Abstract
Mycobacterium abscessus infection tends to occur in patients with an advanced immunocompromised status. We encountered a case of intractable cutaneous M. abscessus infection that developed in a patient with systemic lupus erythematosus (SLE) during maintenance therapy. A 28-year-old woman developed a fever and redness of the skin on her buttocks. General antibacterial therapy was ineffective, and acid-fast bacteria were detected in the biopsy that was conducted to differentiate the dermal symptoms of SLE. The clinical findings eventually improved; however, the symptoms recurred multiple times during treatment. Despite recent advances in SLE treatment, M. abscessus infection remains a considerable complication of SLE.
Keywords: Mycobacterium abscessus, systemic lupus erythematosus, cutaneous lesion, clarithromycin, resistance
Introduction
Mycobacterium abscessus is a type of nontuberculous mycobacteria (NTM) that are widely present in the natural environment, such as in the soil and water. It can infect healthy subjects following an injury or opportunistically in the lung, skin, and soft tissues in immunosuppressed patients; it is often refractory (1). For an early diagnosis, it is important to perform biopsies and acid-fast cultures. The number of reported infections has recently increased. However, there are fewer cases of skin infection than of lung infection during immunosuppressive treatment, and cases complicated with systemic lupus erythematosus (SLE) are rare. When a cutaneous lesion caused by M. abscessus occurs during SLE treatment, it is often difficult to differentiate it from the dermal symptoms caused by SLE. We herein report a patient who developed intractable cutaneous M. abscessus infection during maintenance therapy with low-dose steroids and azathioprine for SLE.
Case Report
A 28-year-old woman developed SLE in 2005, with symptoms of a high fever and arthritis. She had lupus manifestations in the central nervous system with disturbance of consciousness and multiple intracranial lesions, as well as proliferative lupus nephritis (Class IV-G[A/C]+V, ISN/RPS classification). She was treated with high-dose corticosteroid therapy, intravenous injection of high-dose cyclophosphamide (4 g total), tacrolimus, and plasmapheresis. Her symptoms improved, and clinical remission was maintained with daily administration of 11 mg prednisolone and 100 mg azathioprine. In August 2014, she experienced pain of the left buttock and a high temperature of 38.8℃; she was admitted to our hospital with a diagnosis of cellulitis. Palm-sized redness, swelling, and partial induration were observed on the left buttock.
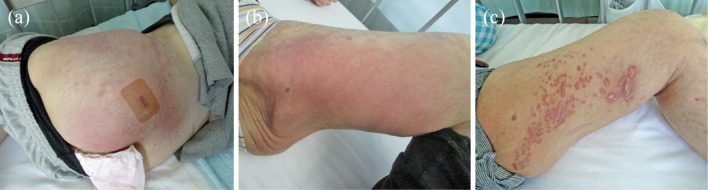
A laboratory examination revealed mild cytopenia: white blood cell count, 4.57×103/μL; hemoglobin, 8.4 g/dL; and platelet count, 10.5×104/μL. Blood cultures were negative, with normal procalcitonin levels; however, C-reactive protein (12.5 mg/dL) levels were elevated. In addition, anti-DNA antibody was not elevated (9.7 IU/mL), and hypocomplementemia was not evident (CH50, 70.6 IU/mL). Despite administering 3 g cefozopran, no therapeutic efficacy was noted, and a fever of 39℃ persisted. The antibiotics were then changed to 3 g cefazolin plus 1,800 mg clindamycin in addition to 3 g meropenem plus 2 g vancomycin. However, the local redness and pain extended further (Fig. 1a).
Figure 1.
Skin lesions of the buttock and the thigh. (a) Broad erythema on the left buttock (after a biopsy). (b) Erythema on the inside of the left thigh at the time of relapse. (c) Multiple subcutaneous indurations after four months of combined antibiotics treatment from the time of relapse.
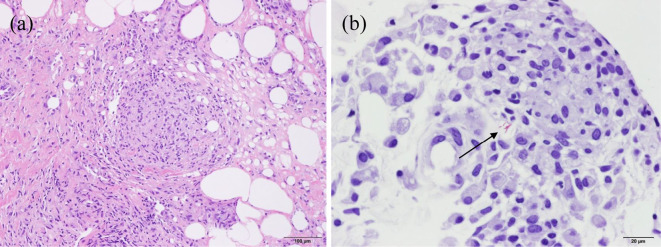
To distinguish between an infectious disease and skin lesion due to SLE, we performed a skin biopsy. The histopathological findings revealed inflammatory cell infiltrates that were mainly composed of lymphocytes and plasma cells extending from the dermis into the subcutaneous fat as well as granuloma formation (Fig. 2a). Furthermore, a microscopic examination of a direct smear showed slight positivity for acid-fast bacilli (+/-, equivalent to Gaffky scale of 1) (Fig. 2b). The polymerase chain reaction test was negative for tubercle bacillus. Accordingly, this patient was diagnosed with cutaneous nontuberculous mycobacteriosis, although the strain was unknown at this point. She was administered 4-drug combination therapy with isoniazid, rifampicin, ethambutol, and 800 mg clarithromycin daily to cover M. avium complex (MAC) and M. kansasii, which are the most common causes of nontuberculous mycobacteriosis. Her fever was immediately alleviated, and the skin findings improved. One week later, M. abscessus was detected from a Mycobacterium culture of the tissues and was considered to be the pathogenic bacteria.
Figure 2.
(a) Histological appearance of the left buttock lesion, showing inflammatory cell infiltration and formation of granuloma (Hematoxylin and Eosin staining, ×100). (b) Acid-fast bacilli (arrow) in the regions (Kinyoun stain, ×400).
Although remission was maintained until the end of September, relapse occurred with a fever and a painful new cutaneous lesion consisting of redness and swelling at the initial infection site on her foot. Based on the possible emergence of resistant bacterium, the antituberculosis drugs were discontinued, except for clarithromycin, and amikacin and imipenem/cilastatin were additionally administered in accordance with the guidelines (ver. 2) of the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) (2). However, a fever of 39℃ persisted, and her general condition gradually worsened. A second skin biopsy, which was performed to clarify either the deterioration of SLE or relapse of M. abscessus, indicated similar histopathological findings to the previous results but showed stronger positivity for acid-fast bacilli (2+; equivalent to Gaffky scale of 5) on the smear specimen; cultivation later also showed M. abscessus. Because of concerns about resistance to clarithromycin, clarithromycin was changed to azithromycin, which contributed to the immediate alleviation of the fever. The drug susceptibility test results, which were obtained 4 weeks later, revealed resistance; the minimal inhibitory concentration of clarithromycin was 0.25 μg/mL at the first biopsy and >32 μg/mL at the second biopsy (Table). Subsequently, azathioprine was reduced to 50 mg daily.
Table.
Minimum Inhibitory Concentrations Using the Broth Microdilution Method.
| Antimicrobial Agent | MIC (µg/mL) | |
|---|---|---|
| First biopsy | Second biopsy | |
| Amikacin | >16 | 16 |
| Clarithromycin | 0.25 | >32 |
| Ethambutol | 128 | 128 |
| Kanamycin | 32 | 8 |
| Levofloxacin | 32 | 16 |
| Rifabutin | >16 | 8 |
| Rifampicin | >32 | >32 |
| Streptomycin | 64 | 32 |
MIC: minimum inhibitory concentrations
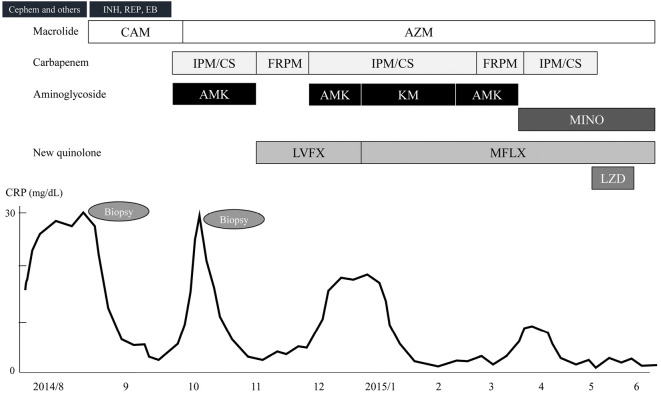
A three-drug combination of azithromycin, amikacin, and imipenem/cilastatin was continued for one month, and her symptoms were stable; therefore, we switched the treatment to oral antibiotics consisting of azithromycin, faropenem, and levofloxacin at the beginning of November. However, she experienced a relapse three weeks later, and cutaneous redness and induration occurred at a new lesion on the wide area of the inside of the left thigh and on the right buttock (Fig. 1b). By switching amikacin and levofloxacin to kanamycin and moxifloxacin, respectively, therapeutic efficacy was achieved. Accordingly, a four-drug combination of macrolide, carbapenem, aminoglycoside, and a new quinolone was continued for four months. The cutaneous lesion gradually improved, and her general condition was stable; however, the subcutaneous induration partially remained (Fig. 1c). She developed high tone deafness during treatment and was diagnosed with auditory nerve disorders because of long-term aminoglycoside use. Therefore, aminoglycoside was switched to minocycline. Although linezolid was also temporarily used, it was discontinued because she developed malaise and thrombocytopenia. Finally, a three-drug combination of azithromycin, moxifloxacin, and minocycline was administered, and she was discharged in the tenth month after hospitalization (Fig. 3). She was then continuously treated in the outpatient department for 12 months and showed no recurrence of skin lesions; in addition, her SLE was stable.
Figure 3.
Clinical course of the patient. INH: isoniazid, REP: rifampicin, EB: ethambutol, CAM: clarithromycin, AZM: azithromycin, IPM/CS: imipenem/cilastatin, FRPM: faropenem, AMK: amikacin, KM: kanamycin, MINO: minocycline, LVFX: levofloxacin, MFLX: minocycline, LZD: linezolid
Discussion
According to the Runyon classification, M. abscessus is in the rapid grower group (Group IV); it was previously considered a subspecies of Mycobacterium chelonae, when it was referred to as M. chelonae subsp abscessus. Currently, the M. abscessus complex consists of three strains: M. abscessus, M. massiliense, and M. bolletii. Among the causes of nontuberculous mycobacteriosis, it is the bacteria associated with the worst outcomes. Multiple lesions tend to form for immunosuppressed patients (3), and the mortality is higher with disseminated infection, particularly for immunosuppressed patients (1). According to the 2007 ATS/IDSA Guidelines, there is currently no available antibiotic regimen; therefore, to improve the cure rate, surgical treatment should be considered (2). Although we considered surgical resection several times during the treatment of this patient, the lesions extended over a wide area of the buttock; therefore, we could not perform surgery.
Regarding antibiotic treatment for these bacteria, amikacin, imipenem/cilastatin, and cefoxitin (which are not yet approved in Japan) are primarily used together with a macrolide such as clarithromycin (2-4). One of the factors that made the treatment difficult was the emergence of resistance to clarithromycin. Initially, although a microscopic examination of a direct smear showed positivity for acid-fast bacilli, the strain was unknown. Based on the epidemiology of NTM in Japan (5), MAC and M. kansasii are the most and second-most common pathogenic strains, respectively. Therefore, we chose combination therapy consisting of clarithromycin, isoniazid, rifampicin, and ethambutol, which covers both MAC and M. kansasii. Additionally, this combination therapy has been used to treat M. marinum, which is commonly reported as prophlogistic bacteria involved in skin infections (6). However, M. abscessus is reportedly resistant to all of the standard antituberculosis drugs (2). Therefore, few drugs other than clarithromycin are available, which was considered one of the causes of the early resistance to clarithromycin. When the strain was determined to be M. abscessus, we considered switching from the antituberculosis drugs to aminoglycoside and carbapenems. However, we eventually waited to switch the therapies because the treatment was very successful, and drug susceptibility was unknown at the time. In hindsight, an early change in the treatment might have avoided the resistance to clarithromycin. Recently, the incidence of M. abscessus as prophlogistic bacteria of cutaneous NTM has increased (7). It is important to use a combination therapy that includes sensitive antibiotics, such as macrolide, aminoglycoside, carbapenem, and new quinolones.
SLE is a typical systemic autoimmune disease that is most commonly found in young women. Corticosteroid and immunosuppressive drugs are commonly used for treatment. However, the treatment may be prolonged in many patients, and measures against opportunistic infection can be a major problem. Based on a review of 25 patients with SLE who had cutaneous NTM (8), 8 (32%) patients were taking immunosuppressants, and 5 of these 8 patients used azathioprine for maintenance therapy. Skin manifestations varied from nodular lesions to ulcerative lesions. The pathogenic bacteria equally included rapid growers in Group IV and slow growers in Groups I-III, according to the Runyon classification. There was only one case caused by M. abscessus (9) in this review, but the proportion of rapid growers in patients with SLE was higher than that in patients with other types of immunosuppression. Because it can be difficult to distinguish cutaneous NTM from SLE-related skin lesions early after the symptoms appear (10), a local biopsy and tests of a smear/culture are essential for a diagnosis, especially for infections with acid-fast bacilli. In fact, our patient did not initially respond to the usual antibiotics. Therefore, it was necessary to make the definitive diagnosis based on the findings of a biopsy.
According to a report of 725 patients with SLE, 11 patients (1.5%) were infected with NTM, and 8 of these had infected soft tissues of the skin (11). Extra-pulmonary involvement was more common in patients with SLE. Because NTM is widely present in soil and water, skin abrasion is the likely route of soft tissue infections. Therefore, for prophylaxis of an NTM infection, it is important to prevent injury and keep the skin clean. For patients infected with NTM, the mean SLE duration was 9.3 years, and the mean cumulative prednisolone dose was 25.82 g. However, for patients with SLE and tuberculosis, the mean SLE duration was 3.7 years, and the mean cumulative prednisolone dose was 11.58 g. Thus, infection with NTM might occur in the presence of more extensive immunosuppression. In previous reports of infection with NTM in immunosuppressed patients, the sample included a number of organ transplant recipients and patients with acquired immunodeficiency syndrome (12,13). However, with advanced understanding of pathology and treatments, stronger immunosuppressive treatment has been more frequently used to treat SLE. B-cell targeted therapy, such as rituximab, belimumab, and epratuzumab, has been suggested as a treatment option (14). Combination therapy including tacrolimus and mycophenolate mofetil has been used for antirejection in transplant patients (15) and has also attracted more attention recently in multitarget therapy for lupus nephritis (16). Furthermore, long-term treatment using steroids and various immunosuppressants is not uncommon for patients with SLE. With this progress in treatment, the incidence of NTM infections is expected to increase. We therefore believe that the possibility of such an infection should be considered when treating SLE. For an early diagnosis, a high level of suspicion is warranted when the therapeutic effect of empirical antibiotic therapy is insufficient, routine cultures are negative, and immunosuppressive treatment is administered for a long period of time.
In conclusion, we reported a patient who developed intractable cutaneous M. abscessus during maintenance treatment of SLE. The treatment of this patient was complicated and prolonged because of multiple factors, including immunosuppression due to long-term treatment of SLE, resistance to clarithromycin, and multiple and enlarged lesions that were difficult to remove. With advancements in techniques for SLE treatment, the prevalence of skin infections due to these bacteria might increase further. In patients with refractory cellulitis that occurs during immunosuppressive treatment, this disease should be suspected, and a Mycobacterium culture of tissues should be actively performed. After the diagnosis, it is important to initiate combination therapy including sensitive antimicrobial agents as early as possible.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful for the assistance of Prof. Ryuhei Okuyama and Dr. Huminao Kamijo, Department of Dermatology, Shinshu University School of Medicine and the helpful suggestions of Dr. Norihisa Ishii, Leprosy Research Center, National Institute of Infectious Diseases, and Dr. Hideaki Nagai, Center for Pulmonary Diseases and Respiratory Disease Division, National Hospital Organization Tokyo National Hospital.
References
- 1.Fukui S, Sekiya N, Takizawa Y, et al. Disseminated Mycobacterium abscessus infection following septic arthritis: a case report and review of the literature. Medicine 94: e861, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol 142: 1287-1292, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infecti Dis 52: 565-571, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K. Serodiagnosis of Mycobacterium avium complex disease in humans: translational research from basic mycobacteriology to clinical medicine. Jpn J Infect Dis 67: 329-332, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother 8: 2965-2978, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Wentworth AB, Drage LA, Wengenack NL, Wilson JW, Lohse CM. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc 88: 38-45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touma Z, Haddad A, Gladman DD, Uleryk EM, Urowitz MB. Skin nontuberculous mycobacterial infection in systemic lupus erythematosus: an unusual skin infection mimicking lupus vasculitis. Semin Arthritis Rheum 42: 498-506, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Sungkanuparph S, Sathapatayavongs B, Pracharktam R. Infections with rapidly growing mycobacteria: report of 20 cases. Int J Infect Dis 7: 198-205, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MM, Wilson HE, Duthie FR, Jones B, Field M. When typical is atypical: mycobacterial infection mimicking cutaneous vasculitis. Rheumatology 41: 685-690, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Mok MY, Wong SS, Chan TM, Fong DY, Wong WS, Lau CS. Non-tuberculous mycobacterial infection in patients with systemic lupus erythematosus. Rheumatology 46: 280-284, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Zumla A, Grange J. Infection and disease caused by environmental mycobacteria. Curr Opin Pulm Med 8: 166-172, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Morales P, Gil A, Santos M. Mycobacterium abscessus infection in transplant recipients. Transplant Proc 42: 3058-3060, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Bernal CB, Zamora LD, Navarra SV. Biologic therapies in systemic lupus erythematosus. Int J Rheum Dis 18: 146-153, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Ekberg H, van Gelder T, Kaplan B, Bernasconi C. Relationship of tacrolimus exposure and mycophenolate mofetil dose with renal function after renal transplantation. Transplantation 92: 82-87, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med 162: 18-26, 2015. [DOI] [PubMed] [Google Scholar]