Abstract
Objective
The objective of this study was to confirm the efficacy of low-dose mizoribine (MZR), an inhibitor of inosine monophosphate dehydrogenase, as part of synchronized methotrexate (MTX) therapy for rheumatoid arthritis (RA) patients with an inadequate response to various combination therapies of MTX, other synthetic disease-modifying anti-rheumatic drugs (DMARDs) and biological DMARDs.
Methods
Low-dose MZR was administered to 56 uncontrolled RA patients being treated with MTX and various biological DMARDs. The observation period was 12 months, and the disease activity was evaluated based on the Disease Activity Score in 28 joints (DAS28)-ESR, Simplified Disease Activity Index (SDAI) and serum MMP-3 level.
Results
All of the disease activity indices were significantly improved within three months, and the serum MMP-3 levels were also significantly decreased around four months after starting low-dose MZR therapy. No patients experienced any adverse effects.
Conclusion
The present preliminary findings suggest that low-dose MZR therapy with MTX should be considered for the treatment of RA patients with an inadequate response to various combination therapies including MTX, other synthetic DMARDs and biological DMARDs or in whom increasing the dose of MTX is difficult for reasons such as adverse effects and complications.
Keywords: DMARDs, methotrexate, mizoribine, rheumatoid arthritis, remission
Introduction
Mizoribine (MZR) is an immunosuppressive agent that is similar to mycophenolate mofetil (MMF) in its inhibitory effect on inosine monophosphate dehydrogenase, a rate-limiting enzyme in the de novo pathway of nucleic acid synthesis (1). The immunosuppressive effect has been suggested to be due to the inhibition of T and B cell proliferation (2). MZR was first isolated from the culture media of Eupenicillium brefeldianum M-2166 in 1974 in Japan (3).
Since MZR was first approved for use in renal transplantation patients (4), it has been thought to be safe and well-tolerated compared with other immunosuppressants, and recent studies have demonstrated its usefulness in the treatment of rheumatoid arthritis (RA) (5,6), systemic lupus erythematosus (SLE) (7,8), nephrotic syndrome (9) and immunoglobulin A (IgA) nephropathy (10). A previous report revealed that MZR can be more easily transferred from the plasma to the synovial fluid in RA patients than methotrexate (MTX) (11).
MZR is conventionally used at a dose of 150 mg daily (12); however, 300 mg weekly as low-dose MZR pulse therapy in combination with MTX was found to be effective in RA patients with an insufficient response to MTX alone (13). This low-dose regimen provides both clinical safety and economic benefits, because the dose is less than one-third of the conventional dose. In addition, increasing the dose of MTX-or using it at all-is difficult for some RA patients, so this presents the need to find an alternative.
Based on these reports, a clinical trial was designed to evaluate the efficacy and safety of low-dose intermittent MZR pulse therapy synchronized with MTX to control the signs and symptoms of RA in patients with an insufficient response to MTX alone or MTX with other synthetic disease-modifying anti-rheumatic drugs (DMARDs) and biological DMARDs.
Materials and Methods
Subjects
This pilot study was conducted from 2010 to 2014 at Juntendo University Urayasu Hospital in Chiba, Japan. All patients who entered into this study had met the following inclusion criteria at the time of their enrollment: 1) a diagnosis of RA according to the 2010 American College of Rheumatology (ACR)-European League of Rheumatology (EULAR) classification criteria for RA (14), 2) a disease duration of at least six months and 3) active disease, which was defined by at least three tender small joints and at least two swollen small joints. The patients who had severe drug hypersensitivity, bone marrow suppression, severe liver dysfunction, severe infection, pregnancy or malignancy were excluded from this study. Additionally, a dose increase for MTX was difficult for all patients due to adverse effects or complications, such as pulmonary and/or renal dysfunction (15).
MZR was initiated at a dose of 50-200 mg orally once a week. Dose regulation was possible depending on each patient's symptoms or adverse drug reactions and was made at the discretion of the investigator. The maximum dose of the intermittent MZR therapy was 400 mg orally per week. The maximum observation period of treatment in the study was scheduled to be 12 months. MTX, other synthetic DMARDs, such as salazosulfapyridine (SASP), biological DMARDs, nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids that were used prior to the study could be continued. Dose reduction was allowed for NSAIDs, DMARDs and steroids during the study period. All patients provided their informed consent to participate in this study, and the local ethics committee of Juntendo Urayasu Hospital approved the study.
Data collection and analyses
The patient medical records were reviewed to identify the main clinical features in terms of the efficacy and safety of MZR therapy. The collected data were the age, sex and treatment history. For the safety evaluation, information about adverse events and the duration of therapy were also recorded. The disease activity was assessed by the swollen and tender joint counts, the erythrocyte sedimentation rate, the C-reactive protein level, the global assessment of the disease activity by the patient, the global assessment of the disease activity by the investigator, the Disease Activity Score in 28 joints (DAS28) following the ACR guidelines and the Simplified Disease Activity Index (SDAI)/the Clinical Disease Activity Index (CDAI) proposed by the ACR-EULAR (16), at the beginning of the study and at monthly intervals thereafter until the end of the study (12 months). In addition, we also assessed remission using the ACR/EULAR criteria, comparing our findings with the DAS28 remission results (17). Missing data were compiled using the last observation carried forward (LOCF) method.
Statistical analyses
The data are presented as the counts or means with the standard error (SE). In the statistical analyses, the paired t-test was used for comparisons between two groups, and the Wilcoxon signed-rank or two-way factorial analysis of variance (ANOVA) test was used for comparisons of the changes in the patients' clinical course over time. The statistical analyses were performed by Kureha Special Laboratory Co. (Tokyo, Japan) using the SAS 9.4 software program (SAS Institute Inc., Cary, NC, USA). All p values were two-sided, and a p value of less than 0.05 was considered to indicate statistical significance.
Results
The patient characteristics at the start of the trial are summarized in Table 1. The mean age of patients was 61.3 (range: 48 to 74) years, and the mean disease duration was 7.5 (range: 0.6 to 14.9) years. Of the 56 patients evaluated by Steinbrocker's radiological stage, 21 were stage I, 16 II, 3 III and 16 stage IV (18). The mean dosage of steroids was 4.2 mg/day (range: 3.0-5.5 mg/day). The mean dose of MTX was 7.5 mg/week (range: 4.0-10.5 mg/week). Other synthetic DMARDs were used in 32% (18/56) of patients, and the numbers of patients who used DMARDs were as follows: SASP was used by 9 patients, bucillamine (BUC) by 1 patient and tacrolimus (TAC) was used by 8 patients. In addition, biological agents were also used by 32% (18/56) of the patients, and the breakdown showed that 10 patients were treated with infliximab (IFX), 4 patients with etanercept (ETN), 1 patient with tocilizumab (TCZ) and 3 patients with abatacept (ABT). There was no dosage increase of MTX, other synthetic DMARDs or biologics within three months before starting this study. There were three incomplete patients due to a worsening of the disease activity within 12 months. None of the patients showed any adverse effects due to the medication throughout this prospective study.
Table 1.
Baseline Patient Characteristics.
| Number of females/males | 42/14 |
| Age, years (range) | 61.3 (48 to 74) |
| Steinbrocker stage | I: 21, II: 16, III: 3, IV: 16 |
| Steinbrocker class | 1: 27, 2: 28 |
| Disease duration, years (range) | 7.5 (0.6 to 14.9) |
| PSL dose, mg/day (range) | 4.2 (3.0 to 5.5) |
| Number of patients treated with PSL | 37 |
| MTX dose, mg/week (range) | 7.5 (4.0 to 10.5) |
| Number of patients treated with MTX | 56 |
| Number of patients treated with SASP | 9 |
| Number of patients treated with BUC | 1 |
| Number of patients treated with TAC | 8 |
| Number of patients treated with IFX | 10 |
| Number of patients treated with ETN | 4 |
| Number of patients treated with TCZ | 1 |
| Number of patietns treated with ABT | 3 |
MTX: methotrexate, PSL: prednisolone, SASP: salazosulfapyridine, BUC: bucillamine, TAC: tacrolimus, IFX: infliximab, ETN: etanercept, TCZ: tocilizumab, ABT: abatacept
The DAS28-ESR value decreased significantly after 3 months compared with baseline (p<0.01), and the effect continued for at least 12 months (p<0.01) (Fig. 1A). The mean DAS28-ESR was improved from 3.50 at baseline to 2.41 at 12 months. The SDAI value also decreased significantly after 3 months compared with baseline (p<0.01), and the effect continued for 12 months (p<0.01). In particular, the mean SDAI was improved from 10.51 at baseline to 3.13 at 12 months (Fig. 1B).
Figure 1.
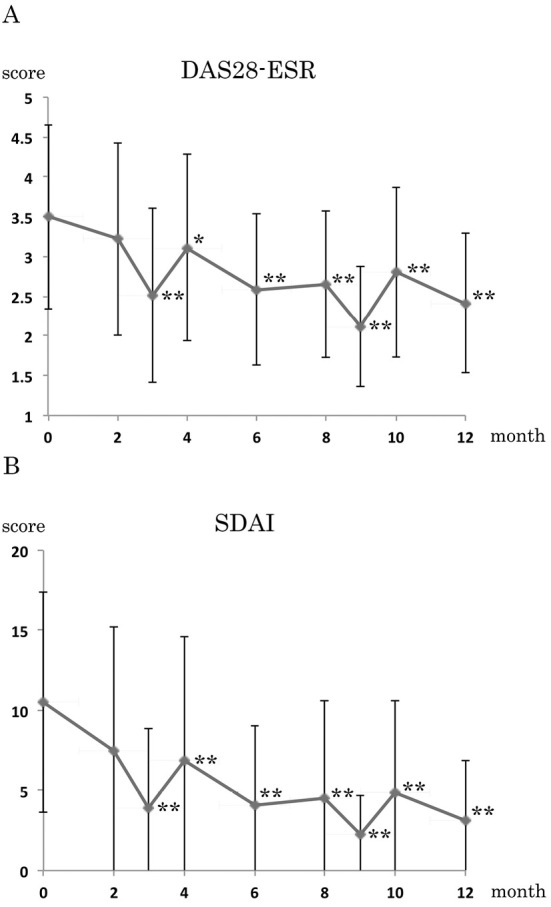
The efficacy as determined by the 28-joint count Disease Activity Score (DAS28) and the Simplified Disease Activity Index (SDAI). (A) The DAS28-ESR and (B) the SDAI. The values are the means ± standard error (SE). Low-dose mizoribine (MZR) pulse therapy led to significant improvement in all of the disease activity indices: each month vs. 0 months (baseline); * p <0.05, ** p <0.01; on both the Wilcoxon signed-rank test and paired t-test for the DAS28-ESR and the Wilcoxon signed-rank test for the SDAI.
To confirm the MZR pulse dose-dependent efficacy, high-dose (201-400 mg/week), moderate-dose (101-200 mg/week) and low-dose (50-100 mg/week) MZR pulse regimens were statistically compared (Fig. 2A). The stratified analysis of MZR therapy showed no significant differences between each dosage group using a two-way factorial ANOVA test. The DAS28-ESR of patients with or without biologics was then statistically analyzed to confirm the effect of biologic DMARDs. MZR pulse therapy showed efficacy in patients using both only synthetic DMARDs and synthetic/biologic DMARDs without any significant differences as determined by a Wilcoxon signed-rank test (Fig. 2B). Both the EULAR response (Table 2) and DAS28 remission rate (Table 3) improved at 6 and 12 months compared with the baseline, respectively. A stratified analysis based on the DAS28-ESR showed that the proportion of patients with remission/low disease activity increased while the proportion of patients with moderate/high disease activity decreased (Table 4). In particular, the proportion of patients in remission was significantly higher at 12 months into the study than at baseline (0 months), as determined by the chi-squared test (p<0.001).
Figure 2.
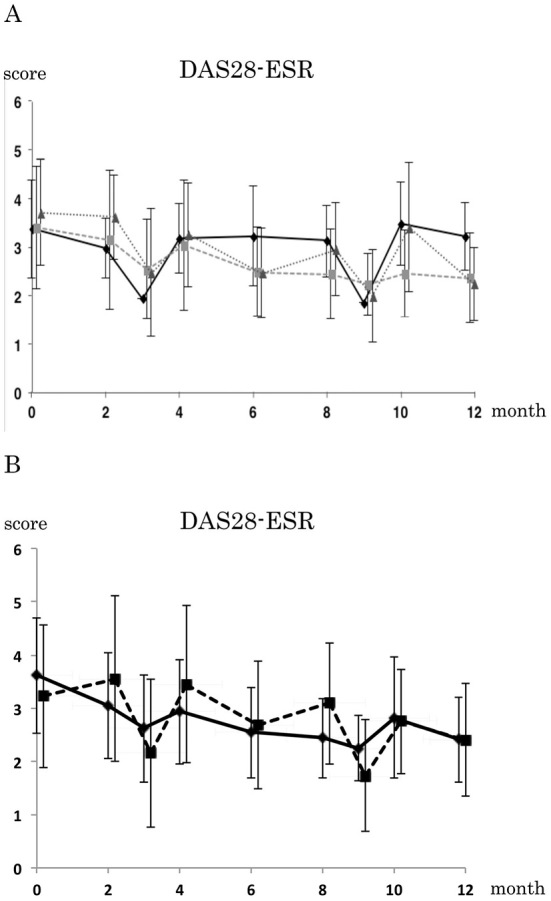
The effects of MZR pulse therapy on patients treated with only synthetic DMARDs or synthetic and biologic DMARDs. A: A comparison of DAS28-ESR between low-dose (50-100 mg/week, n=6), moderate-dose (101-200 mg/week, n=32) and high-dose (201-400 mg/week, n=15) MZR pulse therapy; the solid, large broken and small broken lines represent low-, moderate- and high-dose MZR therapy, respectively. B: The effects of MZR pulse therapy on patients treated with only synthetic DMARDs or synthetic and biologic DMARDs; the broken and solid lines indicate therapy with and without biologic DMARDs, respectively. A stratified analysis of MZR therapy showed no significant differences between each dosage group; two-way factorial ANOVA tests. MZR pulse therapy showed no significant differences in the efficacy between patients using synthetic DMARDs (n=35) and synthetic/biologic DMARDs (n=18); Wilcoxon signed-rank test.
Table 2.
EULAR Response in the Trial.
| DAS28-ESR Baseline | 6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|
| good | moderate | no | good | moderate | no | ||
| ≤3.2 | 11 | 8 | 2 | 11 | 10 | 0 | |
| >3.2 and ≤5.1 | 0 | 29 | 0 | 0 | 28 | 0 | |
| >5.1 | 0 | 5 | 0 | 0 | 4 | 0 | |
| Total | 11 (20.0%) | 42 (76.4%) | 2 (3.6%) | 11 (20.7%) | 42(80.8%) | 0(0.0%) | |
Table 3.
DAS28-ESR Remission Rate in the Trial.
| Remission (DAS28-ESR<2.6) | 6 Months | 12 Months |
|---|---|---|
| Yes | 29 (52.7%) | 36 (67.9%) |
| No | 26 (47.3%) | 17 (32.7%) |
Table 4.
The Stratified Analysis of the Trial Based on DAS28-ESR.
| DAS28-ESR | 0 | 2M | 4M | 6M | 8M | 10M | 12M |
|---|---|---|---|---|---|---|---|
| Remission(≤2.6) | 55.4% | 40.0% | 33.3% | 52.7% | 51.6% | 48.4% | 67.9% |
| Low (>2.6 but ≤3.2) | 8.9% | 16.7% | 26.7% | 29.1% | 25.8% | 9.7% | 10.1% |
| Moderate (>3.2 but ≤5.1) | 28.6% | 40.0% | 36.7% | 18.2% | 22.6% | 38.7% | 22.0% |
| High (>5.1) | 7.1% | 3.3% | 3.3% | 0.0% | 0.0% | 3.2% | 0.0% |
| Chi-squared test | – | p=0.152 | p=0.007 | p<0.001 | p=0.523 | p=0.160 | p<0.001 |
We next assessed the changes in the serum MMP-3 levels, and found that there was a statistically significant reduction in the serum MMP-3 levels after 4 months (p<0.05 to 0.01) (Fig. 3). The mean serum MMP-3 levels dropped from 233.9 ng/mL at baseline to 140.3 ng/mL at 12 months. The serum MMP-3 levels in female patients were higher than in males; however, the differences were not significant, and both groups showed significant reductions in the serum MMP-3 levels (data not shown).
Figure 3.
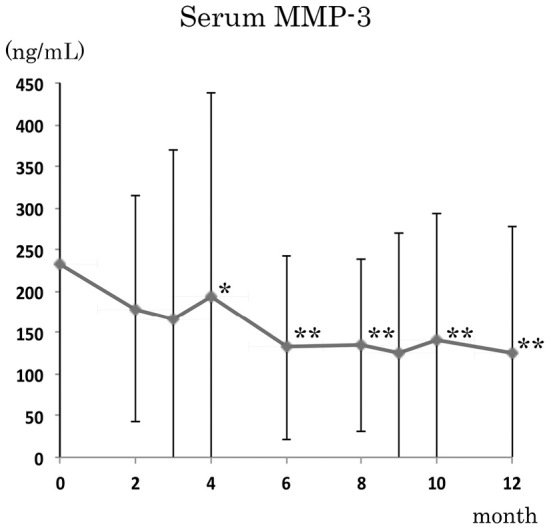
The effects of MZR pulse therapy on the serum MMP-3 levels. There were statistically significant reductions in the serum MMP-3 levels after four months. Each month vs. 0 months (baseline); * p <0.05, ** p <0.01; Wilcoxon signed-rank test.
We also showed that the efficacy of MZR was not dependent on prednisolone (PSL) compared with the dose between the just before starting this study and at the end points of the observation period. The mean dosage of PSL significantly decreased during the observation period, from 4.23 mg/day at baseline to 3.39 mg/day at 12 months (p<0.001) (Fig. 4). The dosages of MTX and biologics did not significantly change throughout the observation period (data not shown). Both SASP and BUC were discontinued in all patients, and NSAIDs were discontinued in seven patients (data not shown).
Figure 4.
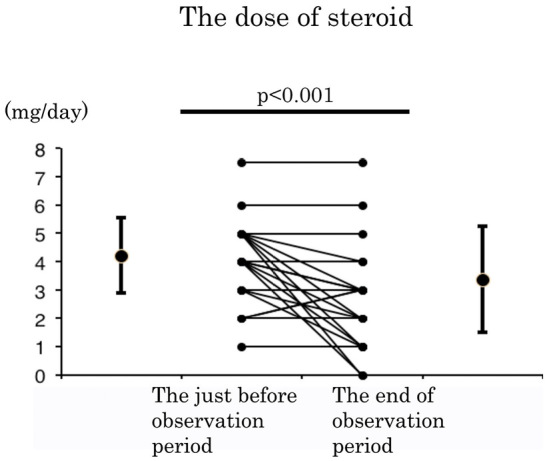
The alteration of steroid dosage throughout this prospective study. The dose of prednisolone (PSL) was significantly decreased during the observation period (p <0.001); Wilcoxon signed-rank test.
Discussion
There are some limitations associated with this study due to its non-controlled nature and the small number of patients enrolled. However, this prospective study demonstrated that low-dose intermittent MZR pulse therapy synchronized with MTX led to re-remission in patients with relapsing RA, which led to significant improvements in the DAS28-ESR and SDAI after three months (Fig. 1). The serum MMP-3 levels were also significantly reduced after four months (Fig. 3). Those data are similar to the results of several previous reports (12,13). The efficacy of low-dose intermittent MZR therapy was previously proven in patients with RA that showed an inadequate response to MTX monotherapy or to IFX, a chimeric antibody against tumor necrosis factor-alpha (13,19). Our study indicates that MZR pulse therapy was effective against RA in patients with an inadequate response to combination therapy, such as MTX plus other DMARDs or biological agents such as SASP, BUC, TAC, IFX, ETN (a dimeric fusion protein against tumor necrosis factor-alpha), TCZ (a humanized monoclonal antibody against the interleukin-6 receptor [IL-6R]) and ABT (a cytotoxic T lymphocyte-associated antigen 4 immunoglobulin fusion protein). While biological agents are extremely effective in improving the disease activity of RA, they did not affect the efficacy of MZR pulse therapy (Fig. 2).
The doses of MTX and steroids used during the study period were not significantly increased throughout the course, and the observed improvement in the disease activity in this study was due solely to the addition of MZR pulse therapy (Fig. 4). These present and previous findings regarding concurrent medications suggest that MZR pulse therapy might be able to salvage RA patients with a wider insufficient response to MTX alone, MTX with other DMARDs and MTX with various biological agents. Additionally, there were no adverse effects throughout the duration of this study in patients of any age, and efficacy was also shown in patients treated with low-dose MTX therapy. We also demonstrated that MZR pulse therapy was effective against MTX-resistant RA, regardless of the MTX amount (4-10.5 mg/week). These results further indicated that MZR pulse therapy could be used safely even in aged patients and patients expected to have an adverse reaction if the MTX dose were increased.
Based on the efficacy, convenience, safety and cost of this treatment, the present preliminary results suggest that low-dose MZR therapy synchronized with MTX should be considered for the treatment of RA patients with an inadequate response to various combination therapies including MTX, other DMARDs and biological agents, or in whom increasing the dose of MTX is difficult due to adverse effects and complications.
The authors state that they have no Conflict of Interest (COI).
Financial support
This study was supported by a JSPS (Japan Society for the Promotion of Science) KAKENHI Grant Number JP15K08107 (to KI).
Acknowledgement
The authors thank rheumatic disease care-certified nurses Mayumi Inoue and Nao Kitajima.
References
- 1.Ishikawa H. Mizoribine and mycophenolate mofetil. Curr Med Chem 6: 575-597, 1999. [PubMed] [Google Scholar]
- 2.Hughes SE, Gruber SA. New immunosuppressive drugs in organ transplantation. J Clin Pharmacol 36: 1081-1092, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno K, Tsujino M, Takada M, Hayashi M, Atsumi K. Studies on bredinin. I. Isolation, characterization and biological properties. J Antibiot (Tokyo) 27: 775-782, 1974. [DOI] [PubMed] [Google Scholar]
- 4.Inou TKR, Takahashi I, Sugimoto H, et al. Clinical trial of Bredinin in renal transplantation. Transplant Proc 12: 526-528, 1980. [PubMed] [Google Scholar]
- 5.Takei S. Mizoribine in the treatment of rheumatoid arthritis and juvenile idiopathic arthritis. Pediatr Int 44: 205-209, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsubo H, Ohkura S, Akimoto M, et al. An investigation of the correlation between blood concentration of mizoribine and its efficacy in treatment of rheumatoid arthritis based on indices of drug survival and improvement in DAS28-CRP. Mod Rheumatol 22: 837-843, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Aihara Y, Miyamae T, Ito S, et al. Mizoribine as an effective combined maintenance therapy with prednisolone in child-onset systemic lupus erythematosus. Pediatr Int 44: 199-204, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Nomura A, Shimizu H, Kishimoto M, et al. Efficacy and safety of multitarget therapy with mizoribine and tacrolimus for systemic lupus erythematosus with or without active nephritis. Lupus 21: 1444-1449, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka K, Ohashi Y, Sakai T, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int 58: 317-324, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K, Nagaoka R, Ohtomo Y, Yamashiro Y. Mizoribine for childhood IgA nephropathy. Nephron 83: 376-377, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Sakai KMH, Tsuji H, Sainou H, Yashima S. Synocial fluid and plasma levels of mizoribine in RA patients. Jpn J Inflamm 14: 521-524, 1994. [Google Scholar]
- 12.Ichinose K, Origuchi T, Kawashiri SY, et al. Efficacy and safety of mizoribine by one single dose administration for patients with rheumatoid arthritis. Intern Med 49: 2211-2218, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Kasama T, Wakabayashi K, Odai T, et al. Effects of low-dose mizoribine pulse therapy in combination with methotrexate in rheumatoid arthritis patients with an insufficient response to methotrexate. Mod Rheumatol 19: 395-400, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69: 1580-1588, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Olivo MA, Siddhanamatha HR, Shea B, Tugwell P, Wells GA, Suarez-Almazor ME. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst Rev 6: CD000957, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res (Hoboken) 63 (Suppl 11): S14-S36, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 70: 404-413, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc 140: 659-662, 1949. [DOI] [PubMed] [Google Scholar]
- 19.Horikoshi M, Ito S, Ishikawa M, et al. Efficacy of mizoribine pulse therapy in patients with rheumatoid arthritis who show a reduced or insufficient response to infliximab. Mod Rheumatol 19: 229-234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


