Abstract
Purpose of review
Nonalcoholic steatohepatitis (NASH), the aggressive form of nonalcoholic fatty liver disease (NAFLD), can progress to cirrhosis and hepatocellular cancer in 5–15% of patients and is rapidly becoming the leading cause for end-stage liver disease. Dietary caloric restriction and exercise, currently the cornerstone of therapy for NAFLD, can be difficult to achieve and maintain, underscoring the dire need for pharmacotherapy. This review presents the agents currently used in managing NAFLD and their pharmacologic targets. It also provides an overview of NAFLD agents currently under development.
Recent findings
Therapies for NASH can be broadly classified into agents that target the metabolic perturbations driving disease pathogenesis (such as insulin resistance and de novo lipogenesis) and agents that target downstream processes including cell stress, apoptosis, inflammation, and fibrosis. Modulation of peroxisome proliferator-activator receptors, farnesoid-X-receptors, and the glucagon-like peptide 1 pathway have been shown to improve liver histology. The intestinal microbiome and metabolic endotoxemia are novel targets that are currently under review. Antioxidants such as vitamin E, and more recently anti-inflammatory agents such as apoptosis signal-regulating kinase 1 inhibitors show promise as therapy for NASH. Several antifibrotic agents including C-C chemokine receptor type 2 and type 5 antagonists have been shown to inhibit the progression of fibrosis toward cirrhosis.
Summary
There are currently several agents in the drug pipeline for NASH. Within the next few years, the availability of therapeutic options for NAFLD will hopefully curb the rising trend of NAFLD-related end stage liver disease.
Keywords: chronic liver disease, cirrhosis, fibrosis, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
INTRODUCTION
Lifestyle modification, consisting of diet and exercise, is the cornerstone of therapy for nonalcoholic fatty liver disease (NAFLD) and has been shown by many studies to improve liver histology [1,2]. However, lifestyle modification is difficult to achieve and to sustain [3]. Recent progress in understanding of NAFLD pathogenesis has led to the development of new therapeutics or the repurposing of currently available drugs that have historically been given for other indications (Fig. 1). In this review, we provide an overview of current therapeutics for NASH and highlight recent findings in NASH pharmacotherapy.
FIGURE 1.
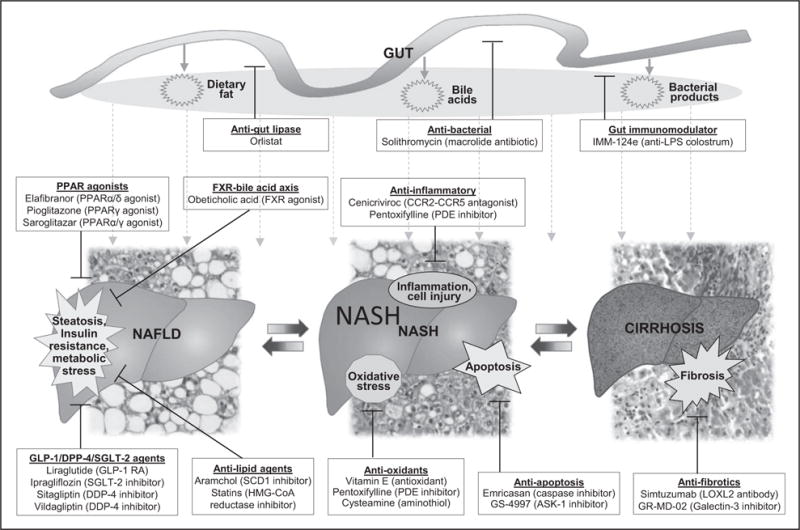
Current and emerging therapies for NAFLD and their mechanisms of action. ASK-1, apoptosis signal-regulating kinase; CCR2–CCR5, chemokine receptors type 2 and type 5; DPP-4, dipeptidyl peptidase-4; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; LOXL2, lysyl oxidase-like 2; LPS, lipopolysaccharide; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PDE, phosphodiesterase; PPAR, peroxisome proliferator-activated receptor; RA, receptor agonist; SCD1, stearoyl coenzyme A desaturase 1; SGLT-2, sodium–glucose co-transporter 2.
TARGETING THE GUT
The portal system supplies about 70% of the total hepatic blood flow [4]. NAFLD is associated with increased gut permeability, allowing transport of gut metabolites and bacterial products into the portal circulation [5].
Gut microbiome
A Number of mechanistic pathways within the gut–liver axis appear to be activated in NAFLD, including lipopolysaccharide production, endogenous alcohol production, and conversion of dietary phosphatidylcholine to choline and hepatotoxic trimethylamine [6]. Presumably, changing the composition of the gut microbiome, either by changing the distribution of the gut bacterial flora, modulating metabolite production, or inhibiting translocation of bacteria or their metabolites to the liver could be beneficial in NAFLD.
Bovine colostrum is enriched with IgG directed against antigens injected into cows immediately prior to calving. An IgG-rich bovine colostrum extract, IMM-124e, generated from cows immunized against lipopolysaccharide was shown to improve insulin sensitivity, glycemic control, and liver enzymes in a small pilot study [7]. A phase 2 trial is currently evaluating 24 weeks of IMM-124e on biopsy-proven NASH (ClinicalTrials.gov identifier NCT02316717). Solithromycin, a macrolide antibiotic with anti-inflammatory properties, has been found to improve NASH in animal studies [8] and is currently being studied in a phase 2 clinical trial (NCT02510599).
Antiobesity medications
Orlistat is a gut lipase inhibitor which decreases the absorption of dietary fats [9]. It has been approved for treatment of obesity and recently became available over the counter in the United States for weight loss. A small pilot study suggested that Orlistat-mediated weight loss is associated with reduction in hepatic steatosis [10]. However, the impact of Orlistat on steatohepatitis and its ability to slow the progression of NASH to cirrhosis remains unknown. Current data does not support the use of orlistat as treatment for NAFLD. However, it can be prescribed as an adjunct medication to help with weight loss in the NAFLD patient population.
TARGETING METABOLIC PATHWAYS
Progression of NAFLD is dependent on a number of metabolic processes in the liver. Medications in this category aim to reduce the accumulation of hepatic fat and resultant metabolic stress.
Peroxisome proliferator-activator receptors
Peroxisome proliferator-activator receptors (PPARs) are nuclear receptors that bind fatty acids and fatty acid derivatives to regulate a number of metabolic processes. The three PPARs α, β/δ, and γ differ in tissue distribution and ligand selectivity [11]. PPARα, expressed in the liver and a number of other tissues including brown adipose tissue and heart, upregulates the expression of genes involved in gluconeogenesis, β-oxidation, and lipid transport. Selective deletion of PPARα in hepatocytes results in hepatic lipid accumulation [12].
PPARδ, also widely expressed in many tissues including the liver, decreases hepatic gluconeogenesis, fatty acid oxidation, improves insulin sensitivity, and inhibits activation of macrophage and Kupffer cells [13]. Recently, a PPARδ agonist MBX-8025 was shown to abolish lipotoxicity and ameliorate NASH in a diabetic mouse model [14]. Elafibranor (GFT-505) is a dual PPARα/δ agonist which has been shown to improve liver, adipose tissue, and peripheral tissue insulin sensitivity and reduce alanine aminotransferase (ALT) levels in patients with metabolic syndrome [15]. In a phase 2b randomized double blind placebo controlled trial (RDBPCT) to study the effect of elafibranor on NASH (GOLDEN-505), 276 patients with biopsy-proven noncirrhotic NASH were randomized to 80 mg/day or 120 mg/day of elafibranor or placebo for 1 year [16■■]. The primary end point, selected as histological resolution of NASH without worsening of fibrosis, was achieved in 23, 21, and 17% of patients who received 80 mg/day, 120 mg/day, and placebo, respectively. There was no statistically significant difference between the groups. Using a more stringent criteria for resolution of NASH, 19% of study participants on elafibranor 120 mg/day and 12% of study participants on placebo achieved NASH resolution (P = 0.045). The inability to demonstrate benefit was thought to be due to the high placebo response rates in study participants with mild to moderate NASH [NAFLD activity score (NAS) 3–5]. Exclusion of study participants with mild disease at baseline showed that the 120 mg/day dose was statistically superior to placebo for both definitions of NASH resolution. Based on these results, a phase 3 trial is currently recruiting NASH study participants with NAS >4 who will be randomized to elafibranor 120 mg/day versus placebo for 72 weeks. Histological primary end point of NASH resolution without worsening of fibrosis, together with a clinical coprimary composite end point based on mortality, cirrhosis, and liver-related outcomes will be assessed (NCT02704403).
PPARγ is primarily expressed in adipose tissue and regulates glucose metabolism, lipogenesis, and adipose tissue differentiation. Thiazolidinediones, including pioglitazone, are PPARγ agonists used in the treatment of diabetes and demonstrated to be effective in NASH [17]. The glitazars are dual PPARα/γ agonists which aim to combine the beneficial effects of activating both PPAR receptors. Saroglitazar, currently the only glitazar in clinical use because of safety concerns with other members of the category, has been shown to improve diabetic dyslipidemia [18,19] and is currently approved in India for this indication. In a mouse model of NASH, saroglitazar was found to reduce steatosis and ALT, and improve liver histology [20]. A subsequent retrospective study of NAFLD patients with dyslipidemia treated with saroglitazar for 24 weeks showed a significant decrease in ALT compared with baseline [21]. A phase 2 open-label study (PRESS VIII) evaluated the effectiveness of saroglitazar among 32 patients with biopsy-proven NASH [22]. After 12 weeks of treatment, a 52% decrease in ALT was shown. A phase 3 RDBPCT is currently ongoing in India to assess the effect of saroglitazar versus placebo for 52 weeks in biopsy-proven noncirrhotic NASH (Clinical Trials Registry-India CTRI/2015/10/006236).
Farnesoid X receptor
Bile acids can negatively regulate bile acid synthesis, decrease hepatic gluconeogenesis, and lipogenesis through interaction with their intracellular receptor, the farnesoid X receptor (FXR). A synthetic bile acid agonist of FXR, obeticholic acid (OCA; 6-ethyl-chenodeoxycholic acid) was evaluated in a phase 2b clinical trial (FLINT) in which 283 study participants with biopsy-proven noncirrhotic NASH (NAS >4) were randomized to OCA 25 mg/day versus placebo for 72 weeks [23■■]. The primary end point of histological improvement, demonstrated as reduction in NAS by two or more points, with no worsening of fibrosis was reached in 45% of study participants on OCA versus 21% of those on placebo (P = 0.0002). Resolution of NASH was demonstrated in 22% of OCA study participants versus 13% of placebo (P = 0.08); and fibrosis score decreased in 35% of OCA study participants versus 19% of placebo (P = 0.004). Study participants on OCA showed a significant decrease in BMI compared to those on placebo (BMI decrease by 0.7 kg/m3 versus gain of 0.1 kg/m3, respectively). OCA treatment, however, decreased high-density lipoprotein cholesterol, while increasing low-density lipoprotein cholesterol, and total cholesterol. These changes in cholesterol occurred primarily at the initiation of the study and improved with continued treatment; whether these changes translate into increased cardiovascular risk remains to be demonstrated. A phase 3 trial to compare the effectiveness of 72 weeks of OCA versus placebo for noncirrhotic biopsy-proven NASH is in its recruitment stages (NCT02548351). A primary histological end point of decreased NAFLD activity or improvement in fibrosis will be assessed. Current pivotal trials for precirrhotic stages of NASH require long-term extension trials to demonstrate that such short-term histological benefits translate into decreased progression to cirrhosis, considered a generally accepted surrogate for full approval by regulatory agencies [24■■].
Incretins, dipeptidyl peptidase-4 inhibitors and sodium–glucose cotransporter 2 inhibitors
Glucagon-like peptide (GLP-1), secreted by intestinal L-cells in response to meal ingestion, improves insulin sensitivity, and increases hepatic glucose uptake and glycogen production [25]. GLP-1 receptor agonists or incretin mimetics, including exenatide and liraglutide are approved for the management of type 2 diabetes mellitus [26]. Recently, liraglutide was investigated in a phase 2 RDBPCT (LEAN trial) to determine the effectiveness of 48 weeks of liraglutide (1.8 mg/day) versus placebo on biopsy-proven NASH [27■■]. The primary end point was histological resolution of NASH without worsening of fibrosis. At the end of the study period, the primary end point was reached by 39% of study participants on liraglutide versus 9% on placebo (P = 0.02). Study participants on liraglutide had an average weight loss of 5.3 kg, with histological responders losing an average of 2.1 kg more than histological nonresponders.
GLP-1 activity can also be augmented by inhibition of dipeptidyl peptidase 4 (DPP-4), an enzyme that degrades GLP-1. Sitagliptin and vildagliptin are DPP-4 inhibitors which have been shown previously to modestly decrease liver fat as measured by magnetic resonance spectroscopy after 24 weeks of treatment [28]. Recently, a RDBPCT in 50 NAFLD patients with prediabetes or early diabetes did not show any improvement in liver fat, liver enzymes, or fibrosis in study participants on sitagliptin (100 mg/day) versus placebo [29]. Based on currently available data, inhibition of DPP-4 does not appear to be beneficial in the treatment of NASH.
The newest class of diabetic medications on the market are sodium–glucose cotransporter 2 (SGLT-2) inhibitors. A recent retrospective study evaluated the effectiveness of SGLT-2 inhibitor, ipragliflozin (50 mg/day), on liver enzymes and fibrosis index (FIB-4) in 50 diabetic NAFLD patients [30]. Over an average follow-up of 451 days, there was a significant decrease in body weight, ALT and FIB-4 compared with baseline values. The average weight loss in patients who achieved normal ALT was 3.94 kg, compared with 1.98 kg in study participants who did not normalize their ALT. Further studies are needed to shed light on the utility of SGLT-2 inhibitors in the treatment of NAFLD.
3-Hydroxy-3-methyl-glutaryl-CoA reductase inhibitors
The 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, popularly known as statins, are safe in patients with NAFLD and other chronic liver diseases [31]. However, a recent study demonstrated the underutilization of statins in patients with NAFLD [32]. A prospective trial in 20 patients with biopsy-proven NASH and dyslipidemia determined the effect of 12 months of rosuvastatin (10 mg/day) on liver histology. In total, 19 out of the 20 patients enrolled demonstrated complete resolution of NASH despite no change in weight compared with baseline [33]. Such data are hard to reconcile with the findings that the majority of study participants in large multicenter cohorts such as the NASH CRN are on statins and yet have severe liver disease. If there is a beneficial effect, the effect size is likely to be modest.
De-novo lipogenesis
Acetyl-CoA carboxylase (ACC) catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, which serves as a building block for fatty acid synthesis while inhibiting fatty acid β-oxidation. In a murine model of NAFLD, inhibition of ACC decreased lipogenesis and hepatic steatosis and improved insulin sensitivity. Recently, a Phase 1 trial in obese but otherwise healthy male volunteers showed dose-dependent inhibition of de-novo lipogenesis by the liver-directed synthetic ACC inhibitor, NDI-010976 [34], thus providing the basis for further studies on ACC inhibition as a potential therapy for NAFLD. The fatty acid-bile acid conjugate aramchol inhibits steroyl CoA desaturase and has been shown to produce a dose-dependent decrease in hepatic steatosis using noninvasive measures [35]. A phase 2b trial of Aramchol (400 and 600 mg/day) in patients with biopsy-proven NASH is currently underway (NCT02279524).
OXIDATIVE STRESS AND INFLAMMATION
Hepatic steatosis is characterized by increased fatty acid β-oxidation and oxidative stress, leading to generation of reactive oxygen species, mitochondrial defect and dysfunction, alteration of cell cycle regulation, and decreased cell viability [36,37]. Targeting of pathways involved in oxidative stress and inflammatory response have shown utility as therapy for NASH.
Antioxidants
The fat-soluble antioxidant vitamin E has been shown to be superior to placebo in achieving histological response and resolution of NASH in a phase 3 RDBPCT (PIVENS) [38]. After 96 weeks of treatment, histological response was achieved in 43% of study participants on vitamin E (800 IU/day), compared with 19% of placebo study participants (P = 0.001). Approximately half of the study participants on vitamin E demonstrated reduction in hepatocyte ballooning and lobular inflammation. Vitamin E had no effect on fibrosis. Despite the favorable safety profile of vitamin E in the PIVENS trial, other studies suggest an increase in all-cause mortality [39] and prostate cancer [40] in study participants on vitamin E; observations that have not been supported by subsequent analyses [41,42]. In a recent study that pooled together data from the PIVENS trial and the placebo arm of the FLINT trial to determine the efficacy of vitamin E in diabetic versus nondiabetic NASH study participants, there was similar improvement in NASH histology in both groups [43]. There was no increase in the incidence of adverse events in study participants treated with vitamin E. Based on demonstrated efficacy in improving the histological features of NASH, vitamin E is currently recommended as first line off-label pharmacotherapy for NASH [44,45].
Cysteamine, an aminothiol that scavenges reactive oxygen species, was shown to improve levels of ALT and AST after 24 weeks of treatment in a small pilot study of 11 children with elevated ALT [46]. In a recent multicenter RDBPCT (CyNCh), 169 children with biopsy-proven NASH (NAS >4) were randomized to 12 months of cysteamine (300 mg for study participants ≤65 kg; 375 mg for study participants 65–80 kg; 450 mg for study participants >80 kg) versus placebo. Although study participants treated with cysteamine showed statistically significant decrease in ALT and lobular inflammation, there was no difference in overall histologic markers of NAFLD or in NAS [47■]. A post hoc analysis of study participants who weighed less than 65 kg showed that 50% of those in the treatment arm reached the primary outcome of histological improvement, compared with 13% of placebo (P = 0.005). Despite the inability to demonstrate histological response overall in the treatment arm, the results highlight several important questions that were raised in an accompanying commentary [48], including the heterogenous nature of pediatric NASH, the lack of robust data on the natural history of NASH in children, and the effect of genetic polymorphisms including patatin-like phospholipase domain-containing protein 3 (PNPLA3) on NASH treatment outcomes.
Immune modulators
Inflammatory cytokines including C-C chemokine ligands type 2 and type 5 (CCL2-CCL5), which are involved in activation and migration of inflammatory cells into the liver and propagation of fibrosis, have been found to be upregulated in NASH. Cenicriviroc, an oral antagonist of the CCL2–CCL5 receptor, showed anti-inflammatory and antifibrotic activity in animal models of fibrosis [49] and decreased serum fibrotic markers when used for treatment of HIV infection in adults without liver disease [50]. An ongoing phase 2a study of cenicriviroc (ORION) is aimed at assessing the effect of 24 weeks of treatment on insulin sensitivity, liver enzymes, and liver imaging in obese patients with insulin resistance and suspected NAFLD (NCT02330549). At the same time, a phase 2b trial (CENTAUR) is investigating the effect of 2 years of cenicriviroc (150 mg daily) or placebo on noncirrhotic NASH and liver fibrosis in patients with T2DM or metabolic syndrome (NCT02217475). Interim analysis at year 1 of the CENTAUR study, in which 289 study participants were randomized, showed twice as many patients with at least one stage improvement in fibrosis and no worsening of steatohepatitis in the treatment group, compared with placebo (P = 0.023) [51]. A similar proportion of patients in the cenicriviroc versus placebo arms achieved improvements in NAS and resolution of steatohepatitis [52]. A phase 3 study (STELLARIS) to evaluate the efficacy and safety of cenicriviroc in patients with NASH fibrosis is expected to start recruitment in 2017 (NCT03028740).
Apoptosis and tumor necrosis factor α
NASH is characterized by enhanced activation of caspases and proinflammatory cytokines (including tumor necrosis factor α) that drive apoptosis and propagate liver injury [53]. Emricasan, an oral pan-caspase inhibitor, improves inflammation, hepatocyte injury, and hepatic fibrosis in mice fed high-fat diet without any effects on hepatic steatosis or features of the metabolic syndrome [54■]. In a recent phase 2 RDBPCT of 38 study participants with noncirrhotic NAFLD, 28 days of emricasan (25 mg twice daily) resulted in a substantial decrease in liver enzymes and cytokeratin 18 fragments, a surrogate of liver apoptosis [55]. A phase 2b trial of emricasan versus placebo (ENCORE-NF) is currently ongoing to evaluate the efficacy of 72 weeks of emricasan (10 mg per day or 100 mg per day) in patients with biopsy-proven NASH. The primary outcome is improvement in fibrosis without worsening of NASH (NCT02686762).
The apoptosis signal-regulating kinase (ASK1), also known as mitogen-activated protein kinase kinase kinase 5, is activated by tumor necrosis factor α, oxidative or endoplasmic reticulum stress, leading to activation of the p38 MAPK/JNK pathway, and resulting in hepatocyte apoptosis and fibrosis [53]. Inhibition of ASK1 reduces liver steatosis and fibrosis in a murine model of diet-induced NASH [56]. An open-label phase 2 trial in 72 NASH patients with stage 2/3 fibrosis randomized to an oral ASK1 inhibitor, selonsertib (formerly called GS-4997; 6 mg or 18 mg/day) versus lysyl oxidase-like 2 antibody simtuzumab (25 mg SC weekly) versus selonsertib and simtuzumab was recently completed [57]. Preliminary analysis showed that patients who received selonsertib (with or without simtuzumab) were more likely to demonstrate decreased hepatic steatosis, decreased fibrotic stage and at least 15% decrease in liver stiffness on magnetic resonance elastography, compared with simtuzumab alone. Antisteatotic and antifibrotic effects of selonsertib were dose dependent [57].
ANTIFIBROTICS
Antifibrotics in NASH aim to counteract progressive fibrosis and resultant complications through blockage of fibrotic pathways or by promoting reversal of fibrosis [58].
Simtuzumab
The lysyl oxidase-like 2 antibody simtuzumab is currently in a phase 2b trial in NASH patients with advanced fibrosis but without cirrhosis (NCT01672866). Study participants are randomized to biweekly SC injections of simtuzumab (75 or 120 mg) versus placebo for 96 weeks, followed by an additional 240 weeks of open-label phase. Simtuzumab is also being investigated in a phase 2b trial in NASH patients with compensated cirrhosis (NCT01672879). The primary end point being assessed is mean change in hepatic venous pressure gradient as well as event-free survival.
Galectin-3
Galectin-3 is expressed primarily in immune cells and is crucial for the development of liver fibrosis. A galectin-3 inhibitor, GR-MD-02, was previously found to decrease disease activity and fibrosis in a murine model [59,60]. A currently ongoing phase 2 study in patients with NASH and advanced fibrosis is evaluating the effect of 16 weeks of drug on hepatic fibrosis as assessed by MRI (NCT02421094). Another phase 2 study is recruiting patients with NASH cirrhosis and portal HTN to assess the efficacy of 1-year treatment of GR-MD-02 in reducing hepatic venous pressure gradient (NCT02462967).
CONCLUSION
There are currently a number of drugs undergoing pivotal trails as potential therapy for NASH. It is anticipated that the first drugs to be approved for NASH will likely become available by 2020. Approval of these agents should herald future trials of combination therapy to prevent NASH progression to cirrhosis and reduce liver-related outcomes.
KEY POINTS.
NASH can progress to cirrhosis and liver cancer and has become a leading cause for chronic liver disease worldwide. It is imperative that effective pharmacotherapies are developed to treat NASH and prevent progression to end-stage liver disease.
Vitamin E and pioglitazone currently remain the first-line off label drugs for NASH. Many agents are currently in intermediate or advanced stages of development, including OCA, elafibranor, and cenicriviroc.
The intestinal microbiome and metabolic endotoxemia are targets that are actively being explored for the development of novel drugs.
It is anticipated that by 2020, some of the agents currently undergoing pivotal trials would be approved for treatment of NASH.
Acknowledgments
None.
Financial support and sponsorship
The work was supported by a training grant T32 DK 7150–40 from NIDDK to A. J. S.
A. J. S. is the President of Sanyal Bio and has stock options in Genfit, NewCo LLC, Akarna, Indalo, and Exhalenz. He has been a paid consultant to Pfizer, Conatus, Novartis, Lilly, Hemoshear, and Salix. He is an unpaid consultant to Intercept, Tobira, Merck, Bristol Myers Squibb, Nitto Denko, Novo Nordisk, Nordic Bio-Science, Syntlogic, Canfite, Jannsen, Gilead, and Galectin. His institution has received grant support from Merck, Astra Zeneca, Bristol Myers, Novartis, Shire, and Conatus.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for nonalcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Stewart KE, Haller DL, Sargeant C, et al. Readiness for behaviour change in nonalcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015;35:936–943. doi: 10.1111/liv.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 6.Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 7.Mizrahi M, Shabat Y, Ben Ya’acov A, et al. Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. J Inflamm Res. 2012;5:141–150. doi: 10.2147/JIR.S35227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes P. Mechanism of anti-NASH effects of Solithromycin in a predictive NASH HCC mouse model. Hepatology. 2015;62:1301A-A. [Google Scholar]
- 9.Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol. 2015;763:64–74. doi: 10.1016/j.ejphar.2015.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen L, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Montagner A, Polizzi A, Fouche E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haczeyni F, Wang H, Barn V, et al. PPAR-δ agonist MBX-8025 abolishes lipotoxicity and reverses NASH in diabetic obese mice. Hepatology. 2016;64:129A. [Google Scholar]
- 15.Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■■.Ratziu V, Harrison SA, Francque S, et al. GOLDEN-505 Investigator Study Group Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. This important study (GOLDEN-505 trial) demonstrated the role of the PPARα/δ pathway in NASH by comparing two different doses of the dual PPARα/δ agonist elafibranor versus placebo in a phase 2b trial in NASH patients. A post hoc analysis of data showed that the higher dose of elafibranor (120 mg/day) resolved NASH, with no worsening of fibrosis using a modified definition for primary outcome. [DOI] [PubMed] [Google Scholar]
- 17.Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai V, Paneerselvam A, Mukhopadhyay S, et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of saroglitazar 2 and 4 mg compared to pioglitazone 45 mg in diabetic dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:132–141. doi: 10.1177/1932296813518680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jani RH, Pai V, Jha P, et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI) Diabetes Technol Ther. 2014;16:63–71. doi: 10.1089/dia.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain MR, Giri SR, Trivedi C, et al. Saroglitazar, a novel PPARalpha/gamma agonist with predominant PPARalpha activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol Res Perspect. 2015;3:e00136. doi: 10.1002/prp2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saboo B, Prajapati A, Joshi S. To Assess the Effect of 4 mg Saroglitazar on patients of diabetes dyslipidemia with nonalcoholic fatty liver disease. Diabetes. 2015;64:A180-A. [Google Scholar]
- 22.Giri S, Bhoi B, Trivedi C, et al. Efficacy of Saroglitazar, a Novel PPARα/γ Agonist in a Mouse Model of Non-Alcoholic Steatohepatitis. Keystone Symposium—Liver Metabolism and Nonalcoholic Fatty Liver Disease (NAFLD) 2015 [Google Scholar]
- 23■■.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for noncirrhotic, nonalcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. This important study (FLINT trial) established the role of FXR in NASH by showing that the FXR bile acid agonist OCA improved histological features of NASH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24■■.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L, American Association for the Study of Liver Diseases; United States Food and Drug Administration Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. The important article presents the trial designs and end points necessary to demonstrate meaningful beneficial effects of NASH pharmacotherapies in development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Hamdah R, Rabiee A, Meneilly GS, et al. Clinical review: the extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metabol. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo XH. The value of short- and long-acting glucagon-like peptide-1 agonists in the management of type 2 diabetes mellitus: experience with exenatide. Curr Med Res Opin. 2016;32:61–76. doi: 10.1185/03007995.2015.1103214. [DOI] [PubMed] [Google Scholar]
- 27■■.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with nonalcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. This important study (LEAN trial) establishes the utility of GLP-1 pathway in NASH by demonstrating that the long-acting GLP-1 analogue, liraglutide, led to histological resolution of NASH. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Nagai Y, Ohta A, et al. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;109:199–205. doi: 10.1016/j.diabres.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for nonalcoholic fatty liver disease: a randomized controlled trial. J Hepatology. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohki T, Akihiro I, Kondo M, et al. SGLT-2 inhibitors improved liver inflammation and fibrosis of NAFLD patients with type 2 diabetes mellitus with a favorable effect of weight reduction. Hepatology. 2016;64:582A. [Google Scholar]
- 31.Pastori D, Polimeni L, Baratta F, et al. The efficacy and safety of statins for the treatment of nonalcoholic fatty liver disease. Dig Liver Dis. 2015;47:4–11. doi: 10.1016/j.dld.2014.07.170. [DOI] [PubMed] [Google Scholar]
- 32.Blais P, Lin M, Kramer JR, et al. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci. 2016;61:1714–1720. doi: 10.1007/s10620-015-4000-6. [DOI] [PubMed] [Google Scholar]
- 33.Kargiotis K, Athyros VG, Giouleme O, et al. Resolution of nonalcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21:7860–7868. doi: 10.3748/wjg.v21.i25.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westlin WF, Blanchette H, Harriman G, et al. NDI-010976, a potent, liver-directed, oral inhibitor of acetyl coa carboxylase for nonalcoholic steatohepatitis: pharmacodynamic effects on hepatic de novo lipogenesis in obese but otherwise healthy adult male volunteers. J Hepatology. 2016;64:S190–S191. [Google Scholar]
- 35.Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2085–2091. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 37.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with nonalcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 40.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abner EL, Schmitt FA, Mendiondo MS, et al. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158–170. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Key TJ, Appleby PN, Travis RC, et al. Endogenous Hormones Nutritional Biomarkers Prostate Cancer Collaborative Group Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr. 2015;102:1142–1157. doi: 10.3945/ajcn.115.114306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kowdley KV, Wilson LA, Van Natta ML, et al. Efficacy and safety of vitamin e in nonalcoholic steatohepatitis patients with and without diabetes: pooled analysis from the PIVENS and FLINT NIDDK NASH CRN trials. Hepatology. 2015;62:264A-A. [Google Scholar]
- 44.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 45.Banini BA, Sanyal AJ. Nonalcoholic fatty liver disease: epidemiology, pathogenesis, natural history, diagnosis, and current treatment options. Clin Med Insights Ther. 2016;2016:75–84. doi: 10.4137/cmt.s18885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dohil R, Schmeltzer S, Cabrera BL, et al. Enteric-coated cysteamine for the treatment of paediatric nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:1036–1044. doi: 10.1111/j.1365-2036.2011.04626.x. [DOI] [PubMed] [Google Scholar]
- 47■.Schwimmer JB, Lavine JE, Wilson LA, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology. 2016;151:1141–1154. doi: 10.1053/j.gastro.2016.08.027. This study further demonstrated the role of oxidative stress in NAFLD by showing that cysteamine, a scavenger of reactive oxygen species reduces serum aminotransferase levels and lobular inflammation, with no effect on histological markers or NAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkhouri N, Feldstein AE. Treating nonalcoholic steatohepatitis (NASH) in children: not a cinch task. Hepatology. 2017 doi: 10.1002/hep.29043. [DOI] [PubMed] [Google Scholar]
- 49.Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PloS one. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson M, Chang W, Jenkins H, et al. Improvements in APRI and FIB-4 fibrosis scores correlate with decreases in sCD14 in HIV-1 infected adults receiving cenicriviroc over 48 weeks. Hepatology. 2014:424A-A. [Google Scholar]
- 51.Friedman S, Sanyal A, Goodman Z, et al. Efficacy and safety study of cenicriviroc for the treatment of nonalcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356–365. doi: 10.1016/j.cct.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Sanyal AJ, Ratzui V, Harrison SA, et al. Cenicriviroc placebo for the treatment of nonalcoholic steatohepatitis with liver fibrosis: results from the year 1 primary analysis of the phase 2b CENTAUR study. Hepatology. 2016;64:1118. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 53.Syn WK, Choi SS, Diehl AM. Apoptosis and cytokines in nonalcoholic steatohepatitis. Clin Liver Dis. 2009;13:565–580. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54■.Barreyro FJ, Holod S, Finocchietto PV, et al. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of nonalcoholic steatohepatitis. Liver Inte. 2015;35:953–966. doi: 10.1111/liv.12570. This study demonstrates the role of caspases in hepatocyte injury and hepatic fibrosis by showing that the oral pan-caspase inhibitor emricasan improves fibrosis without any effects on hepatic steatosis or metabolic syndrome. [DOI] [PubMed] [Google Scholar]
- 55.Shiffman M, Freilich B, Vuppalanchi R, et al. A Placebo-controlled, multicenter, double-blind, randomised trial of emricasan in subjects with nonalcoholic fatty liver disease (Nafld) and raised transaminases. J Hepatology. 2015;62:S282-S. [Google Scholar]
- 56.Budas G, Karnik S, Jonnson T, et al. Reduction of liver steatosis and fibrosis with an ask1 inhibitor in a murine model of NASH is accompanied by improvements in cholesterol, bile acid and lipid metabolism. J Hepatology. 2016;64:S170. [Google Scholar]
- 57.Loomba R, Lawitz E, Mantry PS, et al. GS-4997, an Inhibitor of Apoptosis Signal-Regulating Kinase (ASK1), Alone or in Combination with Simtuzumab for the Treatment of Nonalcoholic Steatohepatitis (NASH): A Randomized, Phase 2 Trial. Hepatology. 2016;64(6):1119A. [Google Scholar]
- 58.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PloS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traber PG, Chou H, Zomer E, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PloS One. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]


