Abstract
Leptospirosis is frequently associated with acute kidney injury. Some survivors are known to progress to chronic kidney disease due to sustained tubulointerstitial inflammation. We present a case of severe leptospirosis with acute renal failure. Although antibiotic therapy resolved the infection, moderate renal dysfunction remained. A renal biopsy demonstrated marked inflammatory infiltration in the tubules and interstitium. Many of the inflammatory cells were CD68-positive monocytes/macrophages, predominantly M1 phenotype. An intermediate dose of oral corticosteroids normalized the patient's serum creatinine levels. We suggest that corticosteroid therapy may be a therapeutic option for some patients with sustained tubulointerstitial nephritis who survive severe leptospirosis.
Keywords: leptospirosis, acute kidney injury, macrophages
Introduction
Leptospirosis is a common zoonotic infection in tropical regions, but it has been identified as an emerging infectious disease even in developed countries (1). The clinical presentations vary from mild flu-like illness to severe septicemia with acute renal failure, hepatic necrosis, pulmonary hemorrhaging, and/or cardiovascular collapse (2). Renal involvement is frequently seen in severe instances of the disease. Such involvement is mainly characterized by pathogen-induced acute tubulointerstitial nephritis as well as acute tubular necrosis caused by hemodynamic alterations and the immune response (3). These pathological alterations have been found in autopsy-based case reports and case series. Even though some patients who survive severe leptospirosis have prolonged renal dysfunction, little evidence has been presented regarding the renal pathology after resolution of the infection in survivors with renal impairment.
Acute kidney injury has been increasingly considered a major cause of chronic kidney disease (CKD) and end-stage renal disease. Survivors of severe leptospirosis were previously assumed to recover their renal function. However, recent reports have suggested that a significant proportion of these patients have incomplete renal recovery and progress to CKD (4). Sustained renal inflammation after resolution of the infection should be attributed to this progression. In particular, persistent monocyte/macrophage (M1 but not M2 phenotype) infiltration probably has an adverse effect, preventing renal histological and functional recovery. It is worth considering a renal biopsy for such patients in order to predict their renal prognosis and establish therapeutic strategies, whenever possible.
In this report, we present a case of severe leptospirosis associated with oligouric renal failure. After successful antibiotic therapy, a renal biopsy revealed sustained tubulointerstitial nephritis. Immunohistochemistry demonstrated the accumulation of macrophages, predominantly M1 phenotype. Oral corticosteroids with tapering improved the patient's renal function completely. This case report suggests the possible benefit of post-infectious corticosteroid therapy for some patients with leptospiral nephropathy in order to prevent the progression to CKD.
Case Report
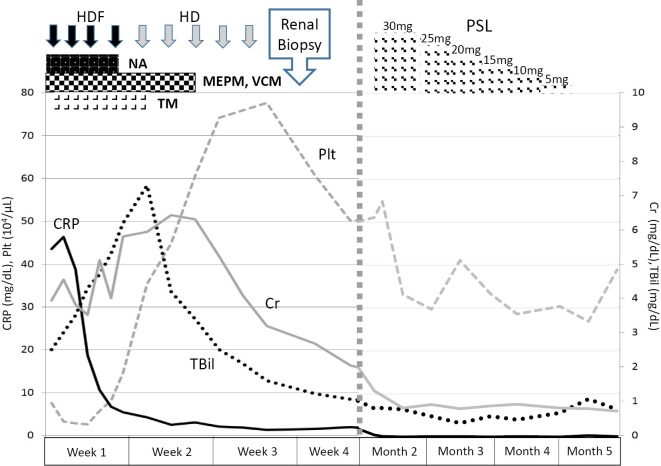
A 63-year-old man presented with fever and general malaise followed by disturbance of consciousness and hypotension. At admission, laboratory testing showed acute renal failure, hepatic injury and disseminated intravascular coagulation (DIC), as shown in Table 1. The elevated procalcitonin (PCT) level of 95.1 ng/mL suggested the presence of a bacterial infection. However, imaging studies, including thoracicoabdominal computed tomography (CT), did not reveal any findings indicating the origin of the infection, and no bacteria were detected by culture testing of blood, urine and cerebrospinal fluid samples. We started broad-spectrum antibiotic therapy with meropenem and vancomycin in addition to norepinephrine infusion and intravenous recombinant thrombomodulin, and then carried out intermittent hemodialysis therapy for oliguric renal failure. The peak values during the treatment were as follows: white blood cells 26,700 /μL, hemoglobin 11.0 g/dL, platelets 28,000 /μL, total-bilirubin 7.3 mg/dL, direct-bilirubin 5.3 mg/dL, blood urea nitrogen 68.7 mg/dL, creatinine 6.30 mg/dL and C-reactive protein (CRP) 43.5 mg/dL. These treatments resulted in improvement in his general condition and laboratory data, including platelet counts and CRP and bilirubin levels (Fig. 1). After three weeks, the patient recovered his urine volume, and hemodialysis therapy withdrawn. On assessing paired serum samples obtained at admission and after four weeks, we found that the agglutinating antibody titers against Leptospira interrogans serovar Hebdomadis andserovar Rachmati had markedly increased to 1:160 and 1:320, respectively, leading to a diagnosis of leptospirosis (Table 2).
Table 1.
Laboratory Data on Admission.
| Blood Count | Chemistry | Immunology | |||||||
| WBC | 26,700 | /μL | TP | 5.0 | g/dL | IgG | 908 | mg/dL | |
| Neu | 87.4 | % | Alb | 1.8 | g/dL | IgA | 164 | mg/dL | |
| Lym | 2.2 | % | T-Bil | 3.0 | mg/dL | IgM | 34 | mg/dL | |
| Eos | 6.6 | % | D-Bil | 2.2 | mg/dL | C3 | 86 | mg/dL | |
| Baso | 0.0 | % | AST | 86 | IU/L | C4 | 32.8 | mg/dL | |
| RBC | 284×104 | /μL | ALT | 24 | IU/L | CH50 | 45 | mg/dL | |
| Hb | 11.0 | g/dL | AMY | 2,512 | IU/L | ANA | ≤40 | (-) | |
| Ht | 32.5 | % | LDH | 336 | IU/L | RF | (-) | ||
| MCV | 114.4 | fL | ALP | 469 | IU/L | MPO-ANCA | <0.50 | (-) | |
| MCH | 38.7 | pg | γGTP | 105 | IU/L | PR3-ANCA | <0.50 | (-) | |
| MCHC | 33.8 | % | UA | 7.7 | mg/dL | GBM Ab | <10 | (-) | |
| Plt | 3.2×104 | /μL | Cr | 4.55 | mg/dL | Coagulation | |||
| BUN | 73.6 | mg/dL | PT | 12.1 | sec | ||||
| Urinalysis | Na | 34 | mEq/L | APTT | 38.4 | sec | |||
| pH | 6.0 | K | 4.7 | mEq/L | Fib | 629 | mg/dL | ||
| SG | 1.010 | Cl | 94 | mEq/L | FDP | 33.2 | μg/mL | ||
| Pro | 4+ | Ca | 7.1 | mg/dL | D-dimer | 12.6 | μg/mL | ||
| OB | 3+ | IP | 7.8 | mg/dL | Infection | ||||
| Uro | ± | T-chol | 124 | mg/dL | HBs Ag | (-) | |||
| Sediment | CK | 284 | mg/dL | HCV Ab | (-) | ||||
| RBC | 10-15 | /HF | CRP | 43.5 | mg/dL | HIV Ab | (-) | ||
| WBC | 5-10 | /HF | HbA1c | 5.7 | % | Parvo IgM | (-) | ||
| ETC | 3-5 | /HF | U-NAG | 116.8 | U/L | PCT | 95.1 | ng/mL | |
SG: specific gravity, Pro: protein, OB: occult blood, Uro: urobilinogen, ETC: epithelial cells, U-NAG: urinary N-acetyl-β-D-glucosaminidase, Ab: antibody, PCT: procalcitonin
Figure 1.
The clinical course of the case from admission to the renal biopsy. HDF: hemodiafiltration, HD: hemodialysis, NA: norepinephrine, MEPM: meropenem, VCM: vancomycin, TM: thrombomodulin, PSL: prednisone (with daily oral dosage)
Table 2.
Results of Microscopic Agglutination Test from Paired Serum Samples at Admission and after Four Weeks.
| The species or serovars of Leptospira | Antibody titer | |
|---|---|---|
| At admission | Four weeks later | |
| L. kirschneri serovar Grippotyphosa | <40 | |
| Leptospira borgpetersenii serovar Castellonis | <40 | |
| L. borgpetersenii serovar Javanica | <40 | |
| L. borgpetersenii serovar Poi | <10 | 80 |
| L. interrogans serovar Australis | <40 | |
| L. interrogans serovar Autumnalis | <10 | 80 |
| L. interrogans serovar Bataviae | <40 | |
| L. interrogans serovar Canicola | <40 | |
| L. interrogans serovar Copenhageni | <40 | |
| L. interrogans serovar Hebdomadis | 10 | 160 |
| L. interrogans serovar Icterohaemorrhagiae | <40 | |
| L. interrogans serovar Kremastos | 20 | 160 |
| L. interrogans serovar Pomona | <40 | |
| L. interrogans serovar Pyrogenes | <40 | |
| L. interrogans serovar Rachmati | <10 | 320 |
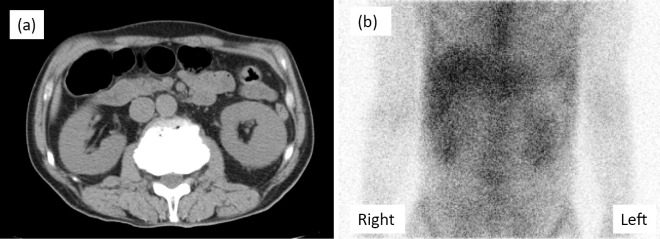
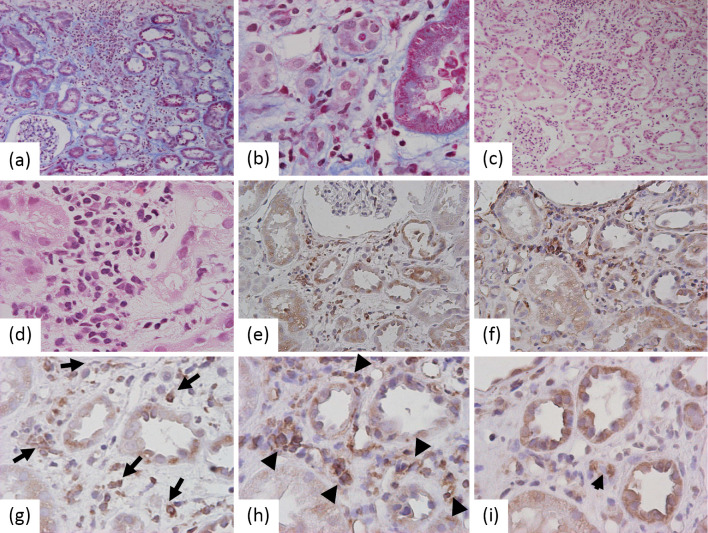
Despite an improvement in the patient's condition and most laboratory data, the renal impairment persisted, with an elevated serum creatinine level between 2.5 and 3.0 mg/dL. CT showed slight bilateral renal swelling. 67Ga-citrate scintigraphy demonstrated prominent accumulation in the bilateral kidneys (Fig. 2). A renal biopsy revealed the renal histological alterations after resolution of leptospiral infection. Detached tubular epithelial cells from the basement membranes as well as regenerating tubules with accumulation of nuclei were observed. We also noted tubulitis lesions with marked mononuclear cell infiltration in the tubules and interstitium (Fig. 3a and b). No apparent changes were observed in the glomeruli. On Hematoxylin and Eosin (H&E) staining, these mononuclear cells were compatible with lymphocytes/monocytes (Fig. 3c and d). Eosinophils and plasma cells were scarcely seen among the mononuclear cells. These findings suggested persistent tubulointerstitial nephritis with regeneration after insult of acute tubular necrosis.
Figure 2.
Renal imaging. a: Computed tomography showed slight bilateral renal swelling. b: 67Ga-citrate scintigraphy demonstrated prominent tracer accumulation in the bilateral kidneys.
Figure 3.
Renal histology of biopsy specimens. a, b: Marked mononuclear cell infiltration in the tubules and interstitium on Masson’s trichrome stained section. c, d: Hematoxylin and Eosin stainingshowed that almost all of the mononuclear cells were lymphocytes/monocytes. 100× and 400× original magnification, respectively. e, g: Immunohistochemistry for CD68 revealed that many of the inflammatory cells were monocytes/macrophages (long arrows). Further study demonstrated that these macrophages were predominantly HLA-DR-positive M1 phenotype (f, h; arrow heads) versus the CD163-positive M2 phenotype (i; short arrow) on serial sections. 200× and 400× original magnification.
To clarify the characteristics of the renal inflammatory cells after resolution of the leptospiral infection, the immunohistochemistry of the renal biopsy specimens was evaluated (see Supplementary Material). In the section containing marked inflammatory infiltration in the renal interstitium, immunohistochemistry demonstrated that some of the mononuclear cells were positive for CD68 antigen, which is a marker for monocytes/macrophages (Fig. 3e). Furthermore, the phenotype of the monocytes/macrophages was confirmed by immunohistochemistry to be HLA-DR (M1 antigen) and CD163 (M2 antigen). HLA-DR-positive mononuclear cells representing M1 macrophages obviously predominated in the renal interstitium (Fig. 3f) compared with CD163-positive M2 macrophages, as shown on the serial sections under high magnification (Fig. 3g-i). Despite the prominent tubulointerstitial inflammation, no leptospiral antigens, including those for L. interrogans serovar Hebdomadis andserovar Rachmati, were stained on the sections with leptospiral serum antibodies (data not shown).
Since antibiotic therapy had resolved his infectious symptoms and increased the PCT levels, oral administration of intermediate-dose corticosteroids at 0.6 mg/kg body weight for sustained tubulointerstitial nephritis was started, with gradual tapering. Four weeks after starting the treatment, his serum creatinine levels returned to the normal range (Fig. 1). There were no instances of re-elevated serum creatinine or recurrent infection.
Discussion
Renal involvement is a well-known complication in leptospiral infection. The major renal histological change in leptospirosis is tubulointerstitial nephritis, which is even observed in patients without renal dysfunction. Tubular necrosis is also common, indicative of acute kidney injury (AKI). Patients with leptospiral AKI usually recover their renal function to the normal range. However, in a previous study of 58 patients with leptospiral AKI, only 12% of survivors had normal creatinine levels at discharge, and the same 12% had still not achieved normal creatinine levels after 90 days (5). Another study followed 22 patients with leptospiral AKI for more than 6 months. The patients with incomplete renal recovery tended to be older and achieved their maximal GFR later than those who recovered well (6). Furthermore, a recent report suggested that a small percentage of patients who survived acute leptospirosis had a persistently abnormal renal function consistent with early-stage CKD over a three-year follow-up period. Sustained tubulointerstitial lymphocyte infiltration might be an important factor influencing the progression to CKD (4).
How can renal inflammation persist after the elimination of leptospiral antigens? The pathogenesis of leptospirosis can be divided into direct effects by the organism and host immune response to the infection. Following acute infection, direct injury from leptospira antigen is caused by proteins present in the outer membrane of the leptospira (OMP). These proteins may activate Toll-like receptor (TLR)-dependent pathways, which leads to tubular injury via cytokines, and also activate the transforming growth factor-β/Smad-mediated fibrosis pathway (7,8). However, in an animal experiment, acute tubulointerstitial nephritis complicated by leptospirosis was demonstrated even in TLR4 and TLR2 double-knockout mice (9), suggesting the pivotal role of a TLR-independent lymphocyte-activating mechanism. Along this line, the presence of far fewer leptospiral antigens in the lung than in the liver or kidney supports an indirect mechanism associated with the host's immune response to the infection. In ocular leptospirosis, the autoimmune response is believed to be the main pathogenesis, where a locally-produced antibody activates the complement cascade (1). Such an indirect immune response may be associated with the persistent renal inflammation seen in leptospirosis.
Renal histology from this case revealed moderate inflammatory infiltration in the tubules and interstitium, with concomitant tubular regeneration. Indeed, such tubulointerstitial lymphocyte infiltration seems to be compatible with the previous report on a renal biopsy in patients with leptospirosis (4), but tubulointerstitial inflammation induced by other causative agents such as antibiotics could not be excluded in this case. Interestingly, immunostaining of his renal specimen did not reveal any leptospiral antigens. Intact leptospira and its fragments have been observed throughout the tubular basement membrane in the acute infection period (10). Additional immunohistochemistry demonstrated that many inflammatory cells were monocytes/macrophages, and the M1 phenotype dominated over the M2 phenotype. Monocytes/macrophages are generally activated by the TLR ligand and interferon gamma in response to bacterial infections, leading to an M1 phenotype. M1 macrophages may induce inflammatory cytokines and reactive oxygen species and promote anti-bacterial immune responses (11). In contrast to M1 macrophages, the M2 phenotype may act later in the anti-inflammatory response to promote healing and angiogenesis. The predominance of the M1 phenotype in renal specimens might reflect on-going active inflammation, possibly through persistent inflammatory mediators.
In the present case, the patient's renal function recovered to the normal range after the administration of oral corticosteroids for persistent renal inflammation. In a previous report, Kyle Minor et al. successfully treated a patient with severe leptospirosis exclusively with intravenous antibiotics and corticosteroids (2). The routine use of corticosteroid has not been well established without concurrent antibiotic therapy. Steroid therapy is usually administered to treat persistent tubulointerstitial nephritis after the removal of the causative drugs or infections. Follow-up without corticosteroids in such a setting could lead to slow improvement of the renal function, but immediate suppression of the renal inflammation and early recovery of the renal function are so crucial that persistent renal inflammation probably causes an increased risk of progression to CKD (12). Indeed, a few patients developing CKD after leptospiral infection have been shown to have persistent renal inflammatory infiltration (4). Furthermore, corticosteroids are suggested to inhibit M1 macrophage activation in favor of M2 macrophage differentiation, leading to kidney repair (11). Considering the tissue-repairing property of M2 macrophages, it may be better to start inhibition of macrophages with corticosteroids after the demonstration of M1 predominance.
Although the current investigation is single case report, the histochemical examination in our case and previous case series suggested that some patients who survive severe leptospirosis may have sustained renal M1 macrophage infiltration. Among patients with a persistently abnormal renal function after resolution of leptospiral infection, a renal biopsy should be considered to provide histological evidence of persistent tubulointerstitial nephritis without residual leptospiral antigens, and if present, oral corticosteroids may be a therapeutic option for preventing the progression to CKD.
Author's disclosure of potential Conflicts of Interest (COI).
Jun Wada: Honoraria, Astellas, Boehringer Ingelheim, Novartis, and Tanabe Mitsubishi; Research funding, Astellas, Bayer, Chugai, Daiichi Sankyo, Kissei, Kyowa Hakko Kirin, MSD, Otsuka, Teijin, Torii, Pfizer, Takeda, and Taisho Toyama.
Acknowledgement
We thank Dr. Nobuo Koizumi of the National Institute of Infectious Disease for the diagnostic assays, including microscopic agglutinating test as well as immunostaining using serum antibodies against leptospiral antigens.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, et al. . Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3: 757-771, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Minor K, Mohan A. Severe leptospirosis: treatment with intravenous corticosteroids and supportive care. Am J Emerg Med 31: 449.e1-449.e2, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Abdulkader RC, Silva MV. The kidney in leptospirosis. Pediatr Nephrol 23: 2111-2120, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Herath NJ, Kularatne SA, Weerakoon KG, Wazil A, Subasinghe N, Ratnatunga NV. Long term outcome of acute kidney injury due to leptospirosis? A longitudinal study in Sri Lanka. BMC Res Notes 7: 398, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant 18: 1128-1134, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Daher Ede F, Zanetta DM, Abdulkader RC. Pattern of renal function recovery after leptospirosis acute renal failure. Nephron Clin Pract 98: c8-c14, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Yang CW. Leptospirosis renal disease: understanding the initiation by Toll-like receptors. Kidney Int 72: 918-925, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nature reviews Microbiology 7: 736-747, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassin C, Picardeau M, Goujon JM, et al. . TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans. J Immunol 183: 2669-2677, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Andrade L, de Francesco Daher E, Seguro AC. Leptospiral nephropathy. Semin Nephrol 28: 383-394, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787-795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez E, Gutierrez E, Galeano C, et al. . Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 73: 940-946, 2008. [DOI] [PubMed] [Google Scholar]