Abstract
Thrombocytopenia, Anasarca, Fever, Reticulin fibrosis/Renal failure, and Organomegaly (TAFRO) syndrome is a recently described systemic inflammatory disorder characterized by thrombocytopenia, anasarca, fever, reticulin fibrosis/renal failure, and organomegaly. It has an acute or subacute onset of unknown etiology, although some pathological features resemble those of multicentric Castleman disease. We here report two cases of TAFRO syndrome. The symptoms and pathological findings in these cases met the 2015 diagnostic criteria. Our cases showed high serum procalcitonin levels, suggesting bacterial infection as an onset trigger. In addition, Case 1 is the first case complicated with adrenal hemorrhaging. Case 2 is the second case of tocilizumab-resistant TAFRO syndrome successfully treated with rituximab.
Keywords: adrenal hemorrhage, Castleman disease, procalcitonin, rituximab, TAFRO syndrome
Introduction
Castleman disease (CD) is an uncommon lymphoproliferative disorder characterized by overexpression of cytokines, particularly interleukin-6 (IL-6) (1). CD can present as two distinct clinical entities: the localized form and the multicentric form, multicentric CD (MCD). In addition to the two clinical subtypes of CD, three histological variants have been recognized: hyaline-vascular type, plasma cell type, and mixed type (2).
In 2010, Takai et al. (3) reported the first three Japanese cases of thrombocytopenia, anasarca, fever, reticulin fibrosis/renal failure, and organomegaly (TAFRO) syndrome, a disorder related to MCD. Thereafter, several meetings held in Japan evaluated the diagnosis and treatment of TAFRO syndrome (4). This syndrome is characterized by thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, and organomegaly. TAFRO syndrome has recently been described in an increasing number of case reports or series (3-25). A careful diagnosis is needed to distinguish TAFRO syndrome from various diseases including viral infections such as human immunodeficiency virus (HIV) infection, rheumatic diseases such as systemic lupus erythematosus, IgG4-related disease, POEMS syndrome (polyneuropathy, organomegaly, endocrine diseases, M-protein, and skin lesions), and malignant lymphoma (20). For the treatment of TAFRO syndrome, immunosuppressive drugs including high-dose corticosteroids and cyclosporin A have been used (20). Tocilizumab (anti-IL-6 receptor antibody) (7,9,10,12,14,15,17,19-22,24,25) and rituximab (anti-CD20 antibody) (7,11,15-17,19,20) have also been used. However, despite these treatments, some patients exhibit a rapidly deteriorating clinical course and died (4). The All Japan TAFRO Syndrome Research Group recently proposed updated diagnostic criteria, disease severity classification, and treatment strategies for TAFRO syndrome (26).
We herein report two cases of TAFRO syndrome with previously unreported findings of high serum procalcitonin (PCT) levels. In addition, Case 1 presented with a previously unreported complication in TAFRO syndrome; adrenal hemorrhaging. Case 2 is the second case of tocilizumab-resistant TAFRO syndrome successfully treated with rituximab.
Case Reports
Case 1
A 48-year-old Japanese man with a 2-month history of general fatigue developed right back pain and edema. He was admitted to a local hospital because of a high level of serum C-reactive protein (CRP) (28.9 mg/dL) and hypoalbuminemia (1.7 g/dL). Soon after admission, he developed a high fever, difficulty of breathing, and oliguria. A computed tomography (CT) scan showed right adrenal hemorrhaging and bilateral thickening of the Gerota's fascia. After treatment with antibiotics and intravenous hydrocortisone, he was referred to our hospital. At that time, the platelet (PLT) count was 61,000 /μL, serum albumin (Alb) 1.5 g/dL, creatinine (Cr) 1.16 mg/dL, estimated glomerular filtration rate (eGFR) 54.3 mL/min/1.73 m2, Na 131 mEq/L, CRP 38.6 mg/dL, IgG4 44.1 mg/dL, and PCT 8.09 ng/mL (normal range, <0.5). Anti-cardiolipin (CL) antibodies, anti-CL/β2-glycoptotein 1 (β2GP1) antibodies, lupus anticoagulants, myeloperoxidase anti-neutrophil cytoplasmic antibodies (MPO-ANCA), and proteinase 3 anti-neutrophil cytoplasmic antibodies (PR3-ANCA) were negative. Urine culture and repeated blood cultures were negative. A CT scan showed bilateral pleural effusion, hepatosplenomegaly, right adrenal hemorrhaging, and systemic lymphadenopathy (Fig. 1A-C). He was treated with antibiotics, intravenous steroids, Alb infusion, and diuretics. His symptoms gradually improved. One month later, he was referred to our department for further examinations.
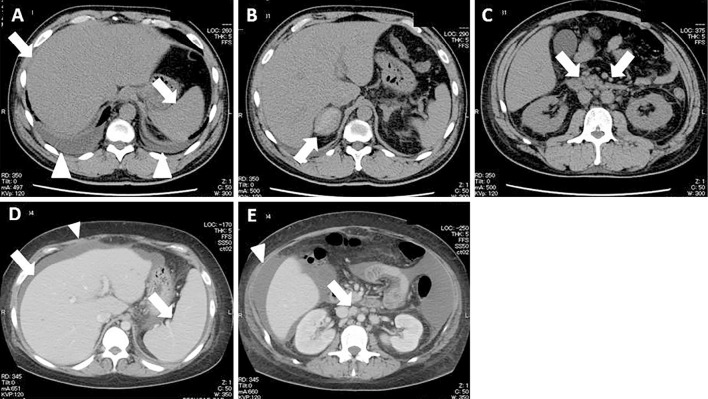
Figure 1.
The CT findings of Case 1 (A-C) and Case 2 (D and E) on admission. An abdominal CT scan shows hepatosplenomegaly (arrows) and bilateral pleural effusion (arrow heads) (A), right adrenal hemorrhaging (arrow) (B), and multiple lymphadenopathy (arrows) (C). An enhanced abdominal CT scan shows hepatosplenomegaly (arrows) and ascites (arrow head) (D), and multiple lymphadenopathy (arrow) and ascites (arrow head) (E).
At the presentation, his body temperature was 36.7℃. A physical examination showed cervical lymph node swelling and edema. A urinalysis showed hematuria (50-99 red blood cells/high power field) without proteinuria. His white blood cell (WBC) count was 14,800 /μL, hemoglobin (Hb) 9.8 g/dL, PLT count 36,000 /μL, activated partial thromboplastin time (APTT) 29.5 sec, prothrombin time-international normalized ratio (PT-INR) 1.23, fibrinogen (Fib) 305.0 mg/dL, fibrinogen degradation product (FDP) 28.0 μg/mL, and D-dimer 12.52 μg/mL. Serum total protein (TP) was 5.1 g/dL, Alb 2.6 g/dL, blood urea nitrogen (BUN) 20.0 mg/dL, Cr 0.42 mg/dL, aspartate transaminase (AST) 89 U/L, alanine transaminase (ALT) 132 U/L, alkaline phosphatase (ALP) 684 U/L, lactate dehydrogenase (LDH) 226 U/L, γ-glutamyl transpeptidase (γ-GTP) 412 U/L, total bilirubin (T. Bil) l.4 mg/dL, Na 139 mEq/L, K 2.5 mEq/L, and Cl 89 mEq/L. Serum CRP was 0.85 mg/dL, antinuclear antibodies (ANA) <20, IgG 1,301 mg/dL, IgA 162 mg/dL, IgM 41 mg/dL, C3 118 mg/dL, C4 27 mg/dL, CH50 60 U/mL, and IL-6 3.8 pg/mL (normal range, <4.0). Serum and urinary protein electrophoresis showed no monoclonal paraproteins. Blood culture and urine culture were negative. There was no evidence of infections caused by hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 8 (HHV-8), HIV, human T-lymphotropic virus (HTLV)-1, toxoplasma, Aspergillus species, or Mycobacterium tuberculosis.
A cervical lymph node biopsy showed atrophic germinal centers with reactive interfollicular plasma cell proliferation (Fig. 2A and B). Immunostaining for CD21 showed follicular dendritic cell proliferation (Fig. 2C). Bone marrow aspiration and a bone marrow biopsy revealed normocellular bone marrow with mild fibrosis without atypical cells (Fig. 2D). On the basis of these pathological findings and symptoms (thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, and organomegaly), he was diagnosed with TAFRO syndrome.
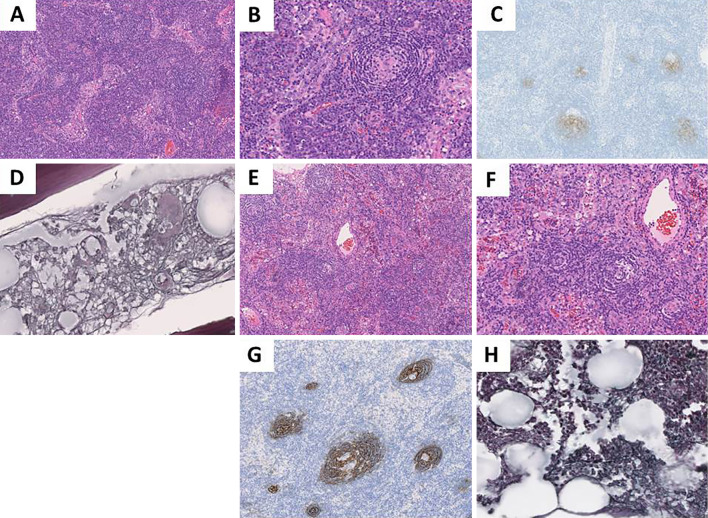
Figure 2.
The pathological findings of cervical lymph nodes and bone marrow of Case 1 (A-D) and Case 2 (E-H). Lymph node biopsy specimens show atrophic germinal centers and interfollicular plasma cell proliferation (A: ×100, B: ×200,), and atrophic germinal center (E: ×100, F: ×200). Hematoxylin-Eosin staining. Lymph node biopsy specimens show CD21+follicular dendritic cell proliferation (C and G: ×100). CD21 immunostaining. Bone marrow biopsy specimens show mild fibrosis (D and H: ×400). Silver staining.
On day 22 after admission to our department, oral prednisolone (PSL: 50 mg/day) therapy was started instead of intravenous steroids, and the dose of PSL was gradually reduced to 30 mg/day. On day 46 after admission, a CT scan showed that the pleural and abdominal fluids had disappeared, the adrenal hematoma had improved, and systemic lymphadenopathy was diminished. His PCT level had decreased to 0.56 ng/mL. He was discharged on day 67. At that time, the PLT count was 260,000 /μL, serum Alb 4.2 g/dL, Cr 0.51 mg/dL, and CRP 0.11 mg/dL.
Case 2
A 27-year-old Japanese woman was admitted to another hospital because of a fever and abdominal pain and distension. On admission, the PLT count was 82,000 /μL, serum Alb 3.0 g/dL, Cr 0.59 mg/dL, and CRP 27.83 mg/dL. She was diagnosed with pelvic inflammatory disease because an ultrasound examination showed ascites. Although she was treated with antibiotics, ascitic fluid accumulated rapidly. She also showed difficulty breathing due to the development of bilateral pleural effusion. Since her anasarca was not controlled, temporal mechanical ventilation, continuous hemodiafiltration, and extracorporeal ultrafiltration were required. A CT scan showed pleural and abdominal fluids, hepatosplenomegaly, and systemic lymphadenopathy. ANA, anti-CL antibodies, anti-CL/β2GP1 antibodies, MPO-ANCA, and PR3-ANCA were negative. The serum levels of IL-6 and vascular endothelial growth factor (VEGF) were 57.5 pg/mL and 534 pg/mL (normal range: <115), respectively. She was considered to have a MCD-like disease based on the pathological findings of a cervical lymph node biopsy. Eleven days after admission, the PLT count was 36,000 /μL, serum Alb 2.0 g/dL, Cr 2.31 g/dL, eGFR 22.3 mL/min/1.73 m2, CRP 27.64 mg/dL, and PCT 8.28 ng/mL. There was no evidence of infections caused by HBV, HCV, or HIV. She was treated with methylprednisolone (mPSL) pulse therapy (1,000 mg/day for 3 days) followed by therapies with a decreasing dose of oral PSL, repeated administration of tocilizumab (8 mg/kg, every 4-6 weeks, 8 infusions), and cyclosporin A. Although her condition gradually improved, she developed thrombocytopenia again when PSL dosage was tapered (2.5 mg/day). Thereafter, she was treated with 35 mg/day of PSL, and was referred to our hospital near her family home. One week after the previous hospital discharge, she developed a high fever and abdominal pain, and visited our outpatient clinic. She was admitted to our department due to high levels of serum CRP (20.0 mg/dL).
At the presentation, her body temperature was 37.4℃. A physical examination showed cervical lymphadenopathy, abdominal distension with undulation and tenderness, and systemic edema. Urinalysis showed proteinuria (0.8 g/gCr) without hematuria. The WBC count was 17,500 /μL, Hb 14.7 g/dL, PLT count 54,000 /μL, APTT 30.5 sec, PT-INR 1.20, Fib 667.0 mg/dL, FDP 22.6 μg/mL, and D-dimer 6.63 μg/mL. TP was 5.3 g/dL, Alb 2.6 g/dL, BUN 29.6 mg/dL, Cr 1.00 mg/dL, eGFR 55.7 mL/min/1.73 m2, AST 24 U/L, ALT42 U/L, ALP 635 U/L, LDH 180 U/L, γ-GTP 261 U/L, and T. Bil 0.7 mg/dL. Serum PCT was 4.24 ng/mL (4 days later, 5.04 ng/mL), ANA <20, IgG 598 mg/dL, IgA 166 mg/dL, IgM 48 mg/dL, C3 109 mg/dL, C4 34 mg/dL, and CH50 56 U/mL. There was no evidence of infections caused by HBV, HCV, CMV, EBV, HHV-8, or Aspergillus species. Cultures of urine, blood, and ascitic fluid were negative. Serum and urinary protein electrophoresis showed no monoclonal paraproteins.
A CT scan showed pleural and abdominal fluid, hepatosplenomegaly, and systemic lymphadenopathy (Fig. 1D and E). A cervical lymph node biopsy showed atrophic germinal centers (Fig. 2E and F). Immunostaining for CD21 showed follicular dendritic cell proliferation (Fig. 2G). There was no pathological evidence of B cell lymphoma. Bone marrow aspiration and a bone marrow biopsy revealed slight fibrosis without atypical cells (Fig. 2H). On the basis of these pathological findings and symptoms (thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, and organomegaly), she was ultimately diagnosed with TAFRO syndrome.
She was initially diagnosed with peritonitis and sepsis and was treated with antibiotics. Her fever and abdominal pain improved immediately, and her serum CRP levels decreased gradually. The PCT level also decreased to 3.84 ng/mL 10 days after taking antibiotics. She was treated with diuretics for massive ascites, and her ascites gradually improved. Two weeks after starting treatment with antibiotics, she was treated with intravenous tocilizumab (8 mg/kg, every 2 weeks, 2 infusions), and the steroid dose was tapered (mPSL 24 mg/day). Her general condition improved and she was discharged on day 41 after admission. At that time, the PLT count was 54,000 /μL, serum Cr 0.44 mg/dL, and CRP 0.04 mg/dL.
Although treatment with tocilizumab was continued at an outpatient clinic, she developed abdominal pain 2 months after discharge. At that time, the PLT count had decreased to 13,000 /μL. She was admitted to our hospital again and treated with rituximab (375 mg/m2, every 3 weeks, 3 infusions) 1 week after mPSL pulse therapy (500 mg/day for 3 days) because tocilizumab therapy could not control her disease progression. She responded well to rituximab therapy, and was discharged on day 49 after admission. At that time, the PLT count was 82,000 /μL, serum Alb 4.1 g/dL, Cr 0.49 mg/dL, and CRP 0.03 mg/dL. One month after discharge, she was well, and the PLT count was 125,000 /μL.
Discussion
According to the 2015 diagnostic criteria for TAFRO syndrome (26), this syndrome is now defined as a systemic inflammatory disorder characterized by thrombocytopenia, anasarca including pleural effusion and ascites, a fever, renal insufficiency, and organomegaly, including hepatosplenomegaly and lymphadenopathy. The pathological findings include MCD-like features on a lymph node biopsy and reticulin myelofibrosis in the bone marrow. The symptoms and pathological findings in our two cases met the 2015 diagnostic criteria for TAFRO syndrome, and the disease severity was regarded as “slightly severe” in Case 1 and “severe” in Case 2. These cases have interesting clinical features: both patients had high levels of serum PCT on admission, Case 1 was the first case of TAFRO syndrome complicated with adrenal hemorrhaging, and Case 2 was refractory to high-dose corticosteroid and tocilizumab therapies but successfully treated with rituximab.
Since patients with adrenal hemorrhaging often present with nonspecific signs and symptoms, the diagnosis in most patients is confirmed on an autopsy (27). Adrenal hemorrhaging may be caused by several factors including infection, congestive heart failure, anticoagulants, trauma, and antiphospholipid syndrome, and undermined causative factors (27-29). There are several reported cases of MCD associated with adrenal tumors (30-32). Debatin et al. (30) reported that a tumor of MCD may have originated from the lymphoid tissue in the adrenal periphery and extended into the adrenal gland itself. In our Case 1, no adrenal tumor was found, but right adrenal hemorrhaging with bilateral thickening of the Gerota's fascia, suggestive of retroperitoneal inflammation, was observed.
To our knowledge, there have been no previous reports describing the serum PCT levels in patients with TAFRO syndrome. PCT seems to be the most helpful laboratory marker for discriminating an infectious fever from a non-infectious one (33) and is a promising marker for the identification of bacterial infections (34). We also reported on the usefulness of serum PCT levels for distinguishing active MPO-ANCA-associated disease and invasive infections (35). Cases 1 and 2 had a high fever and high serum PCT levels in the early clinical course. Although cultures of urine, blood, and ascitic fluid did not reveal any bacterial infections, these patients may have had some bacterial infections that triggered the onset. We showed that measurement of the serum PCT levels was necessary in suspected cases of TAFRO syndrome during the febrile phase. Accumulation of cases is needed to clarify the clinical significance of the elevated levels of serum PCT in the pathogenesis of TAFRO syndrome.
While the pathogenesis of CD is still not fully understood, the central roles of IL-6 and HHV-8 in MCD have been well described (1). However, the majority of Japanese patients with MCD and TAFRO syndrome are negative for HHV-8 (20,26), as in our cases. In the largest TAFRO syndrome case series, including 23 Japanese patients (20), the median serum IL-6 levels were 16.2 pg/mL (range: 6.0-67.3) (normal: <5.0 in this report). Several patients were treated with tocilizumab, but some responded (serum IL-6 levels: 20.2-45.6 pg/mL) while others did not (serum IL-6 levels: 6.0-67.3 pg/mL). These findings suggested that IL-6 may not be the primary pathological cytokine (20). Konishi et al. (15) also suggested the involvement of an IL-2-dependent pathway in addition to IL-6-related pathways in the etiology of TAFRO syndrome, based on their clinical experience.
Although no standard treatment for TAFRO syndrome has yet been established, most patients are treated with corticosteroids, including pulse-therapy as a first-line therapy, and most cases with a poor response to corticosteroids are then treated with cyclosporin A, tocilizumab, and rituximab (3-25). Case 1 with “slightly severe disease activity” was successfully treated with steroids. Case 2 with “severe disease activity” received treatments with tocilizumab and cyclosporin A because of a poor response to mPSL pulse therapy. This patient became resistant to tocilizumab therapy, and was further treated with rituximab. Thereafter, the patient had remarkable clinical improvement and achieved remission.
Rituximab has been increasingly used to treat most chronic B-cell lymphoproliferative disorders, including MCD (2). In the 2015 treatment strategy for TAFRO syndrome (26), treatments with high-dose glucocorticoids, cyclosporin A, tocilizumab, and rituximab are recommended, based on the clinical experiences of Japanese research teams. In previous reports describing the clinical courses of six TAFRO syndrome cases (7,11,15-17,19), treatment with rituximab was effective, except in one case (15). To our knowledge, our Case 2 is the second case of tocilizumab-resistant TAFRO syndrome successfully treated with rituximab (19). According to 16 months of follow-up observations in a previously reported case (16), maintenance therapy with rituximab appears to be effective and safe. Therefore, early use of rituximab should be considered when TAFRO syndrome patients with “severe disease activity” do not respond well to high-dose glucocorticoids, cyclosporin A, or tocilizumab.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Soumerai JD, Sohani AR, Abramson JS. Diagnosis and management of Castleman disease. Cancer Control 21: 266-278, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Casper C, Teltsch DY, Robinson D Jr, et al. . Clinical characteristics and healthcare utilization of patients with multicentric Castleman disease. Br J Haematol 168: 82-93, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsyo Ketsueki 51: 320-325, 2010(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 4.Kawabata H, Takai K, Kojima M, et al. . Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop 53: 57-61, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N, Saeki S. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly: a clinical report five cases. J Clin Exp Hematop 53: 63-68, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Masaki Y, Nakajima A, Iwao H, et al. . Japanese variant of multicentric Castleman's disease associated with serositis and thrombocytopenia - a report of two cases: Is TAFRO syndrome (Castleman-Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop 53: 79-85, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Iwaki N, Sato Y, Tanaka K, et al. . Atypical hyaline vascular-type Castleman's disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J Clin Exp Hematop 53: 87-93, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A: a case report. J Clin Exp Hematop 53: 95-99, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Awano N, Inomata M, Sonoda Y, et al. . A case of multicentric Castleman' disease of mixed-type, which showed constellation of symptoms, i.e., thrombocytopenia, anasarca, anemia, fever, myelofibrosis, and lymphadenopathy. J Clin Exp Hematop 53: 101-105, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Kawabata H, Kotani S, Matsumura Y, et al. . Successful treatment of a patient with multicentric Castleman's disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody. Intern Med 52: 1503-1507, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa T, Kosugi S, Kito M, et al. . Efficacy of rituximab for a variant type of multicentric Castleman's disease termed the TAFRO syndrome. Rinsyo Ketsueki 55: 350-355, 2014(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 12.Kubokawa I, Yachie A, Hayakawa A, et al. . The first report of adolescent TAFRO syndrome, a unique clinicopathologic variant of multicentric Castleman's disease. BMC Pediatrics 14: 139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koduri PR, Parvez M, Kaza S, Pappu P, Anuradha S. Castleman-Kojima disease in a south Asian adolescent. J Clin Exp Hematop 54: 163-166, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Tatekawa S, Umemura K, Fukuyama R, et al. . Thalidomide for tocilizumab-resistant ascites TAFRO syndrome. Clin Case Rep 3: 472-478, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konishi Y, Takahashi S, Nishi K, et al. . Successful treatment of TAFRO syndrome, a variant of multicentric Castleman's disease, with cyclosporine A: possible pathogenetic contribution of interleukin-2. Tohoku J Exp Med 236: 289-295, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Jain P, Verstovsek S, Loghavi S, et al. . Durable remission with rituximab in a patient with an usual variant of Castleman's disease with myelofibrosis - TAFRO syndrome. Am J Hematol 90: 1091-1092, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedesco S, Postacchini L, Manfredi L, et al. . Successful treatment of a Caucasian case of multifocal Castleman's disease with TAFRO syndrome with a pathophysiology targeted therapy - a case report. Exp Hematol Oncol 4: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allegra A, Rotondo F, Russo S, Calabrò L, Maisano V. Castleman-Kojima disease (TAFRO syndrome) in a Caucasian patient: a rare case report and review of the literature. Blood Cells Mol Dis 55: 206-207, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Nagai T, Maemori M, Ando S, et al. . Successful treatment with rituximab in tocilizumab-resistant TAFRO syndrome (translation of Japanese title). Rinsyo Ketsueki 56: 62, 2015(in Japanese, Abstract in English). [Google Scholar]
- 20.Iwaki N, Fajgenbaum DC, Nabel CS, et al. . Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 91: 220-226, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Yamaga Y, Tokuyama K, Kato T, et al. . Successful treatment with cyclosporin A in tocilizumab-resistant TAFRO syndrome. Intern Med 55: 185-190, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Jouvray M, Terriou L, Meignin V, et al. . Pseudo-adult Still's disease, anasarca, thrombotic thrombocytopenic purpura and dysautonomia: an atypical presentation of multicentric Castleman's disease. Discussion of TAFRO syndrome. Rev Med Interne 37: 53-57, 2016(in French, Abctract in English). [DOI] [PubMed] [Google Scholar]
- 23.Kawashima M, Usui T, Okada H, et al. . TAFRO syndrome: 2 cases and review of the literature. Mod Rheumatol Jul 20: 1-5, 2015(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara S, Mochinaga H, Nakata H, et al. . Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol 103: 718-723, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: a case report and systematic review. Mod Rheumatol Feb 17: 1-6, 2016(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 26.Masaki Y, Kawabata H, Takai K, et al. . Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Xarli VP, Steele AA, Davis PJ, Buescher ES, Rios CN, Garcia-Bunuel R. Adrenal hemorrhage in the adult. Medicine (Baltimore) 57: 211-221, 1978. [DOI] [PubMed] [Google Scholar]
- 28.Fujishima N, Komatsuda A, Ohyagi H, et al. . Adrenal insufficiency complicated with antiphospholipid syndrome (APS). Intern Med 45: 963-966, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Dhawan N, Bodukam VK, Thakur K, Singh A, Jenkins D, Bahl J. Idiopathic bilateral adrenal hemorrhage in a 63-year-old male: a case report and review of the literature. Case Rep Urol 2015: 503638, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debatin JF, Spritzer CE, Dunnick NR. Castleman disease of the adrenal gland: MR imaging features. Am J Rent 157: 781-783, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Müssig K, Gallwitz B, Machicao F, Horger M, Häring HU, Kaiserling E. Paraadrenal Castleman disease presenting with adrenal hyperandrogenism. J Endocrinol Invest 29: 172-176, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Otto M, Wieprzowski L, Dzwonkowski J, Ziarkiewicz-Wróblewska B. Castleman's disease - an usual indication for laparoscopic adrenalectomy. Wideochir Inne Tech Maloinwazyine 7: 50-54, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limper M, de Kruif MD, Duits AJ, Brandjes DMP, van Gorp ECM. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect 60: 409-416, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Wacker C, Prkno A, Brunkhorst FM, Sclattmann P. Procalcitonin as a diagnostic marker for sepsis: a systemic review and meta-analysis. Lancet Infect Dis 13: 426-435, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Komatsuda A, Omokawa A, Fujiwara T, et al. . Serum procalcitonin levels in patients with myeloperoxidase-antineutrophil cytoplasmic antibodies-associated glomerulonephritis. Am J Med Sci 343: 136-140, 2012. [DOI] [PubMed] [Google Scholar]