several mechanisms, including Hedgehog and Wnt/β-catenin/Notch signaling pathways (10); however, these pathways are also critical for normal somatic stem cell homeostasis, and several inhibitors designed to block these pathways induce severe toxicity (11). We have previously reported that cGMP, a second messenger that mediates the vasodilation and penile erection induced by sexual arousal, drastically inhibited the CSC properties of PDAC through the suppression of forkhead box O3 (FOXO3) (8). We also confirmed that FOXO3 depletion is sufficient to suppress the CSC phenotype of PDAC cells. Although systemic Foxo3 KO mice exhibit accelerated follicular initiation, they were not associated with a prominent cancer-prone condition or decreased survival time compared with WT mice with the same background (12). These findings suggest that FOXO3 may be an ideal target for eradicating CSCs in PDAC. Moreover, both direct and indirect cGMP induction is widely used for the treatment of erectile dysfunction, pulmonary hypertension, and cardiovascular disease and is therefore clinically applicable; however, little is known regarding the detailed molecular mechanisms of FOXO3-dependent CSC maintenance in PDAC.
In the present study, we revealed that FOXO3 and liver kinase B1 (LKB1), well established tumor suppressor genes, play a central role in CSC maintenance in PDAC. We also revealed that FOXO3 and LKB1 regulate both CD44 expression via PGC-1β and the CD44 expression signaling axis. Moreover, our data indicate that patients with high PDHA1 expression have a poor prognosis. We further confirmed that these molecules are overexpressed in CD44+ cells. Together, these results provide evidence of the molecular mechanism of the cGMP-mediated inhibitory effect on CSCs and suggest a novel approach for targeting the CSCs in patients with PDAC.
Results
The FOXO3/LKB1 axis plays a central role in the CSC properties of pancreatic cancer cells
cGMP acts as a second messenger for regulating vascular homeostasis and penile erection. Recently, we reported that cGMP exerts a suppressive effect on the CSC properties of PDAC (8). cGMP induction via Bay 41-2272 treatment significantly suppressed tumor formation and liver metastasis in vivo (8). In the study, Bay 41-2272 treatment significantly suppressed expression of CD44, the major regulator of CSCs (13–17), in three different PDAC cell lines. Interestingly, the inhibitory effect of cGMP on CSC properties was due to the suppression of FOXO3 (8), a well known tumor suppressor involved in the inhibition of growth and apoptosis in cancer cells (18). We found that FOXO3 was essential for CD44 expression in PDACs (8); however, little is known regarding the molecular mechanisms of such suppression.
We focused on LKB1 as a known “tumor suppressor” similar to FOXO3 and one of the targets of FOXO3. Moreover, previous reports have suggested that abnormal mitochondrial activation is observed in CSCs (7, 19). These results prompted us to evaluate the role of LKB1 as a crucial mediator of mitochondrial activity and the downstream mediator of the FOXO3-dependent mechanisms of CSCs.
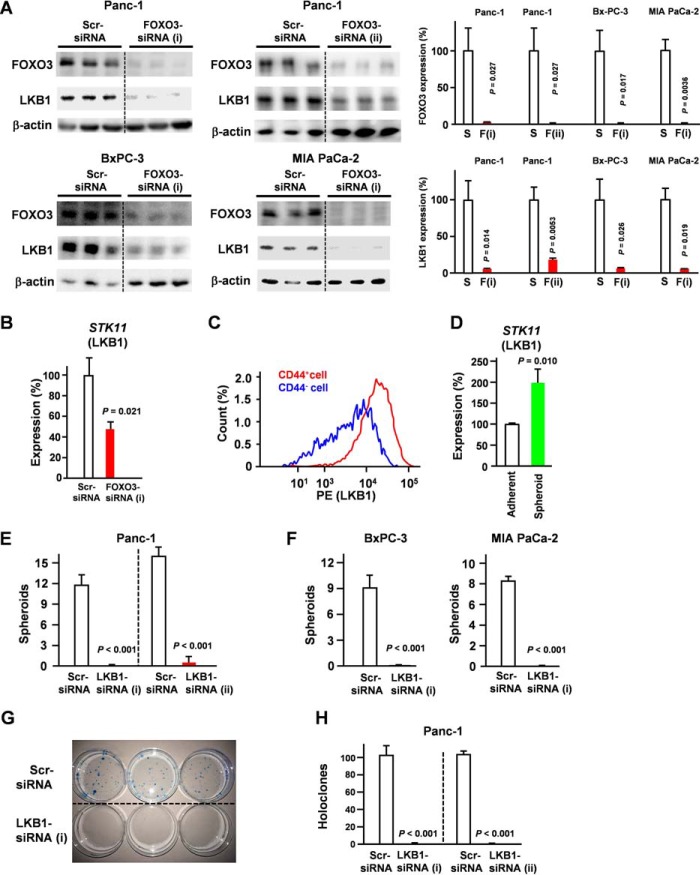
We determined the effect of FOXO3 knockdown on the expression level of LKB1 (Fig. 1A). These data suggest that FOXO3 in PDAC cells primarily regulates the expression of LKB1. Direct binding of FOXO3 to the LKB1 promoter was reported previously (20). We assessed the effect of FOXO3 knockdown on LKB1 levels at the mRNA level. Our results showed that FOXO3 knockdown suppressed LKB1 at the mRNA level (Fig. 1B).
Figure 1.
LKB1 is indispensable for pancreatic cancer stem cells. A, pancreatic cancer cells were transfected with FOXO3 siRNA for 48 h, and LKB1 levels were determined by Western blotting (n = 3). S, scrambled siRNA; F, FOXO3 siRNA. B, Panc-1 cells were transfected with FOXO3 siRNA for 24 h, and STK11 (LKB1) levels were determined by quantitative RT-PCR analysis (n = 3). C, LKB1 expression in Panc-1 cells was compared with that in CD44+ and CD44− cells by flow cytometry. D, quantitative RT-PCR showing that mRNA of STK11 (LKB1) was overexpressed in spheroid cells compared with that in adherent cells (n = 3). E and F, pancreatic cancer cells were transfected with LKB1 siRNA, and spheroid formation was assessed (n = 3). G and H, Panc-1 cells were transfected with LKB1 siRNA, and colony formation was assessed (n = 3). Error bar represents S.E. Scr, scrambled.
LKB1 was up-regulated in CD44+ cells compared with CD44− cells (Fig. 1C). Our results also revealed that LKB1 expression was increased in spheroid cells compared with adherent cells (Fig. 1D). We also found that LKB1 knockdown reduces CSC functionality, including spheroid (Fig. 1, E and F) and colony formation (Fig. 1, G and H).
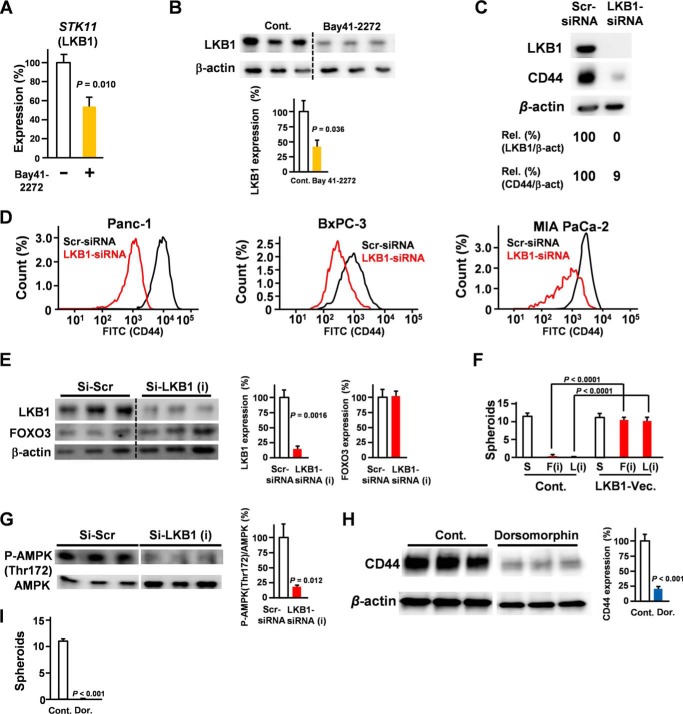
In addition, we confirmed that cGMP induced the suppression of LKB1 at both the mRNA and protein levels (Fig. 2, A and B). To determine the role of LKB1 in pancreatic CSCs, we assessed the effect of LKB1 knockdown on CD44 expression. Our data suggest that silencing LKB1 is sufficient to suppress CD44 expression (Fig. 2, C and D). We assessed the effect of LKB1 knockdown on FOXO3 levels. Our results suggested that LKB1 knockdown did not affect FOXO3 levels (Fig. 2E). To establish functional links between the components, we constructed and performed knock-in experiments with LKB1 (Fig. 2F). Our data showed that ectopic expression of LKB1 rescued inhibition of FOXO3-induced suppression of spheroid formation (Fig. 2F). Considering that silencing of FOXO3 down-regulated LKB1 levels (Fig. 1, A and B), LKB1 inhibition is sufficient to suppress spheroid formation (Fig. 1, E and F), and ectopic expression of LKB1 rescued inhibition of FOXO3-induced suppression on spheroid formation (Fig. 2F), the FOXO3/LKB1 axis plays a crucial role in CSC properties. We assessed the role of LKB1 in AMPK phosphorylation at Thr-172, identified as the major site phosphorylated by AMPK kinase and which plays a crucial role in its activity. Our results showed that silencing of LKB1 down-regulated the AMPK phosphorylation level at Thr-172 (Fig. 2G). Moreover, AMPK inhibitor treatment significantly suppressed CD44 (Fig. 2H) and spheroid formation properties (Fig. 2I).
Figure 2.
LKB1 is essential for CD44 expression. A, Panc-1 cells were treated with Bay 41-2272 (5 μm) for 36 h. Quantitative RT-PCR shows the mRNA expression of STK11 (LKB1) (n = 3). B, Panc-1 cells were treated with Bay 41-2272 for 48 h, and LKB1 expression was examined by Western blotting (n = 3). C, Panc-1 cells were transfected with LKB1 siRNA for 48 h and examined by Western blotting. D, pancreatic cancer cells were transfected with LKB1 siRNA for 48 h and examined by flow cytometry. E, Panc-1 cells were transfected with LKB1 siRNA for 48 h and examined by Western blotting. F, spheroid assay of FOXO3 (F) siRNA, LKB1 (L) siRNA, and LKB1 expression vector (Vec.) (n = 3) is shown. G, Panc-1 cells were transfected with LKB1 siRNA for 48 h and examined for the phospho-AMPK (P-AMPK) (Thr-172) level by Western blotting (n = 3). Lanes were run on the same gel but were noncontiguous (white lines). H, the effect of AMPK inhibitor dorsomorphin (Dor.) (5 μm; 48 h) on CD44 expression in Panc-1 cells was determined by Western blotting (n = 3). Lanes were run on the same gel but were noncontiguous (white lines). I, the effect of AMPK inhibitor dorsomorphin (5 μm; 21 days) on spheroid formation was assessed (n = 3). Error bar represents S.E. Scr, scrambled; Cont., control; Rel., relative; β-act, β-actin.
PGC1-β plays a crucial role in CD44 expression and pancreatic cancer stem cells
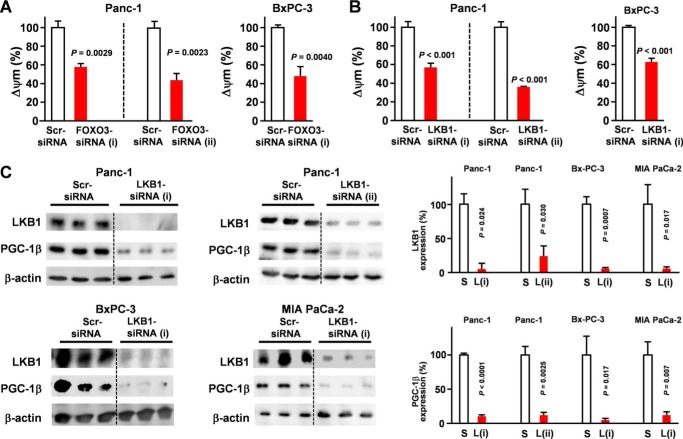
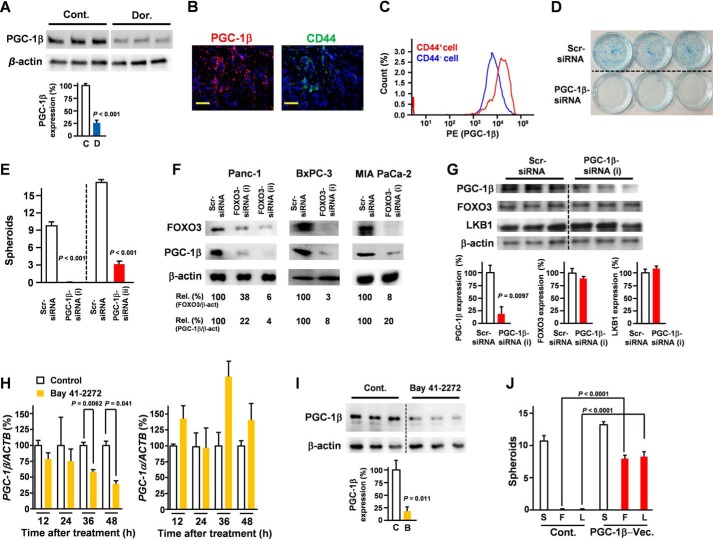
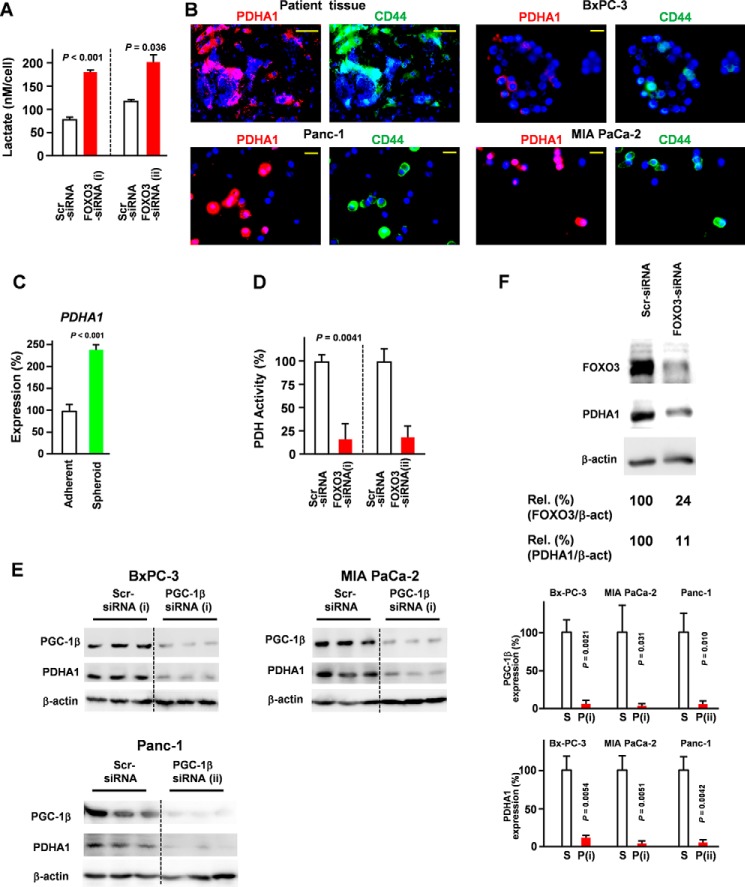
A previous study found that MEK1 and PI3K/mTOR inhibition enriched CSC-like cells with high mitochondrial activity (7); however, the reason that the inhibition of these kinases enriched cells with high mitochondrial activity remains unclear. Interestingly, PI3K/mTOR is an important negative regulator of FOXO3, and the inhibition of these kinases may have caused an accumulation of FOXO3. To determine the role of FOXO3 in the mitochondrial activity of pancreatic cancer cells, the impact of FOXO3 knockdown on mitochondrial membrane potential was evaluated. FOXO3/LKB1 knockdown significantly reduced mitochondrial activity (Fig. 3, A and B), suggesting that FOXO3 and LKB1 play an essential role in the mitochondrial activity of PDAC. PGC is the central regulator of mitochondrial activity. Our microarray data (Gene Expression Omnibus accession number GSE66403) revealed that cGMP induction strongly suppressed PGC-1β, whereas it did not affect PGC-1α. Our data also demonstrated that LKB1 was indispensable for expression of PGC-1β, a key regulator of mitochondrial function (Fig. 3C). A previous study showed that AMPK promotes aberrant PGC-1β expression in colon tumor (21). Consistent with these results, AMPK inhibition suppressed PGC-1β expression levels (Fig. 4A). Our data suggest an LKB1/AMPK/PGC-1β axis. Additionally, PGC-1β is overexpressed in CD44+ cells derived from PDAC patient tissues (Fig. 4B) and PDAC cells (Fig. 4C). Moreover, a PGC-1β knockdown inhibited CSC properties (Fig. 4, D and E). To confirm the role of FOXO3 in the expression of PGC-1β, pancreatic cancer cells were transfected with FOXO3 siRNA. Our data revealed that the down-regulation of FOXO3 was sufficient to suppress PGC-1β expression in pancreatic cancer (Fig. 4F). We evaluated the effect of PGC-1β knockdown on FOXO3 and LKB1 levels and found that it did not affect FOXO3 and LKB1 levels (Fig. 4G). Consistent with our microarray data (Gene Expression Omnibus accession number GSE66403), our quantitative RT-PCR analysis showed that cGMP induction suppressed the PGC-1β expression, whereas the treatment did not suppress PGC-1α (Fig. 4H). Our Western blot analysis showed that cGMP induction suppressed PGC-1β at the protein level (Fig. 4I). Our data showed that ectopic expression of PGC-1β rescued inhibition of FOXO3 and LKB1-induced suppression of spheroid formation (Fig. 4J).
Figure 3.
LKB1 plays a crucial role in PGC-1β expression. A, the effect of FOXO3 knockdown on mitochondrial membrane potential of each PDAC cell line was determined by flow cytometry using JC-1 (n = 3). Δψm, mitochondrial membrane potential. B, the impact of LKB1 knockdown on mitochondrial membrane potential was determined by flow cytometry using JC-1 (n = 3). C, pancreatic cancer cells were transfected with LKB1 siRNA for 48 h. PGC-1β expression was examined by Western blotting (n = 3). S, scrambled siRNA; L, LKB1 siRNA. Error bar represents S.E. Scr, scrambled.
Figure 4.
PGC1-β plays a crucial role in CD44 expression and pancreatic cancer stem cells. A, the effect of AMPK inhibitor dorsomorphin (5 μm; 48 h) on PGC-1β expression in Panc-1 cells was determined by Western blotting (n = 3). C, control (Cont.); D, dorsomorphin. Lanes were run on the same gel but were noncontiguous (white lines). B, immunofluorescence staining for PGC-1β and CD44 on pancreatic cancer patient tissues (magnification, ×60). Scale bar, 50 μm. Representative data from eight patients are shown. C, PGC-1β expression in Panc-1 cells was compared with that in CD44+ and CD44− cells by flow cytometry. D, Panc-1 cells were transfected with PGC-1β siRNA for 48 h, and a colony assay was performed (n = 3). E, Panc-1 cells were transfected with PGC-1β siRNA for 48 h, and a spheroid assay was performed (n = 3). F, pancreatic cancer cells were transfected with FOXO3 siRNA for 48 h. PGC-1β expression in Panc-1 cells was determined by Western blotting (n = 3). G, Panc-1 cells were transfected with PGC-1β siRNA for 48 h, and expression levels were determined by Western blotting (n = 3). H, Panc-1 cells were treated with Bay 41–2272 (5 μm) for the indicated times, and PPARGC1B (PGC-1β) and PPARGC1A (PGC-1α) mRNA levels were measured (n = 3). I, Panc-1 cells were treated with Bay 41-2272 for 48 h, and PGC-1β expression was examined by Western blotting (n = 3). The same samples are used in Fig. 2B. C, control (Cont.); B, Bay 41-2272. J, spheroid assay of FOXO3 siRNA (F), scrambled siRNA (S), LKB1 siRNA (L), and PGC-1β expression vector (Vec.) (n = 3). Lanes were run on the same gel but were noncontiguous (white lines). Error bar represents S.E. Scr, scrambled; Rel., relative.
Considering that silencing of FOXO3/LKB1 down-regulated PGC-1β levels (Figs. 3C and 4F), silencing of PGC-1β is sufficient to suppress spheroid formation (Fig. 4E), and ectopic expression of PGC-1β rescued inhibition of FOXO3 and LKB1-induced suppression of the spheroid formation (Fig. 4J), the axis plays a crucial role in CSC properties. Taken together, the FOXO3/AMPK/LKB1/PGC-1β axis appears to be indispensable for CD44 expression and pancreatic CSC properties.
Mitochondrial enzyme PDHA1 plays a crucial role in CSC properties
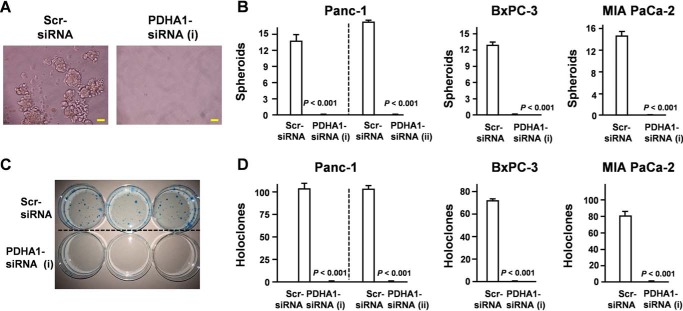
Because FOXO3 knockdown increased the accumulation of lactate (Fig. 5A), we hypothesized that FOXO3 regulates the tricarboxylic acid cycle and glycolysis in pancreatic CSCs. Pyruvate dehydrogenase (PDH) is the key enzyme connecting glycolysis to the tricarboxylic acid cycle (22). Moreover, the active site of the PDHA1 subunit contains the E1 active site and plays a central role in the PDH complex. Although PDHA1 is thought to be a housekeeping gene, it was found to be significantly increased in CD44+ cells (Fig. 5B). Our results also demonstrate that PDHA1 expression was increased in spheroid cells compared with adherent cells (Fig. 5C). These findings prompted us to evaluate whether PDHA1 expression was regulated via the FOXO3/PGC-1β axis. Our data demonstrated that the FOXO3/PGC-1β axis was essential for PDHA1 expression in PDAC (Fig. 5, D–F). To determine the role of PDHA1 in CSC characteristics, we assessed the level of spheroid establishment (Fig. 6, A and B) and colony formation (Fig. 6, C and D). Collectively, our data suggest that PDHA1 knockdown inhibited the development of CSC properties (Fig. 6, A–D).
Figure 5.
PGC-1β plays a crucial role in PDHA1 expression. A, pancreatic cancer cells (Panc-1) were transfected with FOXO3 siRNA, and lactate levels were determined (n = 3). B, immunofluorescence staining for PDHA1 and CD44 in pancreatic cancer patient tissues and pancreatic cell lines Panc-1, BxPC-3, and MIAPaCa-2 cells (magnification, ×60). Representative data from eight patients are shown. Scale bar, 50 μm. C, quantitative RT-PCR showing the mRNA expression of PDHA1 (adherent versus spheres; n = 3). D, Panc-1 cells were transfected with FOXO3 siRNA, and PDH activity was measured (n = 3). E, pancreatic cancer cells were transfected with PGC-1β siRNA for 48 h, and PDHA1 expression was examined by Western blotting (n = 3). S, scrambled siRNA; P, PGC-1β siRNA. F, Western blot of FOXO3 siRNA-transfected Panc-1 cells. Error bar represents S.E. Scr, scrambled; Rel., relative.
Figure 6.
PDHA1 plays a crucial role in pancreatic stem cell properties. A and B, spheroid assay following transfection of pancreatic cancer cells with PDHA1 siRNA (n = 3) (magnification, ×20). Scale bar, 50 μm. C and D, colony assay following transfection of pancreatic cancer cells with PDHA1 siRNA. Error bar represents S.E. Scr, scrambled.
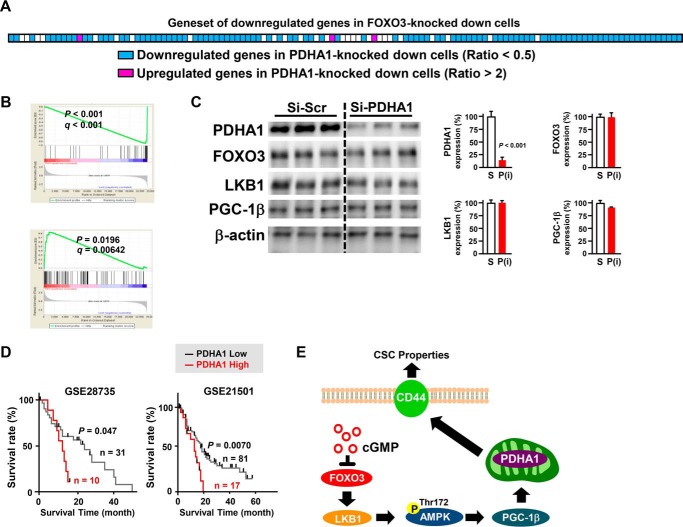
We next assessed the commonality of FOXO3 and PDHA1 knockdown by evaluating the effect of the PDHA1 knockdown on FOXO3-regulated genes. Gene set enrichment analysis revealed that 98 of 128 FOXO3-regulated genes (76.6%) were significantly down-regulated in PDHA1 knockdown cells (Fig. 7, A and B). We also found that PDHA1 knockdown did not affect FOXO3, LKB1, and PGC-1β levels (Fig. 7C). Importantly, patients who had high PDHA1 expression were associated with a poor prognosis compared with those who exhibited low PDHA1 expression (Fig. 7D). Together, the FOXO3/LKB1/PGC-1β/PDHA1 pathway might be crucial for CSC function and CD44 expression.
Figure 7.
Commonality of FOXO3 and PDHA1 knockdown by evaluating the effect of the PDHA1 knockdown on FOXO3-regulated genes. A and B, the effect of a PDHA1 knockdown on FOXO3-regulated genes was evaluated by gene set enrichment analysis. C, pancreatic cancer cells (Panc-1) were transfected with PDHA1 siRNA for 48 h and examined by Western blotting (n = 3). S, scrambled siRNA; P, PDHA1 siRNA. D, the impact of PDHA1 expression on the prognosis of patients with PDAC is shown. The difference in the overall survival was analyzed using the Kaplan-Meier survival model with a log-rank test. E, a novel pathway that impairs pancreatic carcinoma stem cancer cells is shown. Error bar represents S.E. Scr, scrambled.
Discussion
Recent studies have suggested that CSCs exhibit drug-resistant phenotypes, and the survival of CSCs is thought to play a crucial role in the recurrence of several types of cancers following chemotherapy (5, 6, 11, 19). In addition, CSCs are protected by several mechanisms, including enhanced DNA repair capacity and the up-regulation of several multidrug transporters, including P-glycoprotein (also known as MDR-1) and others (10, 19). Abnormally enhanced antiapoptotic pathways and developmental signaling cascades have also been shown to be involved in the resistant phenotype of CSCs. These findings suggest that the establishment of efficient measures for targeting CSCs is the key for eradication therapy.
Recent studies have identified several targets for targeting CSCs, including the Hedgehog, Wnt/β-catenin and Notch signaling pathways (10, 11); however, these signaling pathways are also important for somatic stem cell maintenance (23–25). For instance, in a clinical study using vismodegib (GDC-0449), a small-molecule inhibitor of the Hedgehog pathway, 25% of patients (26 of 104) reported serious adverse events, and 7% of the patients died (7 of 104) due to adverse events (26). In considering selective toxicity, targeting these pathways that are shared with normal stem cells may cause inevitable severe adverse effects, indicating that a novel approach is in high demand.
cGMP is a well known second messenger involved in vascular homeostasis and penile erection. We previously reported that cGMP drastically suppressed the CSC properties in PDAC through the inhibition of FOXO3 (8). Surprisingly, the well known tumor suppressor FOXO3 plays an indispensable role in CSC maintenance, although little is known regarding the specific mechanisms of FOXO3 in the maintenance of CSCs.
In this study, we showed that the FOXO3/LKB1/AMPK/PGC-1β/PDHA1/CD44 axis plays a central role in promoting the CSC phenotype in PDAC cells. Our data also revealed that the inhibition of the LKB1/AMPK/PGC-1β/PDHA1 pathway is sufficient to suppress the CSC properties associated with PDAC, including spheroid formation. Consistent with these data, patients with high PDHA1 expression were associated with a poor prognosis. We also confirmed that the LKB1/PGC-1β/PDHA1/CD44 axis was up-regulated in CD44+, and spheroid cells were shown to exhibit elevated CSC properties.
Systemic FOXO3 knock-out mice are viable and normal except for an enhancement in the activation of ovarian follicles (12). Similar to FOXO3 knock-out mice, PGC-1β knock-out mice also exhibit no overt phenotype except for circadian activity, adaption to the cold, and response to a high-fat diet burden (27). Considering that many mutations in the Hedgehog, Wnt/β-catenin, and Notch signaling pathways are lethal in mice (28, 29), the FOXO3/PGC-1β axis may be a novel target for eradicating pancreatic CSCs.
Several approaches have been established to indirectly or directly up-regulate intracellular cGMP for the treatment of other diseases, including erectile dysfunction, pulmonary hypertension, and cardiovascular disease (30). In addition, nitrites up-regulate cGMP levels through the release of nitric oxide and result in the activation of soluble guanylyl cyclase (31). Recently, the NO-independent soluble guanylyl cyclase activator riociguat has been used to treat pulmonary hypertension (32). Additionally, the pharmacological inhibition of phosphodiesterases, the major negative regulator for cGMP, is also widely used for the treatment of several diseases, such as erectile dysfunction and pulmonary hypertension (33).
In several types of cancers, including hepatic, colon, pancreatic, and breast cancer, CD44 is a well established CSC marker (13–16). Recently, CD44 has been characterized as a master regulator of CSCs, and ectopic CD44 expression endows these cells with the properties of CSCs (13–15); however, no clinically applicable approach has been established to date. Considering our findings, cGMP induction and FOXO3/PGC1-β inhibition may be a novel therapeutic strategy for patients with PDAC.
In cancer cells, the widely observed Warburg effect involves fermentation-based glucose metabolism (34). Conversely, several studies have shown that kinase inhibition, including MEK1 and dual PI3K/mTOR (BEZ235) inhibitors, increased the number of CSC-like cells with enhanced mitochondrial activity (7, 19); however, the reason for such enhanced mitochondrial activity in these surviving cells remains unclear. Although kinases (e.g. Akt, PI3K, mTOR, and Kras) are the preferred molecular targets of therapeutic drugs because they function as strong drivers of cell growth and antiapoptosis signaling, when evaluated from another perspective, these kinases can also function as direct or indirect negative regulators of FOXO3. Thus, the pharmacological inhibition of these kinases may induce unintended FOXO3 accumulation.
Considering its therapeutic index, directly targeting mitochondrial activity may not be practical. Moreover, the abnormal activation of the FOXO3/LKB1/PGC-1β1/PDHA1 axis may be the molecular basis involved in inducing high mitochondrial activity in pancreatic CSCs. Thus, this may provide an ideal method for the selective targeting of mitochondrial activation in CSCs. Furthermore, our study demonstrated that the knockdown of the mitochondrial enzyme PDHA1 was sufficient to suppress expression of CD44, the master regulator of CSCs. This finding indicates that the fate of cancer cells is largely associated with mitochondrial activity.
Taken together, we have demonstrated that the induction of cGMP and inhibition of FOXO3 suppressed the LKB1/PGC-1β pathway and the CSC properties in PDAC. Therefore, these mechanisms may provide a novel therapeutic target for eradicating pancreatic CSCs.
Experimental procedures
Study approval
All human samples in this study were provided after obtaining informed consent from patients in accordance with the Declaration of Helsinki.
Antibodies and reagents
Hoechst33342 (H3570) and Bay 41-2272 (ALX-420-030-M005) were purchased from Invitrogen and Enzo Life Sciences, respectively. A PDH activity kit was obtained from Abcam (ab109902), and the lactate assay kit was from BioVision (K607-100). The cGMP assay kit was provided by Cayman Chemical (581021). The antibodies used for flow cytometry and immunostaining included an APC-labeled anti-CD24 antibody (eBioscience 17-0247), FITC-labeled anti-CD44 antibody (Miltenyi Biotec, 130-095-195), anti-FOXO3 antibody (Abcam, ab109629), anti-CD44 antibody (Abcam, ab51037), anti-LKB1 antibody (Abcam, ab15095), anti-PGC-1β antibody (Abcam, ab176328), anti-PDHA1 antibody (Abcam, ab168379), and anti-β-actin antibody (Sigma-Aldrich, 061M4808). Secondary antibodies were purchased from Invitrogen (Alexa Fluor 488-labeled, A110034 and A11001; Alexa Fluor 555-labeled, A21428 and A21422). Antibody validation is available on the manufacturers' websites.
Cell cultures and cell-based assays
The human pancreatic carcinoma cell lines BxPC-3 and MIAPaCa-2 were provided from the Japanese Collection of Research Bioresources Cell Bank. Panc-1 was obtained from the Riken Cell Bank. All cells were cultured in 10% fetal calf serum, RPMI 1640 medium supplemented with antibiotics (penicillin and streptomycin) at 100% humidity, 37 °C, and 5% CO2. All siRNAs were purchased from Sigma-Aldrich: siScr (SIC-001), siFOXO3 (i) (Hs_FOXO3_9127), siFOXO3 (ii) (Hs_FOXO3_9128), siLKB1 (i) (STK11) (Hs_STK11_2687), siLKB1 (ii) (STK11) (Hs_STK11_2689), siPGC-1β (PPARGC1B) (Hs_PPARGC1B_2923), siPGC-1β (ii) (PPARGC1B) (Hs_PPARGC1B_2924), siPDHA1(i) (Hs_PDHA1_2458), and siPDHA1 (ii) (Hs_PDHA1_2242). For RNAi transfection, LipofectamineTM RNAiMAX transfection reagent (Life Technology) and siRNA were used. Following a 48-h transfection period, the cells were harvested. For the spheroid assay, PDAC cells were seeded into RPMI 1640 medium supplemented with B27 (1:50 dilution; Invitrogen), basic FGF (10 ng/ml; BD Biosciences), EGF (BD Biosciences; 20 ng/ml) at a density of 1 × 103 cells/well and incubated for 21 days in 24-well plates for the spheroid assay (Corning® Ultra-Low Attachment Surface, 3473). To evaluate the effect on holoclones, the cells were seeded into RPMI 1640 medium supplemented with 1% FCS at a density of 1 × 103 cells/5 ml/dish and cultured for 21 days.
Flow cytometry analysis and immunostaining assay
The cells were harvested using Accumax purchased from Innovative Cell Technologies Inc. The cells were blocked with 2.5% BSA,TTBS for 30 min and treated with the following antibodies: FITC-anti-CD44 (1:50 dilution), APC-CD24 (1:50 dilution), and LKB1 (1:300 dilution) for 1 h on ice followed by washing with 2.5% BSA, TTBS. The mitochondrial potential was measured using the Mitochondrial Membrane Potential Assay kit (Cayman Chemical, 10009172) used according to the data sheet specifications. Sections of the patient samples were retrieved under 100 °C in citrate buffer (10 mm, pH 6.0) in an oven and blocked using 2.5% BSA, TTBS. After the blocking step, the slides were incubated with the primary antibody for 7 h at 4 °C. The LKB1, PGC-1β, and PDHA1 antibodies were used at a 1:300 dilution. The anti-CD44 antibody was used at a dilution of 1:50, detected using a secondary antibody (1:100 dilution; 1 h at 4 °C), and stained with Hoechst 33342.
Western blotting and immunofluorescence
The cells were lysed in the above mentioned lysis buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 50 mm NaF, 30 mm Na4P2O7, 1 mm phenylmethanesulfonyl fluoride, 2 mg/ml aprotinin, and 1 mm pervanadate. Western blotting was performed as described previously (8).
Quantitative real-time analysis and microarray analysis
The sequences of the PCR primers used are as follows: PGC-1β, 5′-CTGCTTGGATTTCCAAATGGTTTC-3′ (sense) and 5′-CAGATGCTCCAAGCCAATGCTA-3′ (antisense); β-actin, 5′-CATCCGTA AAGACCTCTATGCCAA-3′ (sense) and 5′-ATGGAGCCACCGATCCACA-3′ (antisense); STK11, 5′-AACGGCCTGGACACCTTCT-3′ (sense) and 5′-CCCTTCCCGATGTTCTC AA-3′ (antisense); and PDHA1, 5′-GAGTCAGTGCTTCAAGCCAACAG-3′ (sense) and 5′-GACACGAGCGTCACCTCCATA-3′ (antisense). The total RNA from the cells was harvested using TRIzol reagent obtained from Invitrogen. Quantitative real-time PCR was performed using the relative standard curve method to quantify the level of target gene expression. SYBR Premix Ex Taq was used for quantitative real-time PCR according to the manufacturer's protocol. In the microarray analysis, the total RNA was determined as described previously (8).
Statistical analysis
All data are presented as the mean ± S.E. Comparisons of the experimental data were examined for statistical differences using a one-way analysis of variance followed by an unpaired Student's t test (two groups) or Tukey's test (multiple comparisons). Statistical analyses were performed using GraphPad (GraphPad Software). Statistical analyses of the survival curves were performed using log-rank analyses of the Kaplan-Meier curves. A p value <0.05 was considered significant.
Author contributions
Mo. K. and M. T. planned the studies. Mo. K., M. T., S. H., C. T., Ma. K., T. N., H. O., J. B., Y. H., K. T., and S. Y. performed the experiments. Mo. K. had responsibility for all data integrity and data analysis. Mo. K., M. T., T. N., K. K., and H. T. discussed the results. H. T. conducted the whole research. H. T. had primary responsibility for the final content. All authors reviewed the manuscript.
Acknowledgments
We appreciate technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University, and the Center for Accelerator and Beam Applied Science, Kyushu University. We thank Takeshi Iwasaki (Kyushu University, Faculty of Medicine) for helpful advice on bioinformatics analysis.
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 22228002 and 15H02448 (to H. T.), a grant-in-aid for JSPS fellows (to M. Kumazoe) (PD), and JSPS KAKENHI Grant 15K18821. The authors declare that they have no conflicts of interest with the contents of this article.
Complete transcriptome profiles are available at the Gene Expression Omnibus under accession number GSE66404.
- PDAC
- pancreatic ductal adenocarcinoma
- AMPK
- AMP-activated protein kinase
- CSC
- cancer stem cell
- FOXO3
- forkhead box O3
- LKB1
- liver kinase B1
- PDH
- pyruvate dehydrogenase
- PGC
- PPAR-γ co-activator
- PPAR
- peroxisome proliferator-activated receptor
- mTOR
- mechanistic target of rapamycin
- APC
- allophycocyanin
- TTBS
- TBS with Tween 20.
References
- 1. Siegel R. L., Miller K. D., and Jemal A. (2015) Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 [DOI] [PubMed] [Google Scholar]
- 2. Vincent A., Herman J., Schulick R., Hruban R. H., and Goggins M. (2011) Pancreatic cancer. Lancet 378, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burris H. A. 3rd, Moore M. J., Andersen J., Green M. R., Rothenberg M. L., Modiano M. R., Cripps M. C., Portenoy R. K., Storniolo A. M., Tarassoff P., Nelson R., Dorr F. A., Stephens C. D., and Von Hoff D. D. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 [DOI] [PubMed] [Google Scholar]
- 4. Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J. L., Gourgou-Bourgade S., de la Fouchardière C., Bennouna J., Bachet J. B., Khemissa-Akouz F., Péré-Vergé D., Delbaldo C., et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 [DOI] [PubMed] [Google Scholar]
- 5. Quint K., Tonigold M., Di Fazio P., Montalbano R., Lingelbach S., Rückert F., Alinger B., Ocker M., and Neureiter D. (2012) Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 41, 2093–2102 [DOI] [PubMed] [Google Scholar]
- 6. Adikrisna R., Tanaka S., Muramatsu S., Aihara A., Ban D., Ochiai T., Irie T., Kudo A., Nakamura N., Yamaoka S., and Arii S. (2012) Identification of pancreatic cancer stem cells and selective toxicity of chemotherapeutic agents. Gastroenterology 143, 234.e7–245.e7 [DOI] [PubMed] [Google Scholar]
- 7. Viale A., Pettazzoni P., Lyssiotis C. A., Ying H., Sánchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V., Kost-Alimova M., Muller F., Colla S., Nezi L., Genovese G., et al. (2014) Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumazoe M., Takai M., Bae J., Hiroi S., Huang Y., Takamatsu K., Won Y., Yamashita M., Hidaka S., Yamashita S., Yamada S., Murata M., Tsukamoto S., and Tachibana H. (2017) FOXO3 is essential for CD44 expression in pancreatic cancer cells. Oncogene 36, 2643–2654 [DOI] [PubMed] [Google Scholar]
- 9. Ohara Y., Oda T., Sugano M., Hashimoto S., Enomoto T., Yamada K., Akashi Y., Miyamoto R., Kobayashi A., Fukunaga K., Morishita Y., and Ohkohchi N. (2013) Histological and prognostic importance of CD44+/CD24+/EpCAM+ expression in clinical pancreatic cancer. Cancer Sci. 104, 1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sergeant G., Vankelecom H., Gremeaux L., and Topal B. (2009) Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat. Rev. Clin. Oncol. 6, 580–586 [DOI] [PubMed] [Google Scholar]
- 11. Takebe N., Harris P. J., Warren R. Q., and Ivy S. P. (2011) Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 8, 97–106 [DOI] [PubMed] [Google Scholar]
- 12. Castrillon D. H., Miao L., Kollipara R., Horner J. W., and DePinho R. A. (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301, 215–218 [DOI] [PubMed] [Google Scholar]
- 13. Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H., Patrawala L., Yan H., Jeter C., Honorio S., Wiggins J. F., Bader A. G., Fagin R., Brown D., and Tang D. G. (2011) The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 17, 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su Y. J., Lai H. M., Chang Y. W., Chen G. Y., and Lee J. L. (2011) Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 30, 3186–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molejon M. I., Tellechea J. I., Loncle C., Gayet O., Gilabert M., Duconseil P., Lopez-Millan M. B., Moutardier V., Gasmi M., Garcia S., Turrini O., Ouaissi M., Poizat F., Dusetti N., and Iovanna J. (2015) Targeting CD44 as a novel therapeutic approach for treating pancreatic cancer recurrence. Oncotarget 6, 7408–742325797268 [Google Scholar]
- 16. Zhao S., Chen C., Chang K., Karnad A., Jagirdar J., Kumar A. P., and Freeman J. W. (2016) CD44 expression level and isoform contributes to pancreatic cancer cell plasticity, invasiveness and response to therapy. Clin. Cancer Res. 22, 5592–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C., Heidt D. G., Dalerba P., Burant C. F., Zhang L., Adsay V., Wicha M., Clarke M. F., and Simeone D. M. (2007) Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 18. Greer E. L., and Brunet A. (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 [DOI] [PubMed] [Google Scholar]
- 19. Roesch A., Vultur A., Bogeski I., Wang H., Zimmermann K. M., Speicher D., Körbel C., Laschke M. W., Gimotty P. A., Philipp S. E., Krause E., Pätzold S., Villanueva J., Krepler C., Fukunaga-Kalabis M., et al. (2013) Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B (high) cells. Cancer Cell 23, 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lützner N., De-Castro Arce J., and Rösl F. (2012) Gene expression of the tumour suppressor LKB1 is mediated by Sp1, NF-Y and FOXO transcription factors. PLoS One 7, e32590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher K. W., Das B., Kim H. S., Clymer B. K., Gehring D., Smith D. R., Costanzo-Garvey D. L., Fernandez M. R., Brattain M. G., Kelly D. L., MacMillan J., White M. A., and Lewis R. E. (2015) AMPK promotes aberrant PGC1β expression to support human colon tumor cell survival. Mol. Cell. Biol. 35, 3866–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wieland O. H. (1983) The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev. Physiol. Biochem. Pharmacol. 96, 123–170 [DOI] [PubMed] [Google Scholar]
- 23. Ahn S., and Joyner A. L. (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897 [DOI] [PubMed] [Google Scholar]
- 24. Varnum-Finney B., Halasz L. M., Sun M., Gridley T., Radtke F., and Bernstein I. D. (2011) Bernstein, Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J. Clin. Investig. 121, 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brack A. S., Conboy I. M., Conboy M. J., Shen J., and Rando T. A. (2008) A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59 [DOI] [PubMed] [Google Scholar]
- 26. Sekulic A., Migden M. R., Oro A. E., Dirix L., Lewis K. D., Hainsworth J. D., Solomon J. A., Yoo S., Arron S. T., Friedlander P. A., Marmur E., Rudin C. M., Chang A. L., Low J. A., Mackey H. M., et al. (2012) Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sonoda J., Mehl I. R., Chong L. W., Nofsinger R. R., and Evans R. M. (2007) PGC-1β controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc. Natl. Acad. Sci. U.S.A. 104, 5223–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammond C. L., and Schulte-Merker S. (2009) Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development 136, 3991–4000 [DOI] [PubMed] [Google Scholar]
- 29. van Amerongen R., and Berns A. (2006) Knockout mouse models to study Wnt signal transduction. Trends Genet. 22, 678–689 [DOI] [PubMed] [Google Scholar]
- 30. Buglioni A., and Burnett J. C. Jr. (2016) New pharmacological strategies to increase cGMP. Annu. Rev. Med. 67, 229–243 [DOI] [PubMed] [Google Scholar]
- 31. Nossaman V. E., Nossaman B. D., and Kadowitz P. J. (2010) Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol. Rev. 18, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sulica R., Fenton R., and Cefali F. (2015) Early observations on the use of riociguat in a large, metropolitan pulmonary arterial hypertension/chronic thromboembolic pulmonary hypertension treatment center. Cardiol. Ther. 4, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lugnier C. (2006) Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol. Ther. 109, 366–398 [DOI] [PubMed] [Google Scholar]
- 34. Warburg O. (1956) On respiratory impairment in cancer cells. Science 124, 269–270 [PubMed] [Google Scholar]