Abstract
Upon infection, the intracellular parasite Toxoplasma gondii co-opts critical functions of its host cell to avoid immune clearance and gain access to nutritional resources. One route by which Toxoplasma co-opts its host cell is through hijacking host organelles, many of which have roles in immunomodulation. Here we demonstrate that Toxoplasma infection results in increased biogenesis of host lipid droplets through rewiring of multiple components of host neutral lipid metabolism. These metabolic changes cause increased responsiveness of host cells to free fatty acid, leading to a radical increase in the esterification of free fatty acids into triacylglycerol. We identified c-Jun kinase and mammalian target of rapamycin (mTOR) as components of two distinct host signaling pathways that modulate the parasite-induced lipid droplet accumulation. We also found that, unlike many host processes dysregulated during Toxoplasma infection, the induction of lipid droplet generation is conserved not only during infection with genetically diverse Toxoplasma strains but also with Neospora caninum, which is closely related to Toxoplasma but has a restricted host range and uses different effector proteins to alter host signaling. Finally, by showing that a Toxoplasma strain deficient in exporting a specific class of effectors is unable to induce lipid droplet accumulation, we demonstrate that the parasite plays an active role in this process. These results indicate that, despite their different host ranges, Toxoplasma and Neospora use a conserved mechanism to co-opt these host organelles, which suggests that lipid droplets play a critical role at the coccidian host-pathogen interface.
Keywords: host-pathogen interaction, lipid droplet, lipid metabolism, parasite, Toxoplasma gondii
Introduction
Lipid droplets (LDs)2 are conserved organelles that act as intracellular stores of neutral lipids such as cholesterol esters and triacylglycerols and as platforms for lipid metabolism (1). These organelles are unique in that they are surrounded by a phospholipid monolayer, rather than a bilayer, and are filled with a neutral lipid core (2). LDs are dynamically generated and turned over in response to metabolic cues (1, 3, 4), and dysregulation of these processes can lead to human diseases such as diabetes and obesity (5). LDs have also been implicated as major players in host-pathogen interaction. Indeed, LDs represent energy-rich prizes for intracellular pathogens. For instance, Chlamydia takes up host lipids by trafficking LDs to the bacterial inclusion (6), and hepatitis C virus and rotavirus co-opt LDs for their replication and assembly (7, 8). LDs may also possess active antimicrobial functions, as invertebrate LDs were recently found to protect against intracellular bacterial infection of Drosophila embryos by directly targeting and killing bacteria (9).
The ubiquitous intracellular parasite Toxoplasma gondii survives and replicates within a parasitophorous vacuole (PV) that protects it from clearance but restricts its access to host resources. Perhaps in response to this challenge, the parasite secretes an array of effector proteins that alter host cell signaling (10–12). Rewiring of host cell functions by the parasite serves the dual purposes of immune evasion (13) and hijacking host resources, such as phospholipids (14, 15) and cholesterol (16), for which the parasite is auxotrophic. Previous work has identified potential interactions between the PV and a number of host organelles, including the ER (17), Golgi (15), and mitochondria (17, 18). In some parasite strains, a polymorphic parasite effector serves to capture host mitochondria against the PV (18), resulting in apparent changes in immune signaling. It is therefore likely that additional effectors directly modulate the functions of other host organelles.
Recent work indicates that LDs accumulate in Toxoplasma-infected murine cells (19, 20), although neither the signaling nor the metabolic changes to the host cell associated with this organellar modulation are understood. Here we demonstrate that Toxoplasma infection radically alters its host cell metabolism, changing how the cells respond to and process fatty acids. These changes result in buildup of neutral lipids in LDs, some of which appear to be captured by the PV. We identify multiple biochemical activities involved in neutral lipid metabolism that are altered by infection and show that these processes are similarly affected by Neospora infection. Furthermore, we demonstrate that this metabolic dysregulation requires secretion of parasite effectors, revealing host LDs as a previously unknown platform for coccidian parasite interaction with their hosts.
Results
Toxoplasma infection causes robust up-regulation of host LDs in human cells
Previous work has indicated that Toxoplasma infection induces an increase in murine host LDs (19, 20). To confirm that this phenomenon is relevant to hosts other than mice, we infected primary human foreskin fibroblasts (HFFs) with the type I RH strain of parasites expressing cytosolic TdTomato. After 24 h, we stained neutral lipids with BODIPY 493/503. Although few LDs were visible without the addition of free fatty acid (FFA) (data not shown), after incubation with oleic acid (OA), parasite-infected cells had significant neutral lipid buildup compared with uninfected cells (Fig. 1). Infection with increasing multiplicities of infection (m.o.i.) led to a more robust increase in host LDs (Fig. 1A), consistent with a dose response to a parasite-derived factor.
Figure 1.
Toxoplasma infection induces accumulation of LDs in host cells. A, representative fluorescent micrographs of HFFs infected with the indicated m.o.i. of RH(TdTomato) Toxoplasma parasites for 24 hpi using the fluorescent neutral lipid dye BODIPY 493/503. All cells were treated for 24 h with 360 μm OA. B, representative images of cells infected with a single m.o.i. of 4 for 2–24 h. All conditions were treated for 24 h with OA. C, quantification of BODIPY intensities of three replicates of ten images each (∼6000 cells/condition) from B. One-way ANOVA comparison to uninfected: ***, p < 0.001. D, quantification of average BODIPY intensity colocalizing with either host nuclei or Toxoplasma. Student's t test: ***, p < 0.001. Scale bars = 20 μm.
To investigate the timing of the up-regulation, HFFs were incubated with OA for a total of 24 h and infected with Toxoplasma for 2–24 h prior to fixation and then stained and imaged (Fig. 1B) as above. Cellular neutral lipid levels were estimated by quantifying BODIPY intensity in all images using an automated CellProfiler pipeline (21) (Fig. 1C). We observed significant increases in BODIPY signal as early as 2 h post infection (hpi) and more substantial differences as infection progressed. As all cells were treated with OA for the same amount of time, these data indicate that, as an infected cell matures, it increases its ability to take up, esterify, and store esterified fatty acids in LDs. We also observed a diffuse BODIPY signal associated with the parasites in OA-treated cells (Fig. 1B, bottom panel). We quantified this parasite-associated signal at 24 hpi and found that it was significantly higher than the BODIPY signal colocalized with host nuclei, which we used as an estimate of nonspecific background (Fig. 1D). This parasite-associated signal suggests that the parasites themselves store neutral lipids when exposed to high levels of FFA.
Parasite-induced LD accumulation occurs without FFA stimulation
To demonstrate that the observed accumulation of LDs is not an artifact of excess FFA, we tested whether LD biogenesis was altered in infected cells that had not been treated with OA. As LDs range in diameter from several microns down to ∼100 nm and often cluster within a cell, they can be difficult to resolve by light microscopy. We therefore used transmission EM to examine cells that were not treated with OA and either infected for 24 h or left uninfected. Consistent with our fluorescence microscopy data of OA-treated cells (Fig. 1), we observed an increase in overall host LD-infected cells compared with uninfected cells (Fig. 2, A–C). Overall, we observed an approximate 2-fold increase in the cellular surface area covered by LDs in infected cells over that in uninfected cells (Fig. 2D). This was partially due to a greater number of droplets in infected cells (Fig. 2E), although infected cells also contained significantly higher numbers of larger LDs (Fig. 2F).
Figure 2.
Infected cells accumulate TAG LDs without OA treatment. A–C, representative transmission electron micrographs of infected (A) versus uninfected (B) cells. The region noted in A is expanded in C, where apparently intravacuolar LDs are indicated by arrowheads. D, quantification of LD area normalized to total host cell area from 27 images for each condition. E, quantification of number of LDs per cell from same data set. In D and E, significance was calculated by Welch's unpaired t test. F, cumulative frequency distribution of individual LD areas from the quantification performed in E. Kolmogorov-Smirnov test: D = 0.10; ***, p < 0.0001.
Neutral lipid synthesis machinery localizes to the ER, and current models indicate that LDs bud from sites on the ER membrane (22). As the Toxoplasma PV is thought to make intimate contacts with a number of host organelles, including the ER (17), it is possible that such contacts might be involved in the observed induction of neutral lipid accumulation in infected cells. If that were the case, we would expect to find concentrated groups of LDs near the PV. Although our EM images do show clusters of LDs in infected cells, we observed no correlation with distance to the PV; clusters of LDs in infected cell often localize quite distant from a PV (Fig. 2A). Strikingly, we also observed round objects of low electron density within the PV, consistent with the EM characteristics of LDs (Fig. 2C, arrowheads), suggesting that neutral lipids are being imported into the PV.
Toxoplasma infection increases triacylglycerol production in host cells
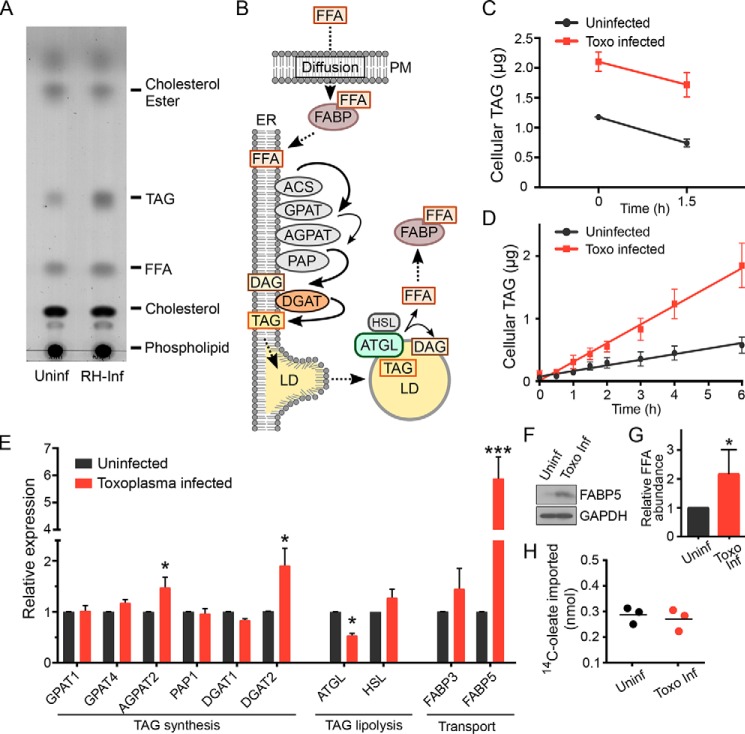
LDs are composed of a variety of neutral lipids, including cholesterol esters, diacylglycerols, and triacylglycerols (TAG). To identify the neutral lipids accumulating in infected cells, at 24 hpi we extracted lipids from infected cells and compared the neutral lipid levels with those extracted from uninfected cells. As addition of FFA would be expected to drive the increase of TAG over other neutral lipids, we did not treat cells with OA. To control for lipids found in parasites, parasites were mechanically released from identically treated infected cells and purified from cell debris by filtration. These free parasites were mixed with uninfected host cells in organic solvent to extract neutral lipids. All “uninfected” samples therefore contained a mix of parasite and host-derived lipids and thus provided a true baseline against which to specifically measure changes in host neutral lipid levels because of infection. We resolved extracted neutral lipids by TLC. Quantifying individual lipid levels by densitometric comparison with lipid standards revealed that parasite infection increased TAG levels by 3-fold but that other neutral lipids, including cholesterol esters, remained largely unaltered (Fig. 3A). Taken together with our EM images, these data demonstrate that Toxoplasma infection of human cells triggers a specific up-regulation in TAG accumulation and the biogenesis of new lipid droplets.
Figure 3.
Toxoplasma infection alters host TAG synthesis. A, TLC of neutral lipids extracted from uninfected (Uninf) HFFs compared with those infected with the Toxoplasma RH strain (RH-Inf). Cells had not been treated with OA. B, diagram of cellular TAG metabolism that leads to LD formation. Enzymes and transporters are indicated as ovals and lipids as squares. Host proteins whose transcripts are altered upon parasite infection are colored. Rate-limiting reactions are indicated by thinner lines. Note that HSL and ATGL catalyze the same reaction (TAG hydrolysis). PM, plasma membrane; ACS, acyl-CoA synthetase; GPAT, glycerol-3-phosphate acyltransferase; AGPAT, 1-acylglycerol-3-phosphate acyltransferase; PAP, phosphatidate phosphatase; DAG, diacylglycerol; HSL, hormone-sensitive lipase. C, quantification of lipolysis rate. Cellular TAG was quantified after OA washout and combined with triacsin C to inhibit TAG synthesis. Cells were harvested at the indicated times, and neutral lipids were extracted and quantified as described under “Experimental procedures” (n = 3). D, quantification of TAG accumulation. HFFs were infected for 16 h or left uninfected and pulsed with 360 μm OA for the indicated times, after which total TAG levels were quantified (n = 3). Data were fit linearly using GraphPad Prism: kuninf = 0.090 ± 0.007 μg/h; kinf = 0.30 ± 0.01 μg/h. Toxo, Toxoplasma. E, host transcript levels encoding proteins associated with neutral lipid metabolism were quantified by qPCR after 24-h infection with Toxoplasma RH without OA treatment and compared with uninfected cells. One-way ANOVA: *, p < 0.05; ***, p < 0.0001. F, Western blot of cellular lysates of uninfected and Toxoplasma-infected cells probed with anti-FABP5 and GAPDH as a loading control. G, relative FFA abundance was quantified by densitometric analysis of TLC. H, uptake of [14C]oleate by uninfected and infected cells was compared after 1-h incubation.
LDs are maintained in a dynamic equilibrium by continuous synthesis and hydrolysis (lipolysis) of neutral lipids (1). The accumulation of LDs we observe in infected cells could therefore be due either to an increased rate of synthesis, due to a reduction in the rate of lipolysis, or both. We thus sought to quantify the contributions of TAG synthesis and lipolysis to Toxoplasma-induced LD accumulation. To measure the rate of lipolysis, Toxoplasma-infected and uninfected HFFs were treated with 60 μm OA to induce high levels of LD accumulation. After 16 h, OA was washed out, and 6 μm triacsin C, an inhibitor of neutral lipid synthesis, was added to the medium. By measuring the amount of cellular TAG over time, we observed no significant difference between the lipolysis rates in infected and uninfected cells (Fig. 3C), suggesting that Toxoplasma is inducing changes in biogenesis and growth, but not turnover, of host LDs.
We next sought to measure the rate of LD accumulation in infected and uninfected cells. After overnight infection, HFFs were incubated with OA for 0–6 h, and cellular TAG was quantified over time as above. Consistent with our observation that infected HFFs begin accumulating host LDs shortly after infection (Fig. 1), we observed a substantially increased rate of LD accumulation in Toxoplasma-infected cells compared with uninfected controls (Fig. 3D).
The increased response of infected cells to FFA stimulation could have its roots in a variety of regulatory mechanisms, from transcriptional to post-translational. We used qPCR to quantify the transcript levels of host enzymes involved in LD maintenance (Fig. 3B) at 24 hpi but without stimulation with OA (Fig. 3E). Although we observed no change in lipolysis rate because of infection, we did observe that the transcript levels for adipose triglyceride lipase (ATGL), which encodes the enzyme that catalyzes the rate-limiting step in TAG lipolysis, were reduced by 1.9 ± 0.1-fold in infected cells. Changes in transcript levels were not limited to proteins involved in lipolysis. Most notably, we observed a 1.9 ± 0.3-fold increase in the transcript for the enzyme diacylglycerol O-acyltransferase (DGAT2), which converts DAG and FFA into TAG (23), as well as a 5.9 ± 0.8-fold increase in the fatty acid-binding protein 5 (FABP5), which is required for efficient transport of FFA between organellar membranes. Consistent with the increase in its transcript, FABP5 protein levels were substantially increased in infected cells (Fig. 3F). Increased levels of FABP5 protein would be expected to bind FFA, potentially increasing the amount of FFA available in a cell. We quantified FFA levels from our lipid extractions (e.g. Fig. 3A) by densitometry and found that infected cells had 2.2 ± 0.4-fold more FFA than uninfected cells (Fig. 3G). We also tested whether infection increased FFA import into a cell using radiolabeled OA but saw no significant difference between uptake in infected versus uninfected cells (Fig. 3H). These data are consistent with the known role of FABP5 in intracellular FFA transport rather than in import.
Taken together, these data suggest that the parasite-driven increase in host LD accumulation is caused, in part, by changes in transcripts that encode for proteins involved in both the intracellular transport and synthesis of lipids. Given the multiple layers of regulation of cellular metabolism, it is unlikely that transcriptional changes are entirely responsible for the observed changes in LD accumulation upon infection.
We went on to quantify the DGAT activity in uninfected and Toxoplasma-infected cells according to the method described under “Experimental procedures.” We observed no significant difference in DGAT-specific activities between uninfected and infected cells that had not been treated with OA (Fig. 4A). Strikingly, when treated for 6 h with 360 μm OA, Toxoplasma-infected cells, but not uninfected cells, showed a 2.7 ± 0.4-fold increase in DGAT activity. There was no apparent change in DGAT2 protein levels in our membrane fractions after OA stimulation (Fig. 4B), suggesting that DGAT activity is being posttranslationally regulated differently in infected versus uninfected cells.
Figure 4.

Toxoplasma induces posttranslational up-regulation in cellular DGAT activity. A, DGAT activity was measured from isolated cellular membranes from uninfected (Uninf) or Toxoplasma-infected (Toxo inf.) cells with and without the addition of 360 μm OA for 6 h prior to lysis (n = 3). Significance was tested by one-way ANOVA. n.s., not significant. TG, triacylglycerol. B, Western blot probing with anti-DGAT2 in isolated membranes from A. HSP90B1 was used as a loading control. Equal masses of each sample were loaded.
Parasite-induced LD accumulation requires host mTOR and JNK signaling
We next sought to determine which host signaling pathways modulate the changes to neutral lipid metabolism triggered by parasite infection. Given the diversity of possible signaling networks manipulated by Toxoplasma to up-regulate LDs, we adopted a pharmacological strategy to identify potential players. We chose the inhibitor of mTOR signaling Torin-1 as a likely first candidate. mTOR is a central regulator of cellular metabolism; its inhibition reduces global protein translation (24) and would thus be expected to act against parasite up-regulation of host metabolic proteins. In addition, mTOR inhibition increases autophagic breakdown of LDs, or lipophagy (3), which could partially counter the accumulation we found associated with Toxoplasma infection. As expected, Torin-1 treatment during infection largely blocked Toxoplasma-induced accumulation of cellular TAG and, thus, the associated built-up LDs (Fig. 5, A and B), indicating that we could indeed pharmacologically inhibit this up-regulation.
Figure 5.
Pharmacological inhibition of Toxoplasma-induced LD accumulation. A, fluorescent micrographs of HFFs that were infected with RH(TdTomato) Toxoplasma and treated with the indicated drugs for 24 h. To visualize LD accumulation, cells were pulsed with 360 μm OA for the last 6 h of infection. Scale bars = 20 μm. B, cellular TAG was quantified for HFFs infected for 24 h and treated with the indicated drugs (n = 3). All infected samples were normalized to an uninfected (Uninf) control. One-way ANOVA: *, p < 0.05;**, p < 0.001; ***, p < 0.0001. C and D, qPCR comparing host transcript levels of DGAT2 (C) and FABP5 (D) after infection and drug treatment. All samples are normalized to infected (Inf)/DMSO control. Significance was tested by one-way ANOVA. B–D, drugs that showed no significant effect on LD accumulation are shaded light gray.
To identify other host signaling pathways that may be involved, confluent HFFs were infected and treated for 24 h with inhibitors of signaling through HIF1α, c-Myc, EGF receptor (EGFR), or the MAPKs ERK1/2, p38, or JNK. We incubated cells with OA for the last 6 h of infection, stained for neutral lipids with BODIPY 493/503, and imaged. The majority of drugs we tested showed no significant ability to block parasite-induced LD accumulation (Fig. 5 and data not shown), including the HIF1α inhibitor PX-478. We were thus surprised to find that the ALK4/5 inhibitor SB505124, which was shown previously to inhibit Toxoplasma-induced HIF1α activation (25), was able to partially inhibit LD accumulation in infected cells. More recently, SB505124 was also found to block Toxoplasma replication by inhibiting a parasite kinase (26). Given that neither direct inhibition of host HIF1α nor HIF1α knockout (data not shown) had any effect on LD accumulation, we reasoned that parasite growth is required for the observed phenotypes. This suggests that SB505124-mediated killing of parasites was causing the reduction in LD buildup, perhaps by inhibiting the release of an effector required for the observed phenotypes.
To our surprise, we noted that one other drug, the widely used JNK inhibitor SP600125, also appeared to block parasite replication (Fig. 5A). We observed, however, that SP600125 treatment much more robustly inhibited LD accumulation than SB505124 (Fig. 5), suggesting that its activity against host JNK may also be relevant. To test this, we used a recently developed JNK inhibitor (JNK-in-8, Ref. 27) with greatly improved specificity and an unrelated chemical scaffold to SP600125. Consistent with a requirement for host JNK activity in parasite-induced LD biogenesis, treating infected cells with JNK-in-8 substantially reduced TAG accumulation in infected cells (Fig. 5, A and B) but did not block parasite replication. As JNK-in-8 is an irreversible inhibitor, we were able to eliminate any confounding direct effect it may have on the parasites by pretreating HFFs with drug for 2 h and washing out at the time of infection. Even though such inhibition would only persist until new JNK protein had been synthesized, we found that JNK-in-8 pretreatment blocked ∼30% of the TAG accumulation induced by Toxoplasma infection (Fig. 5B). We therefore conclude that efficient Toxoplasma induction of host LDs appears to require both JNK and mTOR signaling to be uninhibited.
Somewhat surprisingly, although both Torin-1 and JNK-in-8 treatment significantly attenuated LD buildup in infected cells, their effects on host metabolic transcript levels were mild. Torin-1 partially blocked Toxoplasma-induced changes to FABP5 transcript levels, whereas JNK-in-8 showed no significant effect. Neither drug significantly reduced the effect of infection on DGAT2 transcript levels (Fig. 5, C and D). That the attenuation of LD accumulation by Torin-1 and JNK-in-8 treatment was far stronger than the inhibition of Toxoplasma-induced transcriptional changes suggests that the observed effect on TAG synthesis of these drugs is largely posttranscriptional.
Host LD accumulation is conserved across Toxoplasma strains and the related parasite Neospora
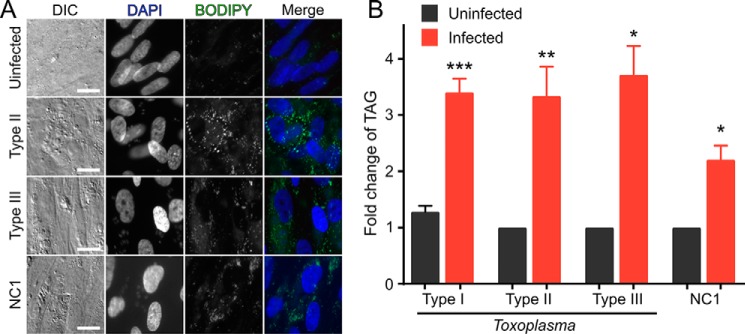
Distinct evolutionary pressure on individual Toxoplasma strains has led to a number of polymorphic effectors that cause strain-specific differences in host cell function (13, 28). We thus sought to determine whether the ability to up-regulate host LDs was limited to specific Toxoplasma strains. We elected to test the three genetically divergent strains (called types I, II, and III) that are the predominant global isolates (29) for the ability to induce host LD accumulation. HFFs were infected with either the type II ME49 or type III CEP strain, treated with OA, and imaged as above. Infection of cells with each strain yielded a robust up-regulation of host LDs (Fig. 6A) similar to what we had observed with the type I RH strain (Fig. 1). By using TLC to quantify neutral lipids that had been extracted from cells infected with any of the three Toxoplasma strains, we demonstrated that infection with each strain yields an approximately 2- to 4-fold increase in cellular TAG over uninfected cells (Fig. 6B).
Figure 6.
LD induction is conserved in both Toxoplasma and Neospora. A, representative fluorescent micrographs of cells infected with three divergent strains of Toxoplasma (type I RH, type II ME49, and type III CEP) and the related parasite Neospora (NC1). Scale bars = 20 μm. DIC, differential interference contrast. B, quantification of -fold-change in cellular TAG after infection (n = 3; type I, n = 5; type II, n = 4). Ratio paired t test: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Neospora caninum is a coccidian parasite that is closely related to T. gondii but has effector molecules that have largely diverged in function from those in Toxoplasma (30). Indeed, the majority of functions that have been ascribed to specific Toxoplasma effector proteins to date appear to be unconserved in the two organisms (30). Also, unlike Toxoplasma, Neospora is unable to productively infect humans in vivo. We repeated our imaging and neutral lipid extraction on HFFs that had been infected with the Neospora NC1 strain. To our surprise, Neospora infection also resulted in a substantial accumulation of TAG in host LDs in infected human fibroblasts (Fig. 6). Thus, host up-regulation of LDs upon infection appears to be conserved among this family of parasites.
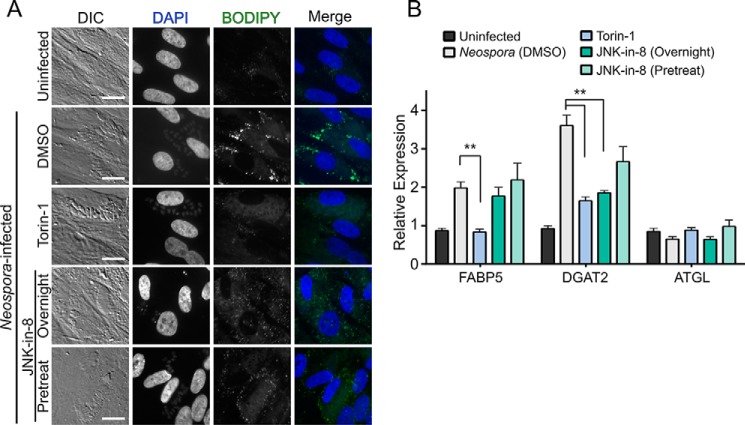
As we have demonstrated that Neospora infection also induces LD accumulation, we next asked whether both parasites required similar host pathways to modulate host neutral lipid metabolism. We thus tested the panel of inhibitors we used above for an ability to attenuate Neospora-induced effects on host LD accumulation. Consistent with our data for Toxoplasma infection, we observed no significant change in transcription or LD accumulation for the majority of drugs we tested (data not shown). Also, similar to our data with Toxoplasma, treatment with both Torin-1 and JNK-in-8 efficiently blocked LD accumulation (Fig. 7A). In contrast to their global effect on LD biogenesis, these two drugs showed strikingly different effects on Neospora-induced changes to host transcripts than what we observed with treatment of Toxoplasma-infected cells. In Neospora-infected cells, Torin-1 treatment substantially attenuated the increase of both FABP5 and DGAT2 transcripts (Fig. 7B). On the other hand, JNK-in-8 did not significantly affect FABP5 transcript levels but strongly attenuated the Neospora-associated increase in the level of DGAT2 transcript (Fig. 7B). The distinct effects of the two drugs suggest that the observed changes in host metabolic transcript levels are differentially regulated by host mTOR and JNK. Furthermore, the differential effect of the drugs on Neospora- versus Toxoplasma-infected cells suggests that, although both parasites appear to drive LD biogenesis by similar alterations to host neutral lipid metabolism, the changes induced by the parasites are differentially sensitive to regulation by JNK and mTOR.
Figure 7.
Pharmacological inhibition of Neospora-induced LD accumulation. A, representative fluorescent micrographs of HFFs that were infected with Neospora and imaged as in Fig. 6. Scale bars = 20 μm. DIC, differential interference contrast. B, qPCR comparing changes in host transcript levels induced by Neospora infection with and without treatment with the indicated drugs. All samples are normalized to an uninfected control. One-way ANOVA: **, p < 0.001.
Recently, Toxoplasma strains were found to up-regulate and activate the transcription factor c-Myc, and this up-regulation was suggested to be downstream of JNK (31). c-Myc can drive the transcription of FABP5 (32), although the transcription factor has also been associated with the negative regulation of LDs (33). When we directly inhibited c-Myc function with either of the drugs KJ Pyr 9 or 10058-F4, we saw neither a significant change in LD accumulation in Toxoplasma-infected cells nor a significant effect on relevant host transcript levels. We have shown, however, that infection with Neospora up-regulates host LDs to levels similar to what we observe with Toxoplasma (Fig. 6), and Neospora infection does not activate host c-Myc (31). Taken together, these data suggest that, although c-Myc may play a minor role in the ability of Toxoplasma to affect host neutral lipid metabolism, other pathways must be playing a dominant role in cells infected with both parasites.
An effector exported from the parasitophorous vacuole is required to induce host LD up-regulation
Toxoplasma secretes effectors from specialized organelles as it invades a given host cell and exports effectors from the PV into the host cytosol when infection has been established. Recently, knockout of the Toxoplasma protein MYR1 was demonstrated to specifically block the export of effectors from the PV without disrupting other effector functions (34). We used such Δmyr1 parasites to test whether a parasite effector exported from the PV is required for the up-regulation of host LDs. Strikingly, HFFs infected with RHΔmyr1 parasites and treated with OA showed no change in neutral lipid storage by microscopy or TLC compared with uninfected cells (Fig. 8, A and B). In addition, infection with RHΔmyr1 led to no significant changes in any of the transcripts of host genes involved in lipid metabolism that we had identified as correlated with LD up-regulation during wild-type infection (Fig. 8C). RHΔmyr1 parasites also did not induce changes to FABP5 protein levels or to mTOR activity (Fig. 8D). Both host cell transcriptional changes and LD accumulation were restored by infection with a strain in which the MYR1 gene had been complemented (Fig. 8). These data strongly suggest that the activities of one or more effectors exported from the PV into the host cytosol are required for parasite-induced changes in host neutral lipid metabolism.
Figure 8.
MYR1-mediated export is required for Toxoplasma-induced LD accumulation. A, representative fluorescence micrographs of BODIPY-stained neutral lipids of HFFs infected for 24 h after infection with the indicated strain. DIC, differential interference contrast. B, TAG quantification of cells treated with OA and infected with the indicated strain for 24 h (n = 3). C, qPCR quantification of host transcripts of cells infected for 24 h with the indicated strain without the addition of OA. D, Western blots of lysates from cells infected with wild-type RH or RHΔmyr1 parasites or left uninfected (Uninf) and probed with the indicated antibodies.
Discussion
We have demonstrated that infection of primary human cells with the coccidian parasites Toxoplasma and Neospora leads to a rewiring of host cell lipid metabolism that ultimately results in robust accumulation of TAG in host LDs. We showed that this LD accumulation appears to be driven initially by changes in transcript levels encoding the host proteins that modulate critical steps in host neutral lipid homeostasis (Fig. 3). This includes up-regulation of FABPs that transport FFA across cytosolic spaces to various organellar membranes as well as key enzymes in TAG synthesis.
In our biochemical analysis, we also observed that Toxoplasma infection causes an increase in the host cell responsiveness to additional FFA. Regulation of TAG synthesis and LD biogenesis occurs both transcriptionally and posttranslationally (35, 36), and our data indicate that Toxoplasma infection of mammalian cells alters LD accumulation at both mechanistic levels. In particular, DGAT activity in infected cells is robustly induced by OA stimulation (Fig. 4). The alteration of the host cellular response to FFA is, to our knowledge, the first example of a change in cellular dose/response to a metabolite caused by Toxoplasma infection. In addition, although Toxoplasma strongly dampens cellular responses to inflammatory cytokines such as IFNγ (37, 38), we believe this is the first report of Toxoplasma infection increasing sensitivity to a cellular stimulus.
The changes Toxoplasma infection induces in host neutral lipid metabolism are striking in their breadth. Although infection with diverse intracellular pathogens alters LD homeostasis, few of these interactions have been defined at a biochemical level. For those that have been examined mechanistically, the cellular changes are much more limited than what we observed with Toxoplasma infection. For instance, hepatitis C virus (HCV) infection causes LD buildup by specifically blocking TAG lipolysis without affecting synthesis (39). Also, Leishmania infection alters transcripts indicated to increase FFA availability and TAG synthesis but has not been reported to alter the regulation of enzymatic activity (40). Toxoplasma may therefore prove to be an ideal system for further elucidating the roles of LDs in intracellular host-pathogen interaction.
Recent work has identified an increasing number of host signaling networks that are actively manipulated during Toxoplasma infection, many of which have been implicated in the regulation of mammalian LDs in other contexts. For instance, HIF1α is activated by Toxoplasma infection (25), and in glioblastoma, its activity regulates fatty acid uptake and LD formation, largely through the induction of FABP expression (41). Both EGF receptor signaling (42) and c-Myc (31) are similarly activated by all strains of Toxoplasma, and both pathways have been shown to up-regulate FABP5 expression in cancer (32, 43). In addition, host MAPK signaling is activated by all strains of Toxoplasma through multiple mechanisms (12, 42, 44). Importantly, MAPK signaling regulates a variety of metabolic processes, including host LD formation in pathogenic contexts (45, 46). Given the diverse signaling pathways that can regulate neutral lipid metabolism, we were surprised that our pharmacological inhibition of host signaling revealed a specific role for mTOR and JNK in parasite-mediated dysregulation of LD biogenesis. This suggests that the host-parasite interaction may be modulating host metabolism through a unique mechanism.
Notably, we observed increased LD accumulation after infection with each of the three globally predominant Toxoplasma strains as well as with the related parasite N. caninum. Our data from transcriptional analyses and pharmacological perturbation of host signaling suggest that infection with the two parasites affects host LDs through dysregulation of the same host cellular pathways. The conservation of this phenotype across the parasite family raises the possibility that LD accumulation may be a host-driven response to protozoal infection. Indeed, LDs have been reported to provide antimicrobial activities in invertebrates (9). JNK-mediated induction of LDs in glia in animals prone to neurodegeneration leads to production of reactive oxygen species associated with cellular damage (47). Given that JNK activity appears to modulate the effects we report here (Fig. 5), and also given that reactive oxygen species are an important innate defense against pathogens, the potential that LDs have an underappreciated role in the innate immune response to parasites deserves further study.
In fact, infection with a wide range of pathogens alters host neutral lipid metabolism, including viruses (8, 39), bacteria (6, 48, 49), and protozoa (40, 50). In the majority of these cases, however, the interaction with host lipid droplets is either required for pathogen replication or otherwise provides a growth advantage to infectious agents. Akin to what has been reported for Chlamydia (51), we observed what appear to be LDs within the Toxoplasma PV (Fig. 2). LDs are unlikely to have been generated in situ, as this would require the full complement of metabolic enzymes to be secreted by the parasite into the PV. It therefore appears more likely that host neutral lipids are transferred into the PV either through fusion of LDs with the PV membrane or import of whole LDs into the PV lumen, as has been reported for other organelles (52, 15). Regardless of the mechanism of import, their presence within the PV suggests that Toxoplasma may use host LDs as a lipid source. Given the size of a lipid droplet, the parasite would be unable to directly endocytose the organelles. Therefore, accessing the lipids would seem to require PV-resident enzymes able to release esterified FFAs and cholesterol from within the LD core. Although no such proteins have yet been identified, Toxoplasma secretes hundreds of uncharacterized effectors into the PV space, providing a bounty of candidates.
Although blocking a potential energy source might be expected to slow parasite growth, we observed no growth rate deficiency from pharmacologically blocking LD up-regulation. This could be due to the relaxed requirements for in vitro growth versus the intense competition the parasite faces during in vivo infection. This may also be compounded by the fact that Toxoplasma appears to have evolved multiple independent methods to obtain required resources. For instance, although Toxoplasma may access host cholesterol esters (53), it also manipulates cholesterol homeostasis through dysregulation of the transcription of cholesterol metabolic enzymes (54) and by redirecting LDL trafficking (16, 52).
The interaction of host LDs with the parasite likely exceeds their classic role as lipid stores, as mammalian LDs are involved in immune activation (55–58), and their accumulation has been associated with both the generation of (47) and protection from reactive oxygen species (41). The Δmyr1 parasite strain we identified as unable to up-regulate host TAG accumulation has been reported previously to grow normally in vitro but shows a striking attenuation in its in vivo growth and virulence to mice (34). These mutant parasites are completely defective in effector translocation from the PV into the host cytosol, which strongly argues that a parasite-derived factor drives the host metabolic changes we have observed. Identifying the specific effector or effectors responsible for this host dysregulation will be required to tease apart the role for this process in pathogenesis and to better elucidate any benefits the host or parasite derives from this manipulation of organellar biogenesis and cellular metabolic homeostasis.
Experimental procedures
Parasite and host cell culture
HFFs were grown in Dulbecco's modified Eagle's medium (HyClone) supplemented with 10% fetal bovine serum (Sigma), 2 mm l-glutamine (HyClone), and 100 units/ml penicillin/streptomycin (Sigma). Toxoplasma and Neospora tachyzoites were maintained in confluent monolayers of HFFs. All cells and parasites were routinely tested to be Mycoplasma-negative. All experiments were conducted with confluent monolayers of HFFs.
Neutral lipid staining and fluorescent microscopy
HFF cells were grown on coverslips in 24-well plates until confluency and were infected with parasites. Where m.o.i. is not indicated, an m.o.i. of 4 was used. For most experiments, infected HFF cells were supplemented with 0.36 mm oleic acid (Sigma) solubilized in 0.064 mm fatty acid-free BSA (Sigma), and the cells were grown for another 6 h. The cells were rinsed twice with PBS and fixed with 4% paraformaldehyde/4% sucrose in PBS at room temperature for 15 min. After two washes with PBS, cells were stained for neutral lipids with BODIPY 493/503 (Invitrogen) and then mounted with mounting medium containing DAPI (Vector Laboratories). Cells were imaged on an AxioObserver equipped with an AxioCam 503 using a ×20/0.8 numerical aperture objective (Zeiss).
Quantitative automated image analysis
Fluorescent micrographs were analyzed with CellProfiler (21) using a pipeline that identified host cell nuclei, identified parasites by TdTomato signal, quantified background in the BODIPY channel, masked the BODIPY channel to ignore the signal in parasites, and quantified total BODIPY intensity normalized by host cell number (i.e. number of nuclei).
Transmission electron microscopy
Cells were fixed on MatTek dishes with 2.5% (v/v) glutaraldehyde in 0.1 m sodium cacodylate buffer. After three rinses in 0.1 m sodium cacodylate buffer, they were post-fixed with 1% osmium tetroxide and 0.8% K3[Fe(CN6)] in 0.1 m sodium cacodylate buffer for 1 h at room temperature. Cells were rinsed with water and stained en bloc with 2% aqueous uranyl acetate overnight. After three rinses with water, the specimens were dehydrated with increasing concentrations of ethanol, infiltrated with Embed-812 resin, and polymerized in a 70 °C oven overnight. Blocks were sectioned with a diamond knife (Diatome) on a Leica Ultracut UC7 ultramicrotome, collected onto copper grids, and post-stained with 2% uranyl acetate in water and lead citrate. Images were acquired on a Tecnai G2 spirit transmission electron microscope (FEI) equipped with a LaB6 source at 120 kV. LD sizes were measured manually from 27 images/condition using the Fiji distribution of ImageJ (59). Total droplet area per cell was measured and divided by the area of each cell to define the percent cellular area covered by LDs. The distributions were graphed using GraphPad Prism version 7.02.
Neutral lipid extraction and thin-layer chromatography
Where indicated, uninfected or parasitized cells were grown in medium supplemented with OA solubilized in fatty acid-free BSA (Sigma). Cells were washed with PBS twice, and then lipids were extracted in 4 ml 2:1 chloroform:methanol. Lipids were further extracted in 3 ml of chloroform:methanol and washed with 1 m KCl. The purified lipids were dried under N2 and dissolved in 150 μl of 1:1 chloroform:methanol. Samples and serially diluted lipid standards were separated by thin-layer chromatography on 20-cm TLC Silica Gel 60 plates (EMD Millipore) in 80:20:1 hexane:diethyl ether:acetic acid. Plates were charred to visualize and quantify lipids as in Ref. 60. For quantification, lipid bands were digitally scanned, and their intensities were determined by ImageJ. Concentrations were calculated relative to six to eight serial dilutions of lipid standards in a range of 125 ng to 25 μg of TAG. Standards were fit to a hyperbola using GraphPad Prism. Care was taken to collect experimental values within the useful dynamic range of the standard curve.
Analysis of host transcript levels by quantitative PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the protocol of the manufacturer. RNA was first treated with RNase-free DNase I (Thermo Scientific) to remove the genomic DNA and then reverse-transcribed into cDNA in the presence of RNase inhibitor (Applied Biosystems) using an ABI high-capacity cDNA RT kit (Invitrogen) or iScript RT Supermix (Bio-Rad). Host transcript levels were quantified using SYBR Green (Invitrogen) using an Applied Biosystems 7700 Sequence Detection System. All reactions were conducted in triplicate, and analyzed results represent at least three independent biological samples. The expression levels of human genes were normalized to human U36B4 and quantified by the ΔΔCt method (61). Statistical analysis was conducted with GraphPad Prism using unpaired t test (two samples) or one-way ANOVA (three or more samples). Primer sequences were as follows: U36B4 F: CGAGGGCACCTGGAAAAC, R: CACATTCCCCCGGATATGA; GPAT1 F: GCCTGTGGAGTGTAGCAAGA, R: ACCGGTTTCTGACTTTGGCT; GPAT4 F: AGATGCTGTCCAGTTTGCGA, R: TGCTCCTCCTTGAACGTGTC; AGPAT2 F: CTCCAACCACCAGAGCATCC, R: GCTGCCGGTTGATGAAGAAG; PAP1 F: CCACTCTTGCCCATGATCGA, R: GTCATCCAAGTAGACGCCGT; DGAT1 F: CCGGACAATCTGACCTACCG, R: CCTGGAGCTGGGTGAAGAAC; DGAT2 F: CAAGAAAGGTGGCAGGAGGT, R: GGTCAGCAGGTTGTGTGTCT; ATGL F: ACCAACACCAGCATCCAGTT, R: TCCCTGCTTGCACATCTCTC; HSL F: AAGGGATGCTTCTATGGCCG, R: CGTTGCGTTTGTAGTGCTCC; FABP3 F: GTGGAGTTCGATGAGACAACAGC, R: TGGTCTCTTGCCCGTCCCATTT; FABP5 F: CCTGTCCAAAGTGATGATGG, R: CAGCATCAGGAGTGGGATG.
Pharmacological perturbation of host signaling
For analysis of host signaling networks required for LD accumulation, host HFFs were infected with RH parasites for 24 h and simultaneously treated with the following drugs at the indicated concentrations: Torin-1 (Tocris, 200 nm), PX478 (Med Chem Express, 100 μm), SB505124 (Cayman Chemical, 10 μm), KJ Pyr 9 (Cayman Chemical, 10 μm), 10058-F4 (Cayman Chemical, 100 μm), erlotinib (Cayman Chemical, 2.5 μm), gefitinib (Tocris, 1 μm), PD325901 (Tocris, 500 nm), SB203580 (Tocris, 10 μm), BIRB 796 (Tocris, 1 μm), SP600125 (Tocris, 25 μm), and JNK-in-8 (Cayman Chemical, 3 μm). For JNK-in-8 pretreatment, HFFs were incubated with 10 μm inhibitor for 2 h and quickly washed three times with PBS, and parasites were added with fresh medium without inhibitor.
DGAT biochemical activity
For each sample, two 15-cm dishes of confluent HFFs were scraped into buffer A (20 mm Tris 7.8 and 250 mm sucrose) and lysed by nitrogen bomb. Cellular membranes were sedimented for 1 h by ultracentrifugation at 120,000 × g. Isolated membranes were washed twice with buffer A and sedimented as above. Protein concentrations were measured by Amido Black. Enzyme activity was measured as in Ref. 62 with modifications. For membrane fractions, 50 μg of membrane protein was used, which was within the linear range of the assay. Activities were calculated essentially as in Ref. 62, although lipids were chloroform-extracted before separation by TLC and quantification by scintillation. Dependence on diacylglycerol was tested in some experiments by leaving out that exogenous substrate.
Radiolabeled oleate uptake
FFA uptake was measured essentially as described in Ref. 63. Briefly, HFFs were infected overnight with RH or left uninfected. Cells were incubated in serum-free medium containing 1 μm [14C]oleate (PerkinElmer Life Sciences). The medium was removed after 1 h, and the cells were washed three times in PBS + 0.1% fatty acid-free BSA. Cells were scraped into radioimmune precipitation assay buffer, and radioactivity was quantified by scintillation.
Author contributions
X. H. and M. L. R. designed the study, analyzed all data, and wrote the paper. X. H. and D. B. performed all experiments.
Acknowledgments
We thank the Goodman laboratory for assistance with neutral lipid biochemistry as well as many thoughtful discussions and advice regarding the project. The RHΔmyr1 and complement parasites were a kind gift from John Boothroyd. We thank Gustavo Arrizabalaga, Jim Collins, Mike Henne, Anita Koshy, and Eva LaDow for helpful comments on the manuscript.
This research was funded in part by National Institutes of Health Grant 1K22AI097345 (to M. L. R.).The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- LD
- lipid droplet
- PV
- parasitophorous vacuole
- ER
- endoplasmic reticulum
- HFF
- human foreskin fibroblast
- OA
- oleic acid
- m.o.i.
- multiplicity of infection
- hpi
- hours post-infection
- TAG
- triacylglycerol
- qPCR
- quantitative PCR
- ATGL
- adipose triglyceride lipase
- DGAT
- diacylglycerol O-acyltransferase'
- mTOR
- mammalian target of rapamycin
- FABP
- fatty acid-binding protein
- ANOVA
- analysis of variance.
References
- 1. Hashemi H. F., and Goodman J. M. (2015) The life cycle of lipid droplets. Curr. Opin. Cell Biol. 33, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujimoto T., and Parton R. G. (2011) Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a004838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu K., and Czaja M. J. (2013) Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Andrea S. (2015) Lipid droplet mobilization: the different ways to loosen the purse strings. Biochimie 10.1016/j.biochi.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 5. Krahmer N., Farese R. V. Jr., and Walther T. C. (2013) Balancing the fat: lipid droplets and human disease. EMBO Mol. Med. 5, 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cocchiaro J. L., Kumar Y., Fischer E. R., Hackstadt T., and Valdivia R. H. (2008) Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc. Natl. Acad. Sci. U.S.A. 105, 9379–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dansako H., Hiramoto H., Ikeda M., Wakita T., and Kato N. (2014) Rab18 is required for viral assembly of hepatitis C virus through trafficking of the core protein to lipid droplets. Virology 462, 166–174 [DOI] [PubMed] [Google Scholar]
- 8. Crawford S. E., and Desselberger U. (2016) Lipid droplets form complexes with viroplasms and are crucial for rotavirus replication. Curr. Opin. Virol. 19, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anand P., Cermelli S., Li Z., Kassan A., Bosch M., Sigua R., Huang L., Ouellette A. J., Pol A., Welte M. A., and Gross S. P. (2012) A novel role for lipid droplets in the organismal antibacterial response. eLife 1, e00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saeij J. P., Coller S., Boyle J. P., Jerome M. E., White M. W., and Boothroyd J. C. (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445, 324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosowski E. E., Lu D., Julien L., Rodda L., Gaiser R. A., Jensen K. D., and Saeij J. P. (2011) Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208, 195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun L., Brenier-Pinchart M.-P., Yogavel M., Curt-Varesano A., Curt-Bertini R.-L., Hussain T., Kieffer-Jaquinod S., Coute Y., Pelloux H., Tardieux I., Sharma A., Belrhali H., Bougdour A., and Hakimi M.-A. (2013) A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med. 210, 2071–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunter C. A., and Sibley L. D. (2012) Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charron A. J., and Sibley L. D. (2002) Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115, 3049–3059 [DOI] [PubMed] [Google Scholar]
- 15. Romano J. D., Sonda S., Bergbower E., Smith M. E., and Coppens I. (2013) Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole. Mol. Biol. Cell 24, 1974–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coppens I., Sinai A. P., and Joiner K. A. (2000) Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149, 167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinai A. P., Webster P., and Joiner K. A. (1997) Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 110, 2117–2128 [DOI] [PubMed] [Google Scholar]
- 18. Pernas L., Adomako-Ankomah Y., Shastri A. J., Ewald S. E., Treeck M., Boyle J. P., and Boothroyd J. C. (2014) Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol. 12, e1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mota L. A., Roberto Neto J., Monteiro V. G., Lobato C. S., Oliveira M. A., Cunha Md., D'Ávila H., Seabra S. H., Bozza P. T., and DaMatta R. A. (2014) Culture of mouse peritoneal macrophages with mouse serum induces lipid bodies that associate with the parasitophorous vacuole and decrease their microbicidal capacity against Toxoplasma gondii. Mem. Inst. Oswaldo Cruz 109, 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomes A. F., Magalhães K. G., Rodrigues R. M., de Carvalho L., Molinaro R., Bozza P. T., and Barbosa H. S. (2014) Toxoplasma gondii-skeletal muscle cells interaction increases lipid droplet biogenesis and positively modulates the production of IL-12, IFN-g and PGE2. Parasit. Vectors 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamentsky L., Jones T. R., Fraser A., Bray M.-A., Logan D. J., Madden K. L., Ljosa V., Rueden C., Eliceiri K. W., and Carpenter A. E. (2011) Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics 27, 1179–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barbosa A. D., Savage D. B., and Siniossoglou S. (2015) Lipid droplet-organelle interactions: emerging roles in lipid metabolism. Curr. Opin. Cell Biol. 35, 91–97 [DOI] [PubMed] [Google Scholar]
- 23. Yen C.-L., Stone S. J., Koliwad S., Harris C., and Farese R. V. Jr. (2008) DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49, 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma X. M., and Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 25. Wiley M., Sweeney K. R., Chan D. A., Brown K. M., McMurtrey C., Howard E. W., Giaccia A. J., and Blader I. J. (2010) Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1α subunit via type I activin-like receptor kinase receptor signaling. J. Biol. Chem. 285, 26852–26860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown K. M., Suvorova E., Farrell A., McLain A., Dittmar A., Wiley G. B., Marth G., Gaffney P. M., Gubbels M. J., White M., and Blader I. J. (2014) Forward genetic screening identifies a small molecule that blocks Toxoplasma gondii growth by inhibiting both host- and parasite-encoded kinases. PLoS Pathog. 10, e1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang T., Inesta-Vaquera F., Niepel M., Zhang J., Ficarro S. B., Machleidt T., Xie T., Marto J. A., Kim N., Sim T., Laughlin J. D., Park H., LoGrasso P. V., Patricelli M., Nomanbhoy T. K., et al. (2012) Discovery of potent and selective covalent inhibitors of JNK. Chem. Biol. 19, 140–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boothroyd J. C. (2013) Have it your way: how polymorphic, injected kinases and pseudokinases enable Toxoplasma to subvert host defenses. PLoS Pathog. 9, e1003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyle J. P., Rajasekar B., Saeij J. P., Ajioka J. W., Berriman M., Paulsen I., Roos D. S., Sibley L. D., White M. W., and Boothroyd J. C. (2006) Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 103, 10514–10519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saeij J. P., Boyle J. P., Coller S., Taylor S., Sibley L. D., Brooke-Powell E. T., Ajioka J. W., and Boothroyd J. C. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314, 1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franco M., Shastri A. J., and Boothroyd J. C. (2014) Infection by Toxoplasma gondii specifically induces host c-Myc and the genes this pivotal transcription factor regulates. Eukaryot. Cell 13, 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawaguchi K., Kinameri A., Suzuki S., Senga S., Ke Y., and Fujii H. (2016) The cancer-promoting gene fatty acid-binding protein 5 (FABP5) is epigenetically regulated during human prostate carcinogenesis. Biochem. J. 473, 449–461 [DOI] [PubMed] [Google Scholar]
- 33. Zirath H., Frenzel A., Oliynyk G., Segerström L., Westermark U. K., Larsson K., Munksgaard Persson M., Hultenby K., Lehtiö J., Einvik C., Påhlman S., Kogner P., Jakobsson P.-J., and Henriksson M. A. (2013) MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl. Acad. Sci. U.S.A. 110, 10258–10263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franco M., Panas M. W., Marino N. D., Lee M.-C., Buchholz K. R., Kelly F. D., Bednarski J. J., Sleckman B. P., Pourmand N., and Boothroyd J. C. (2016) A novel secreted protein, MYR1, is central to Toxoplasma's manipulation of host cells. mBio 7, e02231–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Markgraf D. F., Klemm R. W., Junker M., Hannibal-Bach H. K., Ejsing C. S., and Rapoport T. A. (2014) An ER protein functionally couples neutral lipid metabolism on lipid droplets to membrane lipid synthesis in the ER. Cell Rep. 6, 44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu J., Li Y., Zou F., Xu S., and Liu P. (2015) Phosphorylation and function of DGAT1 in skeletal muscle cells. Biophys. Rep. 1, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olias P., Etheridge R. D., Zhang Y., Holtzman M. J., and Sibley L. D. (2016) Toxoplasma effector recruits the Mi-2/NuRD complex to repress STAT1 transcription and block IFN-γ-dependent gene expression. Cell Host Microbe. 20, 72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gay G., Braun L., Brenier-Pinchart M.-P., Vollaire J., Josserand V., Bertini R.-L., Varesano A., Touquet B., De Bock P.-J., Coute Y., Tardieux I., Bougdour A., and Hakimi M.-A. (2016) Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-γ-mediated host defenses. J. Exp. Med. 10.1084/jem.20160340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris C., Herker E., Farese R. V. Jr., and Ott M. (2011) Hepatitis C virus core protein decreases lipid droplet turnover: a mechanism for core-induced steatosis. J. Biol. Chem. 286, 42615–42625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lecoeur H., Giraud E., Prévost M.-C., Milon G., and Lang T. (2013) Reprogramming neutral lipid metabolism in mouse dendritic leucocytes hosting live Leishmania amazonensis amastigotes. PLoS Negl. Trop. Dis. 7, e2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bensaad K., Favaro E., Lewis C. A., Peck B., Lord S., Collins J. M., Pinnick K. E., Wigfield S., Buffa F. M., Li J.-L., Zhang Q., Wakelam M. J., Karpe F., Schulze A., and Harris A. L. (2014) Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 9, 349–365 [DOI] [PubMed] [Google Scholar]
- 42. Muniz-Feliciano L., Van Grol J., Portillo J.-A., Liew L., Liu B., Carlin C. R., Carruthers V. B., Matthews S., and Subauste C. S. (2013) Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 9, e1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kannan-Thulasiraman P., Seachrist D. D., Mahabeleshwar G. H., Jain M. K., and Noy N. (2010) Fatty acid-binding protein 5 and PPARβ/δ are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth. J. Biol. Chem. 285, 19106–19115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morgado P., Ong Y.-C., Boothroyd J. C., and Lodoen M. B. (2011) Toxoplasma gondii induces B7–2 expression through activation of JNK signal transduction. Infect. Immun. 79, 4401–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng B., Wu X., Sun S., Wu Q., Mei C., Xu Q., Wu J., and He P. (2014) MAPK-PPARα/γ signal transduction pathways are involved in Chlamydia pneumoniae-induced macrophage-derived foam cell formation. Microb. Pathog. 69, 1–8 [DOI] [PubMed] [Google Scholar]
- 46. Levi L., Lobo G., Doud M. K., von Lintig J., Seachrist D., Tochtrop G. P., and Noy N. (2013) Genetic ablation of the fatty acid-binding protein FABP5 suppresses HER2-induced mammary tumorigenesis. Cancer Res. 73, 4770–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B. H., Quintana A., and Bellen H. J. (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gilk S. D. (2012) Role of lipids in Coxiella burnetii infection. Adv. Exp. Med. Biol. 984, 199–213 [DOI] [PubMed] [Google Scholar]
- 49. Mattos K. A., Oliveira V. G., D'Avila H., Rodrigues L. S., Pinheiro R. O., Sarno E. N., Pessolani M. C., and Bozza P. T. (2011) TLR6-driven lipid droplets in Mycobacterium leprae-infected Schwann cells: immunoinflammatory platforms associated with bacterial persistence. J. Immunol. 187, 2548–2558 [DOI] [PubMed] [Google Scholar]
- 50. Toledo D. A. M., D'Avila H., and Melo R. C. N. (2016) Host lipid bodies as platforms for intracellular survival of protozoan parasites. Front. Immunol. 10.3389/fimmu.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumar Y., Cocchiaro J., and Valdivia R. H. (2006) The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr. Biol. CB. 16, 1646–1651 [DOI] [PubMed] [Google Scholar]
- 52. Coppens I., Dunn J. D., Romano J. D., Pypaert M., Zhang H., Boothroyd J. C., and Joiner K. A. (2006) Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125, 261–274 [DOI] [PubMed] [Google Scholar]
- 53. Sonda S., Ting L. M., Novak S., Kim K., Maher J. J., Farese R. V. Jr., and Ernst J. D. (2001) Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J. Biol. Chem. 276, 34434–34440 [DOI] [PubMed] [Google Scholar]
- 54. Blader I. J., Manger I. D., and Boothroyd J. C. (2001) Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J. Biol. Chem. 276, 24223–24231 [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y., Li Q., Rao E., Sun Y., Grossmann M. E., Morris R. J., Cleary M. P., and Li B. (2015) Epidermal fatty acid binding protein promotes skin inflammation induced by high-fat diet. Immunity. 42, 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Fröhlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., Tabas I., Levak-Frank S., and Kratky D. (2010) Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285, 20192–20201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aflaki E., Radovic B., Chandak P. G., Kolb D., Eisenberg T., Ring J., Fertschai I., Uellen A., Wolinski H., Kohlwein S.-D., Zechner R., Levak-Frank S., Sattler W., Graier W. F., Malli R., et al. (2011) Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J. Biol. Chem. 286, 7418–7428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Araújo-Santos T., Prates D. B., Andrade B. B., Nascimento D. O., Clarêncio J., Entringer P. F., Carneiro A. B., Silva-Neto M. A., Miranda J. C., Brodskyn C. I., Barral A., Bozza P. T., and Borges V. M. (2010) Lutzomyia longipalpis saliva triggers lipid body formation and prostaglandin E2 production in murine macrophages. PLoS Negl. Trop. Dis. 4, e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adeyo O., Horn P. J., Lee S., Binns D. D., Chandrahas A., Chapman K. D., and Goodman J. M. (2011) The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 192, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 62. Cartwright B. R., Binns D. D., Hilton C. L., Han S., Gao Q., and Goodman J. M. (2015) Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 26, 726–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dubikovskaya E., Chudnovskiy R., Karateev G., Park H. M., and Stahl A. (2014) Measurement of long-chain fatty acid uptake into adipocytes. Methods Enzymol. 538, 107–134 [DOI] [PMC free article] [PubMed] [Google Scholar]