Abstract
We report a case of reactive arthritis (ReA) triggered by Yersinia enterocolitica enteritis. A 24-year-old Japanese man developed polyarthritis in the lower limbs. Two weeks prior to these symptoms, he noted diarrhea, right lower abdominal pain and a fever. Y. enterocolitica was not isolated from a stool culture; however, he was diagnosed with ReA based on the colonoscopic findings of a high anti-Y. enterocolitica antibody titer and HLA-B27 antigen positivity. Following treatment with methotrexate and steroids, his arthritis improved. This is the first reported Japanese case of ReA in the English literature after a gastrointestinal infection caused by Y. enterocolitica.
Keywords: HLA-B27, reactive arthritis, Yersinia enterocolitica enteritis
Introduction
Reactive arthritis (ReA) is a non-purulent joint inflammation that can be triggered by bacterial infections in the urogenital tract or gut and genetic factors, such as human leucocyte antigen (HLA)-B27 (1). Yersinia enterocolitica is a well-established trigger of ReA (2), and several case reports of ReA triggered by Y. enterocolitica have been reported in Western countries (3,4). However, no Japanese cases of Y. enterocolitica triggered by ReA have been reported. To our knowledge, the present report describes the first Japanese patient with ReA that developed two weeks after the onset of acute Y. enterocolitica enteritis.
Case Report
A 24-year-old Japanese man presented with a 2-week of history of a fever, central abdominal pain and frequent bowel movements without blood, and the pain shifted to his right iliac region. Based on these findings, he had been diagnosed with acute enteritis and treated with an anti-diarrheal drug. Two weeks later, he presented with polyarthritis of the left knee and left ankle joints and was referred to our hospital. On admission, his body temperature was 37.5℃, pulse rate was regular at 78 beats/min and blood pressure was 120/78 mmHg. He had arthralgia in the left swollen knee and ankle joints. He also had arthralgia in the bilateral sacroiliac joints. The laboratory findings at that time were as follows: hemoglobin was 13.3 g/dL, white blood count was 10,300 /μL, erythrocyte sedimentation rate was 51 mm/h, and C-reactive protein was 14.1 mg/dL. The findings from liver and renal function tests were normal. Anti-citrullinated peptide antibody and antinuclear antibody tests were negative. Urinalysis revealed no abnormal findings (Table).
Table.
Laboratory Findings.
| Peripheral blood | Blood glucose test | ||
| Red blood cells | 450×104/μL | Glucose | 101mg/dL (80-112) |
| Hemoglobin | 13.3g/dL | HbA1c | 5.7% (4.6-6.2) |
| White blood cells | 10,300/μL | Immunological test | |
| Neut | 80.9% | IgA | 264mg/dL (110-410) |
| Ly | 11.9% | IgG | 1,210mg/dL (870-1700) |
| Eo | 1.0% | IgM | 157mg/dL (33-190) |
| Ba | 0.3% | C3 | 154mg/dL (86-160) |
| Mo | 5.9% | C4 | 26mg/dL (17-45) |
| Platlet | 22.4×104/μL | CH50 | 45.4U/mL (30-50) |
| Erythrocyte sedimentation rate | 51mm/hr | Rheumatoid factor | (-) |
| Blood chemistry | ASLO | (-) | |
| Total protein | 7.0g/dL (6.7-8.3) | ANA | (-) |
| Albumin | 3.4g/dL (4.0-5.0) | MMP-3 | 171.7ng/mL (36.9-121) |
| Total bilirubin | 0.6mg/dL (0.3-1.2) | Anti-citrullinated peptide antibody | (-) |
| Glutamic-oxaloacetic transaminase | 20 IU/L (13-33) | Anti-SS-A antibody | (-) |
| Glutamic-pyruvic transaminase | 36 IU/L (8-42) | Anti-SS-B antibody | (-) |
| Lactate dehydrogenase | 187 IU/L (119-229) | Proteinase-3 anti-neutrophil cytoplasmic antibody | (-) |
| Alkaline phosphatase | 338 IU/L (115-359) | Myeloperoxidase anti-neutrophil cytoplasmic antibody | (-) |
| γ-glutamyltransferase | 80 IU/L (10-47) | Yersinia enterocolitica antibody | 1:5120 (<1:20) |
| Creatinine kinase | 46 IU (62-287) | (Quantitative Agglutination Test) | |
| Total cholesterol | 182mg/dL (128-220) | Genetic test | |
| Blood urea nitrogen | 14mg/dL (8-22) | Human Leukocyte Antigen | A2 A24 |
| Creatinine | 1.0mg/dL (0.6-1.1) | B27 B60 | |
| Na | 137mEq/L (138-146) | Urinalysis | |
| K | 4.5mEq/L (3.6-4.9) | Uric protein | (-) |
| Cl | 103mEq/L (99-109) | Occult blood | (-) |
| Uric acid | 5.8mg/dL (3.6-7.0) | Glucose | (-) |
| Ca | 8.9mg/dL (8.7-10.3) | Stool culture | |
| Triglyceride | 68mg/dL (30-150) | Escherichia coli | (-) |
| C-reactive protein | 14.12mg/dL (<0.3) | Acid-fast bacilli | (-) |
| Ferritin | 558ng/mL (20-250) |
ASLO: anti-streptolisyn-O, ANA: anti-nuclear antibody, MMP-3: Matrix Metalloproteinase-3
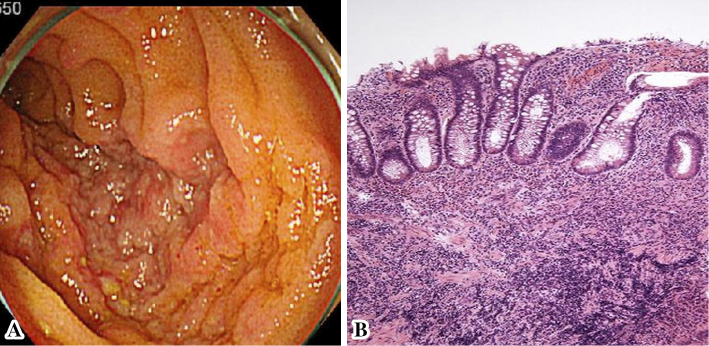
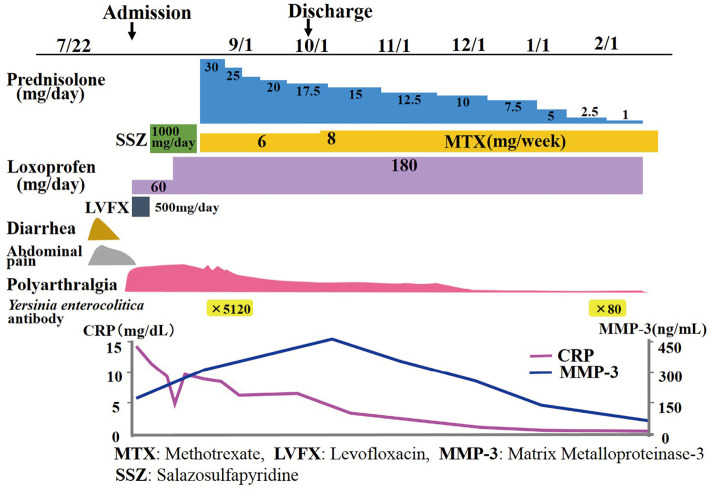
Abdominal computed tomography demonstrated inflammatory changes in the terminal ileum with enlarged regional mesenteric lymph modes (Fig. 1). Colonoscopy showed marked swelling in the mucosa with small ulcerations in the terminal ileum (Fig. 2A). A pathological examination demonstrated inflammatory infiltrates in the lamina propria with crypt abscess (Fig. 2B). A stool culture for Y. enterocolitica was negative; however, antibodies for Y. enterocolitica were positive with elevated titers (×5,120; normal range <×20). Serotyping of HLA class 1 was positive for B27. Y. enterocolitica-triggered ReA was subsequently diagnosed, and he was treated with levofloxacin (500 mg/day) for 7 days. His polyarthralgia did not improve; therefore, sulfasalazine (SSZ) treatment (1,000 mg/day) was started. However, the polyarthralgia was sustained; therefore, methotrexate (MTX; 8 mg/week) combined with steroid treatments was started. The patient's condition subsequently improved, and his elevated erythrocyte sedimentation rate and C-reactive protein level normalized under tapered steroid therapy plus MTX (8 mg/week) (Fig. 3).
Figure 1.
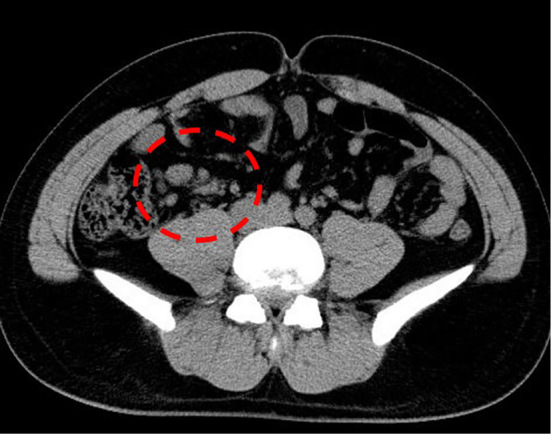
Abdominal computed tomography showing regional mesenteric lymphadenopathy.
Figure 2.
A: Colonoscopic photographs showing multiple mucosal aphthae and edematous changes in the ileocecal mucosa. B: A histopathological examination of the ileocecal mucosa showing inflammatory cell infiltration and crypt abscess.
Figure 3.
Clinical course.
Discussion
ReA is a type of sterile synovitis that occurs after a gastrointestinal or urogenital infection and is characterized by asymmetric arthritis predominantly affecting the lower limbs (5). The present patient developed Y. enterocolitica enteritis, followed by asymmetrical polyarthritis in the legs. In Y. enterocolitica infection, pseudoappendicitis, terminal ileitis, and mesenteric lymphadenopathy caused by Y. enterocolitica (6) should be kept in mind for patients presenting with symptoms resembling appendicitis. The present case had these clinical manifestations.
Enteropathogenic Yersinia spp. are the most frequent causative agents of human diarrhea in developed countries (7). However, the incidence of yersiniosis is largely underestimated, due to difficulty in isolating Yersinia from poly-contaminated stool cultures (8). Serology as a diagnostic tool for Y. enterocolitica infection has become more refined through the use of purified Yersinia outer membrane proteins (9).
The possibility that acute enteritis in the present case was caused by other enteropathic microorganisms could not be ruled out, Y. enterocolitica infection, at least in part, contributes to the occurrence of acute enteritis according to the serological findings.
In a study concerning ReA in German rheumatology clinics, as the causative enteric bacteria, Salmonella 11/33 (33%) and Yersinia 6/33 (18%) were isolated in patients with enteric ReA (10). Therefore, the frequency of ReA in adults after Yersinia enteric infection may not be unusual in Western countries. However, the frequency of HLA-B27 positivity is lower in Japan than in Western counties (11); thus, Yersinia-triggered ReA may be overlooked in Japanese patients. HLA-B27 has been shown to be a useful prognostic marker of ReA, and patients positive for HLA-B27 antigens are more likely to develop chronic or severe arthritis than those negative for it (12). HLA-B27-positive individuals are also thought to be less efficient at eliminating intracellular enteric bacteria, including Yersinia, than HLA-B27-negative individuals (13). The present patient exhibited HLA-B27 positivity, which may have contributed to the development of ReA following the failure to eliminate intracellular organisms or the presentation of arthrogenic peptides. Whether or not to use antibiotics in ReA is controversial. Clinical and experimental studies have clearly demonstrated that early and vigorous treatment of a triggering infection before the development of ReA is effective (14). However, in an experimental model of ReA induced by intravenous application Yersinia enterocolitica into rat, ciprofloxacin treatment after inoculation of the microbe, a definite effect was seen but later administration of ciprofloxacin had no effect (15,16). Therefore, the routine use of antibiotics to treat ReA has not been established.
In chronic and severe ReA, disease-modifying antirheumatic drugs (DMARDs) are recommended for treatment (17). The most frequently used DMARD is SSZ, which has limited effectiveness in patients with ReA (18). Another DMARD is MTX, which may be used as an alternative to SSZ in patients who are intolerant to SSZ (19). The systemic use of corticosteroids is not indicated for cases of severe joint involvement, except in short courses (1). Corticosteroids are a potent group of drugs for treating ReA. The drug should therefore be continued for a brief period in low dose and stopped gradually. If the patient later suffers from chronic arthralgia, corticosteroids should be avoided altogether. In these instances, corticosteroids have a poor therapeutic effect and cause more harm than benefit (20). Because the present patient had sustained active arthritis and was intolerant to SSZ, he was treated with prednisolone (30 mg/day) plus MTX (8 mg/week), and the steroids were subsequently tapered after his symptoms improved.
In conclusion, we described, to our knowledge, the first case of a Japanese patient with ReA that developed after acute Y. enterocolitica enteritis. Physicians should consider Y. enterocolitica infection in the differential diagnosis of patients with symptoms resembling appendicitis as well as ReA.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Flores D, Marquez J, Garza M, Espinoza LR. Reactive arthritis: newer developments. Rheum Dis Clin North Am 29: 37-59, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Granfors K, Jalkanen S, von Essen R, et al. . Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med 320: 216-221, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Aho K. Yersinia reactive arthritis. Br J Rheumatol 22: 41-45, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Foley JA, Mathews JA. Reactive arthritis due to Yersinia enterocolitica. Clin Rheumatol 3: 385-387, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol 25: 347-357, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Perdikogianni C, Galanakis E, Michalakis M, et al. . Yersinia enterocolitica infection mimicking surgical conditions. Pediatr Surg Int 22: 589-592, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Rosner BM, Stark K, Werber D. Epidemiology of reported Yersinia enterocolitica infections in Germany, 2001-2008. BMC Public Health 10: 337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savin C, Leclercq A, Carniel E. Evaluation of a single procedure allowing the isolation of enteropathogenic Yersinia along with other bacterial enteropathogens from human stools. PLoS One 7: e41176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogkamp-Korstanje JA, de Koning J, Samsom JP. Incidence of human infection with Yersinia enterocolitica serotypes O3, O8, and O9 and the use of indirect immunofluorescence in diagnosis. J Infect Dis 153: 138-141, 1986. [DOI] [PubMed] [Google Scholar]
- 10.Fendler C, Laitko S, Sörensen H, et al. . Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann Rheum Dis 60: 337-343, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan NJ. HLA-B27: what's new? Rheumatology (Oxford) 49: 621-631, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Yu D, Kuipers JG. Role of bacteria and HLA-B27 in the pathogenesis of reactive arthritis. Rheum Dis Clin North Am 29: 21-36, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Rihl M, Klos A, Köhler L, Kuipers JG. Infection and musculoskeletal conditions: Reactive arthritis. Best Pract Res Clin Rheumatol 20: 1119-1137, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Sieper J, Braun J. Treatment of reactive arthritis with antibiotics. Br J Rheumatol 37: 717-720, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Gripenberg-Lerche C, Söderström KO, Toivanen A, Toivanen P. Antibiotic prophylaxis and treatment of reactive arthritis. Lessons from an animal model. Arthritis Rheum 39: 1238-1243, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Toivanen A, Toivanen P. Experimental Yersinia-triggered reactive arthritis; effect of a 3-week course with ciprofloxacin. Br J Rheumatol 36: 541-546, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Palazzi C, Olivieri I, D'Amico E, Pennese E, Petricca A. Management of reactive arthritis. Expert Opin Pharmacother 5: 61-70, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Clegg DO, Reda DJ, Weisman MH, et al. . Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter's syndrome). A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 39: 2021-2027, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher HR., Jr Reactive arthritis. Rheum Dis Clin North Am 24: 261-273, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Toivanen A. Managing reactive arthritis. Rheumatology (Oxford) 39: 117-119, 2000. [DOI] [PubMed] [Google Scholar]