Abstract
The coexistence of acute myeloid leukemia (AML) with Behçet's disease (BD) is rare. The optimum treatment for AML-associated BD has not been established. We herein report a patient with BD who developed AML with myelodysplasia-related changes. Induction chemotherapy caused complete remission of the AML but worsened the BD. Thereafter, AML was treated with azacitidine. The BD was steroid-dependent. Tacrolimus was added, which improved the BD. The patient underwent allogeneic hematopoietic stem cell transplantation (HSCT) and remains in complete remission for both diseases. Allogeneic HSCT was found to be a potent therapeutic option for AML-associated BD. In addition, azacitidine and tacrolimus were shown to be a suitable bridging regimen before HSCT.
Keywords: Behçet's disease, acute myeloid leukemia, tacrolimus, azacitidine, allogeneic hematopoietic stem cell transplantation
Introduction
Behçet's disease (BD) is a systemic inflammatory disorder caused by vasculitis that is characterized by recurrent oral and genital ulcers, eye and skin lesions, and intestinal tract involvement. Recently, there have been several case reports of BD associated with myelodysplastic syndrome (MDS). The majority of patients with MDS who presented with symptoms of BD had trisomy 8, and most had intestinal manifestations (1-3). Intestinal BD is usually refractory to treatment and results in life-threatening comorbidities. Therefore, the majority of patients with BD and MDS involving trisomy 8 fail to respond to conventional treatment and have a poor prognosis (1,3). The optimum treatment of BD associated with MDS has not been established.
Recently, several cases of MDS-associated BD have been treated with allogeneic hematopoietic stem cell transplantation (HSCT), which resulted in remission of both the MDS and BD (4-6). Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) is an acute leukemia with an excess of blasts showing the morphological features of myelodysplasia, or a prior history of a MDS or myelodysplastic/myeloproliferative neoplasm, or MDS-related cytogenetic abnormalities (7). The coexistence of BD and AML is extremely rare, and only a single case has been reported (8).
We herein report a case of AML-MRC-associated BD that was successfully treated with azacitidine, tacrolimus, and allogeneic HSCT. Azacitidine and tacrolimus may be a suitable bridging regimen before HSCT. The publication of this case report was approved by the Institutional Review Board of Yamaguchi University School Hospital (No. 20160224-7-1).
Case Report
A 38-year-old Japanese man had a history of recurrent oral ulcers for more than 3 months. He subsequently developed a high fever, genital ulcers, and folliculitis and was admitted to a local hospital. Colonoscopy revealed multiple active ulcers in the terminal ileum and throughout the entire colon. He was diagnosed with BD according to the diagnostic criteria (9). Other signs or symptoms of BD, such as ocular lesions, central nervous system involvement, or vascular manifestation were not present. Furthermore, he did not have human leukocyte antigen-B*51 or -A*26, which are associated with BD pathogenesis (10). His symptoms were relieved by 30 mg prednisolone and mesalazine. However, a reduction of the steroid dose resulted in symptom recurrence. Therefore, the steroid dose was increased, and continuous administration of 15 mg prednisolone was required. One month later, he developed monocytosis. Five months later, his anemia progressed, and blasts appeared in the peripheral blood. At this time, he was referred to our hospital for further evaluation.
The laboratory data on admission revealed a leukocyte count of 10.4×109/L with 35% monocytes and 24% blasts, a hemoglobin concentration of 8.6 g/dL, and a platelet count of 7.1×1010/L. A bone marrow examination showed hypercellularity and trilineage dysplasia with 29% blasts, which were positive for CD13 and CD33. A chromosomal analysis revealed the presence of trisomy 8 in 20 out of 20 metaphases. Based on these findings, the patient was diagnosed with AML-MRC associated with BD. Colonoscopy revealed an ulcer in the terminal ileum and aphthae in the ascending colon. Pathological studies did not find evidence of leukemic cell infiltration. There were no noticeable symptoms of BD when 15 mg prednisolone and mesalazine was administered. He subsequently received induction chemotherapy with idarubicin and cytarabine.
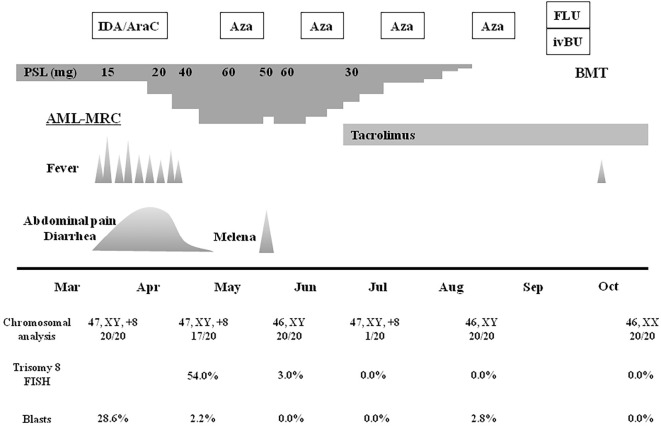
During the myelosuppression period, the patient suffered from a high fever, abdominal pain, and frequent diarrhea. Broad-spectrum antibiotics were ineffective, and a stool culture indicated no evidence of infection. Despite marked neutropenia, we considered that his BD had worsened due to mucosal injury and occult intestinal infection caused by chemotherapy. Thus, the prednisolone dose was increased to 40 mg, and his symptoms abated. However, colonoscopy revealed multiple active ulcers in the terminal ileum and entire colon, so the prednisolone dose was further increased to 60 mg (Fig. 1). Cytomegalovirus colitis was ruled out by pathological immunostaining studies. He achieved hematological complete remission of AML-MRC with 2.2% blasts in bone marrow cells. However, chromosomal and fluorescence in situ hybridization analyses revealed the persistence of trisomy 8 (Fig. 2).
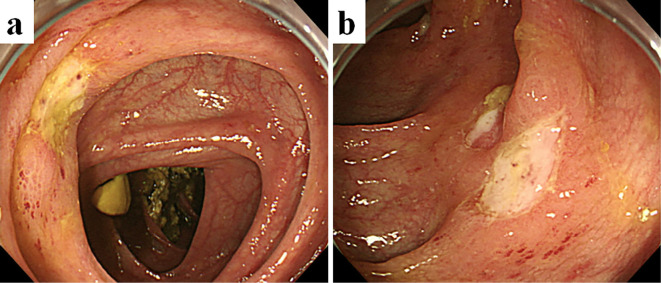
Figure 1.
Colonoscopic image after induction chemotherapy, revealing multiple active ulcers in the terminal ileum (a) and entire colon (b).
Figure 2.
The clinical course of the patient. All chromosomal and fluorescence in situ hybridization (FISH) analyses and percentages of blasts were analyzed using bone marrow cells. AML-MRC: acute myeloid leukemia with myelodysplasia-related changes, AraC: cytarabine, Aza: azacitidine, BMT: bone marrow transplantation, FLU: fludarabine, IDA: idarubicin, ivBU: intravenous busulfan, PSL: prednisolone
After making a diagnosis of AML-MRC, we searched for unrelated donors from the Japan Marrow Donor Program. Consolidation chemotherapy was initially planned; however, an intensive regimen would induce further mucosal injury and intestinal infection, thereby worsening his BD. Therefore, azacitidine (75 mg/m2/day), which has been reported to cause less mucosal injury, was administered for 7 days in a 4-week cycle. During azacitidine therapy, an attempt was made to reduce the prednisolone dose. However, when the dose was reduced to 50 mg, the patient suffered from melena. Therefore, because we had planned on administering tacrolimus for graft-versus-host disease (GVHD) prophylaxis after allogeneic HSCT and since tacrolimus was expected to have a dose-sparing effect on the steroid, we initiated oral tacrolimus to adjust the serum trough level from 10 to 15 ng/mL.
Two months later, prednisolone was tapered off completely without BD recurrence. Colonoscopy revealed complete disappearance of the ulcers. He also remained in complete remission for AML-MRC, and chromosomal and fluorescence in situ hybridization analyses revealed complete disappearance of trisomy 8 (Fig. 2). After 4 cycles of azacitidine therapy, the patient underwent bone marrow transplantation containing 2.4×108 nucleated cells/kg from a human leukocyte antigen-matched unrelated female donor. Fludarabine (30 mg/m2/day for 6 days) and busulfan (3.2 mg/kg/day for 4 days) were administered as the conditioning regimen. GVHD prophylaxis comprising of methotrexate and tacrolimus was administered consecutively. After the conditioning regimen, mild mucosal injury developed but improved, along with the recovery of the neutrophil count. Neutropenic fever occurred, but there was no apparent infection. Neither mild mucosal injury nor accelerated recovery of the neutrophil count by administration of granulocyte colony-stimulating factor induced BD recurrence. Engraftment was achieved with a neutrophil count above 0.5×109/L on Day 14, and complete chimerism was confirmed by fluorescence in situ hybridization of sex chromosomes on Day 28 after HSCT. No symptoms of acute GVHD developed.
Two months after HSCT, the patient developed arthritis as a symptom of chronic GVHD, which was successfully treated with 2.5 mg prednisolone and salazosulfapyridine. The dose was tapered off, and tacrolimus and prednisolone were discontinued at 14 and 17 months after HSCT, respectively. Moderate chronic GVHD involving the skin and liver recurred just after discontinuation of immunosuppressants; therefore, tacrolimus was readministered and continued. At forty-five months after HSCT, the patient is doing well, without any signs or symptoms of BD or AML-MRC.
Discussion
In the present case, at the diagnosis of AML-MRC, the patient's BD was stabilized using a low-dose steroid. However, his intestinal BD worsened during induction chemotherapy. Overproduction of inflammatory cytokines and abnormal neutrophil function are thought to be involved in the etiology of BD (2,3). Intensive chemotherapy or an intestinal infection can damage the recipient's tissues, thereby inducing production of inflammatory cytokines (11), which can cause BD recurrence (2). Therefore, to prevent worsening of his BD, we planned on treating the patient's AML-MRC while avoiding mucosal injury and an intestinal infection as much as possible. Azacitidine is a DNA hypomethylating agent with proven efficacy in treating AML (12). The adverse effects of azacitidine are acceptable in most patients, and mucosal injury is rare. The efficacy of maintenance treatment with azacitidine in patients with complete remission of AML has also been reported (13). Furthermore, pretransplantation azacitidine therapy has been reported to serve as an adequate bridge to allogeneic HSCT (14). Therefore, the patient received azacitidine as consolidation chemotherapy. Allogeneic HSCT is the only curative therapy for AML-MRC (15). Myeloablative conditioning regimens, especially those including total body irradiation, can cause serious mucosal injury (11). However, the combination of fludarabine and busulfan is an effective and well-tolerated myeloablative conditioning regimen that is associated with limited mucosal toxicity, a low transplant-related mortality rate, and antitumor activity comparable to other conventional regimens (16). Therefore, the current patient underwent allogeneic HSCT with this regimen.
The patient's worsened BD caused by the chemotherapy improved by increasing the steroid dose. However, a reduction of the steroid dose resulted in symptom recurrence, i.e., his BD seemed to be steroid-dependent. The administration of a high-dose steroid before or during HSCT can cause serious comorbidities, including transplant-related mortality. Tacrolimus has been used for prophylaxis and treatment of GVHD (17). Furthermore, a previous report showed that tacrolimus had therapeutic effects on intestinal BD that was refractory to other immunosuppressants (18). Therefore, in this case, tacrolimus was initiated before HSCT, which improved his BD and allowed discontinuation of the steroid. Tacrolimus was continued for the prevention and treatment of GVHD.
We conclude that allogeneic HSCT might be a potent therapeutic option for AML-associated BD. Furthermore, azacitidine and tacrolimus might be a suitable bridging regimen before HSCT. Further accumulation of clinical cases is therefore necessary to validate this conclusion.
Author's disclosure of potential Conflicts of Interest (COI).
Yukio Tanizawa: Honoraria, Astellas Pharma Inc.
Acknowledgement
The authors would like to thank all of the participants, physicians, and staff involved in this report.
References
- 1.Tada Y, Koarada S, Haruta Y, Mitamura M, Ohta A, Nagasawa K. The association of Behçet's disease with myelodysplastic syndrome in Japan: a review of the literature. Clin Exp Rheumatol 24: S115-S119, 2006. [PubMed] [Google Scholar]
- 2.Kawabata H, Sawaki T, Kawanami T, et al. Myelodysplastic syndrome complicated with inflammatory intestinal ulcers: significance of trisomy 8. Intern Med 45: 1309-1314, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Toyonaga T, Nakase H, Matsuura M, et al. Refractoriness of intestinal Behçet's disease with myelodysplastic syndrome involving trisomy 8 to medical therapies: our case experience and review of the literature. Digestion 88: 217-221, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Yamato K. Successful cord blood stem cell transplantation for myelodysplastic syndrome with Behçet disease. Int J Hematol 77: 82-85, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Tomonari A, Tojo A, Takahashi T, et al. Resolution of Behçet's disease after HLA-mismatched unrelated cord blood transplantation for myelodysplastic syndrome. Ann Hematol 83: 464-466, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Nonami A, Takenaka K, Sumida C, et al. Successful treatment of myelodysplastic syndrome (MDS)-related intestinal Behçet's disease by up-front cord blood transplantation. Intern Med 46: 1753-1756, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg OK, Seetharam M, Ren L, et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood 113: 1906-1908, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Lim SH, Hulsey M, Esler WV. Resolution of Behcet's disease after non-myeloablative allogeneic stem cell transplant for acute myeloid leukaemia. Rheumatology 48: 88-91, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet 335: 1078-1080, 1990. [PubMed] [Google Scholar]
- 10.Gül A. Pathogenesis of Behçet's disease: autoinflammatory features and beyond. Semin Immunopathol 37: 413-418, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 373: 1550-1561, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126: 291-299, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grövdal M, Karimi M, Khan R, et al. Maintenance treatment with azacytidine for patients with high-risk myelodysplastic syndromes (MDS) or acute myeloid leukaemia following MDS in complete remission after induction chemotherapy. Br J Haematol 150: 293-302, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Nishihori T, Perkins J, Mishra A, et al. Pretransplantation 5-azacitidine in high-risk myelodysplastic syndrome. Biol Blood Marrow Transplant 20: 776-780, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Ikegawa S, Doki N, Kurosawa S, et al. Allogeneic hematopoietic stem cell transplant overcomes poor prognosis of acute myeloid leukemia with myelodysplasia-related changes. Leuk Lymphoma 57: 76-80, 2016. [DOI] [PubMed] [Google Scholar]
- 16.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 104: 857-864, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Jamil MO, Mineishi S. State-of-the-art acute and chronic GVHD treatment. Int J Hematol 101: 452-466, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K, Nakase H, Chiba T. Efficacy of oral tacrolimus on intestinal Behcet's disease. Inflamm Bowel Dis 16: 188-189, 2010. [DOI] [PubMed] [Google Scholar]