Abstract
Key points
Ligating the femoral artery of a rat for 72 h, a model for peripheral artery disease, causes an exaggerated exercise pressor reflex in response to muscle contraction.
Likewise, the hindlimb muscles of rats with ligated femoral arteries show increased levels of reactive oxygen species.
Infusion of tiron, a superoxide scavenger, attenuated the exaggerated pressor reflex and reduced reactive oxygen species production in rats with ligated femoral arteries.
Conversely, we found no effect of tiron infusion on the pressor reflex in rats with patent femoral arteries.
These results suggest a role of reactive oxygen species with respect to causing the exaggerated pressor response to contraction seen in rats with ligated arteries and peripheral artery disease.
Abstract
Contraction of muscle evokes the exercise pressor reflex (EPR), which is expressed partly by increases in heart rate and arterial pressure. Patients with peripheral artery disease (PAD) show an exaggerated EPR, sometimes report pain when walking and are at risk for cardiac arrthymias. Previous research suggested that reactive oxygen species (ROS) mediate the exaggerated EPR associated with PAD. To examine the effects of ROS on the EPR, we infused a superoxide scavenger, tiron, into the superficial epigastric artery of decerebrated rats. In some, we simulated PAD by ligating a femoral artery for 72 h before the experiment. The peak EPR in ‘ligated’ rats during saline infusion averaged 31 ± 4 mmHg, whereas the peak EPR in these rats during tiron infusion averaged 13 ± 2 mmHg (n = 12; P < 0.001); the attenuating effect of tiron on the EPR was partly reversed when saline was reinfused into the superficial epigastric artery (21 ± 2 mmHg; P < 0.01 vs. tiron). The peak EPR in ‘ligated’ rats was also attenuated (n = 7; P < 0.01) by infusion of gp91ds‐tat, a peptide that blocks the activity of NAD(P)H oxidase. Tiron infusion had no effect on the EPR in rats with patent femoral arteries (n = 9). Western blots showed that the triceps surae muscles of ‘ligated’ rats expressed more Nox2 and p67phox, which are components of NADPH oxidase, compared to triceps surae muscles of ‘freely perfused’ rats. Tiron added to muscle homogenates reduced ROS production in vitro. The results of the present study provide further evidence indicating that ROS mediates the exaggeration of EPR in rats with simulated PAD.
Keywords: decerebrate rats, femoral artery ligation, neural control of the circulation, superoxide ions, sympathetic nervous system, tiron
Key points
Ligating the femoral artery of a rat for 72 h, a model for peripheral artery disease, causes an exaggerated exercise pressor reflex in response to muscle contraction.
Likewise, the hindlimb muscles of rats with ligated femoral arteries show increased levels of reactive oxygen species.
Infusion of tiron, a superoxide scavenger, attenuated the exaggerated pressor reflex and reduced reactive oxygen species production in rats with ligated femoral arteries.
Conversely, we found no effect of tiron infusion on the pressor reflex in rats with patent femoral arteries.
These results suggest a role of reactive oxygen species with respect to causing the exaggerated pressor response to contraction seen in rats with ligated arteries and peripheral artery disease.
Abbreviations
- DHE
dihydroethidium
- DRG
dorsal root ganglion
- EPR
exercise pressor reflex
- HR
heart rate
- MAP
mean arterial (blood) pressure
- PAD
peripheral artery disease
- ROS
reactive oxygen species
Introduction
The exercise pressor reflex is evoked by contraction of skeletal muscles and consists of increases in arterial pressure, heart rate and cardiac output (Coote et al. 1971; McCloskey & Mitchell, 1972). In health, the exercise pressor reflex functions to increase perfusion of the contracting muscles (Amann et al. 2011; O'Leary et al. 1999). Its afferent limb is comprised of thinly myelinated group III afferents, which are responsive to mechanical stimuli, and unmyelinated group IV afferents, which are responsive to metabolic by‐products of contraction (Kaufman et al. 1983; Rotto & Kaufman, 1988). The term ‘thin fibre muscle afferents’ has been used frequently to describe the group III and IV afferents comprising the sensory limb of the exercise pressor reflex arc (Mitchell et al. 1983).
The role played by superoxide ions in evoking the exercise pressor reflex in the ‘freely perfused’ hindlimbs of healthy animals and humans is controversial. For example, in decerebrate rats, Wang et al. (2009) reported that femoral arterial infusion of tempol, a superoxide dismutase mimetic, attenuated the reflex. By contrast, in healthy, decerebrate rats, Koba et al. (2009, 2013) reported that femoral arterial infusion of either tempol or tiron, the latter being a superoxide scavenger, had no effect on the exercise pressor reflex. In healthy humans, Muller et al. (2012, 2013) found that i.v. infusion of ascorbic acid, an anti‐oxidant, had no effect on the reflex. Unlike the controversy concerning the role played by superoxide ions in evoking the exercise pressor reflex in health, there is general agreement that superoxide ions play a significant role in evoking the exercise pressor reflex in heart failure. Specifically, both Wang et al. (2009) and Koba et al. (2009) reported that tempol attenuated the exaggerated exercise pressor reflex seen in rats with heart failure.
Our laboratory has also investigated the role played by superoxide ions in evoking the exercise pressor reflex in both health and disease. Consistent with the findings reported by Koba et al. (2009, 2013), and in contrast to those reported by Wang et al. (2009), we found that neither tempol, nor tiron attenuated the exercise pressor reflex in healthy rats with freely perfused arteries (McCord et al. 2011). These contrasting findings might be explained by differences in the methods used to administer anti‐oxidants. Specifically, both McCord et al. (2011) and Koba et al. (2009, 2013) injected anti‐oxidants as a bolus 10–15 min before evoking the exercise pressor reflex, whereas Wang et al. (2009) infused an anti‐oxidant while the reflex was being evoked. Consequently, in the experiments reported by McCord et al. (2011) and Koba et al. (2009, 2013), the possibility exists that the anti‐oxidants had either been washed out of the contracting muscles or had lost their ability to reduce the concentration of superoxide ions. Therefore, one aim of the present study was to examine the exercise pressor reflex in healthy rats when an anti‐oxidant was being constantly infused into the arterial supply of the contracting muscles.
We have also investigated the effect of anti‐oxidants on the exercise pressor reflex in rats with simulated peripheral artery disease, which was induced by ligating a femoral artery 72 h before performing the experiments (Tsuchimochi et al. 2010). We reported that tempol, but not tiron, attenuated the exercise pressor reflex in rats with ligated femoral arteries (McCord et al. 2011). In a subsequent study, we found that the attenuating effect of tempol on the exercise pressor reflex in rats with ligated femoral arteries was secondary to the ability of this compound to open KATP channels, the concentration of which was found to be elevated in the dorsal root ganglia innervating the contracting hindlimbs of the rats with ligated femoral arteries (Yamauchi et al. 2012). In both reports from our laboratory (McCord et al. 2011; Yamauchi et al. 2012), we injected tempol and tiron into the arterial circulation of the hindlimb 10–15 min before its muscles were contracted. Our findings that neither tiron, nor tempol attenuated the exercise pressor reflex as a result of their anti‐oxidant properties were surprising because they contrasted with the findings of Muller et al. (2012), who found that i.v. infusion of ascorbic acid during exercise attenuated the reflex in patients with peripheral artery disease. In our experiments with ‘freely perfused’ rats, we were concerned that the anti‐oxidant effect of either tempol or tiron in our experiments with ‘ligated’ rats was not present while we contracted the hindlimb muscles. Therefore, our second aim was to examine the exercise pressor reflex in rats with ligated femoral arteries when the hindlimb muscles were contracted while an anti‐oxidant was being constantly infused.
Methods
Ethical approval
The Institutional Care and Use Committee of the Pennsylvania State University College of Medicine approved all of the procedures. The authors understand and conform to the ethical principles and guidelines of The Journal of Physiology, as outlined by Grundy (2015).
General
Experiments were performed on 4–6‐month‐old male, Sprague–‐Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 300–450 g. Rats were housed within the central animal facility at the Pennsylvania State University College of Medicine, with ad libitum access to food and water and were routinely monitored by veterinary and animal care staff. Seventy‐two hours before the experiment, some of the rats underwent surgery to ligate one femoral artery. The rats were anaesthetized in an induction chamber, followed by a nose cone, to a surgical plane with a mixture of 4% isoflurane and 100% oxygen. The femoral artery was isolated and 5‐0 suture was tied tightly around it ∼3 mm distal to the inguinal ligament. The wound was sutured and a small drop (0.05 ml) of bupivacaine was applied. Rats were returned to their cage and allowed 72 h to recover following the femoral artery occlusion procedure, which does not affect normal cage activity (Taylor et al. 2008). Rats that underwent this surgery are referred to as ‘ligated’, whereas those who did not have their femoral artery ligated are referred to as ‘freely perfused.’
On the day of the experiment, rats were anaesthetized in an induction chamber, followed by a nose cone, to a surgical plane with isoflurane (4%) in oxygen. The trachea was cannulated and the lungs mechanically ventilated with 2–3% isoflurone in oxygen. The carotid arteries and one jugular vein were cannulated (PE‐50) to measure arterial blood pressure and to administer drugs and fluids, respectively. Arterial blood gases and pH were measured using an automated blood gas analyser (ABL 80; Radiometer, Brea, CA, USA). and arterial pH were maintained within normal ranges either by adjusting ventilation, by adding oxygen to the inspired gas mixture, or by an i.v. injection of sodium bicarbonate (8.5%). Body temperature was maintained between 36.5 and 38.0°C by use of a heating pad. Arterial blood pressure was measured by attaching one carotid cannula to a Statham P23XL strain gauge (AMETEK Power Instruments; Rochester, NY, USA). Heart rate was calculated from the arterial pressure pulse (Spike2; Cambridge Electronics Design, Cambridge, UK). In one hindlimb of the rats in which we needed to infuse saline and tiron (Sigma‐Aldrich Corp., St Louis, MO, USA) into the arterial supply of the contracting muscles, the superficial epigastric artery, which is a side branch of the femoral artery, was cannulated. In some rats, a snare was placed around the iliac artery and vein supplying the contracting triceps surae muscles.
The rats were secured in a customized Kopf spinal frame (David Kopf Instruments, Tujunga, CA, USA) by clamps placed on the rostral lumbar vertebra and the pelvis. The left ankle was secured in a clamp and the knee was stabilized so that the leg did not move during contraction of the hindlimb. The calcaneal bone was severed and attached to a force transducer (Model FT 10; Natus Neurology Grass, Warwick, RI, USA). A pre‐collicular decerebration was performed and all neural tissue rostral to the superior colliculi was removed (Smith et al. 2001; Tsuchimochi et al. 2010). Bleeding was controlled and the cranial vault was filled with gauze. Anaesthesia was discontinued immediately after decerebration. The tibial nerve was isolated, a shielded stimulating electrode was placed underneath it, and the leg was covered in gauze soaked in saline.
Experimental protocols
In both ‘freely perfused’ and ‘ligated’ rats, we measured the pressor and cardioaccelerator responses to static contraction of the triceps surae muscles (i.e. the exercise pressor reflex). Contraction was evoked for 30 s and was caused by electrically stimulating the tibial nerve (40 Hz; 0.01 ms pulse duration, two times motor threshold). The pressor and cardioaccelerator responses to contraction were assessed in the order: (i) during infusion of saline into the superficial epigastric artery (2 ml h−1); (ii) during infusion of tiron (2 ml h−1; 200 mg ml−1) into the superficial epigastric artery; and (iii) during infusion of saline into the superficial epigastric artery (i.e. washout). The infusions started 10 min before contraction and lasted throughout the 30 s contraction period. Tiron was dissolved in saline (0.9%). At the end of every experiment, we paralyzed the rats with pancuronium bromide (0.5 mg kg−1; i.v.) and electrically stimulated the tibial nerve for 30 s; we did this using the same frequency, pulse duration and current as those used in rats without neuromuscular blockade. Pancuronium bromide was only administered following the decerebration procedure. In every experiment in which we infused tiron into the superficial epigastric artery, we infused Evan's blue dye into this artery through the same catheter to determine whether the infusate entered the circulation of the triceps surae muscles. At the conclusion of every animal experiment, rats were anaesthetized with 3–4% isoflurane in oxygen and killed by i.v. administration of 3 mg kg−1 potassium chloride.
In ‘ligated’ rats, we measured the pressor and cardioaccelerator responses to static contraction for 30 s while infusing tiron i.v. Only ‘ligated’ rats were studied because intra‐arterial infusion of tiron had no effect on the exercise pressor reflex in ‘freely perfused’ rats (see Results). The aim of these experiments was to determine whether attenuation of the exercise pressor reflex by intra‐arterial infusion of tiron was caused by circulation of the anti‐oxidant to the spinal cord and/or brain. The time period, rate of infusion and concentration of tiron infused i.v. duplicated those used when tiron was infused into the superficial epigastric artery.
In ‘ligated’ rats, we measured the pressor and cardioaccelerator responses to static contraction for 30 s when infusing tiron i.v. but with a snare tightened around the iliac artery and vein that supplied the contracting triceps surae muscles. The ‘ligated’ rats used in these experiments were different from those used in the experiments in which tiron was infused i.v. without snaring the iliac artery and vein. The aim of our occluding the iliac artery and vein in these ‘ligated’ rats was to minimize the access of the i.v. infusion of tiron to the contracting triceps surae muscles via the collateral circulation.
In ‘ligated’ rats paralyzed with pancuronium bromide (0.5 mg kg−1; i.v.), we measured the pressor and cardioaccelerator responses to electrical stimulation of the tibial nerve for 30 s while infusing tiron i.v. The tibial nerve was stimulated at 40 Hz, with 0.01 ms pulse durations, but with a current intensity that was 10–20 times the motor threshold. Our intent was to deliberately evoke a pressor response that was similar to that evoked by static contraction in ‘ligated’ rats. The aim of these experiments was to further determine whether tiron infusion attenuated the exercise pressor reflex by its action on the spinal and/or medullary circuits involved in expressing the reflex.
In an additional set of seven ‘ligated’ rats, we measured the pressor and cardioaccelerator responses to static contraction of the triceps surae muscles while infusing the NADPH oxidase inhibiting peptide, gp91ds‐tat (AnaSpec, Fremont, CA, USA) into the superficial epigastric artery. Gp91ds‐tat attaches to the binding site of p47phox, preventing its association with gp91phox (Rey et al. 2001). As with tiron, gp91ds‐tat was infused at a rate of 2 ml h−1. The concentration used was 80 μg ml−1 (30 μm), resulting in a 1 μm final concentration (Park et al. 2008) within the volume of the leg (15 ml). The aim of these experiments was to determine whether NAD(P)H oxidase derived reactive oxygen species (ROS) were attributable to the exaggerated pressor responses observed in ‘ligated’ rats.
In the last series of experiments performed in ‘freely perfused’ rats, we measured the pressor and cardioaccelerator responses to static contraction for 30 s both while infusing tiron into the superficial epigastric artery and simultaneously occluding the iliac artery and vein supplying the contracting triceps surae muscles. The aim of these experiments was to determine whether the lack of effect of tiron infusion on the exercise pressor reflex in ‘freely perfused’ rats was a result of washout of the infusate from triceps surae muscles that were receiving normal arterial blood flow. The rats used in these experiments were different from the ‘freely perfused’ rats used in the experiments described above.
Detection of ROS in skeletal muscle cross‐sections
Dihydroethidium (DHE) (Life Technologies, Grand Island, NY, USA) was used to confirm in situ sequestration of ROS with Tiron infusion as adapted from a previous study (Zhao et al. 2006). DHE is a blue fluorescent dye that is oxidized to 2‐hydroxyethidium in the presence of ROS and, in turn, intercalates into DNA to emit a red fluorescence signal. Decerebrate, ‘ligated’ rats underwent either saline (n = 2) or tiron (n = 2) infusion into the superficial epigastric artery. The plantaris muscles were subsequently dissected, immersed in Tissue‐Tek® OCT compound (Sakura, Torrance, CA, USA) and frozen in isopentane cooled with liquid nitrogen. Muscle cross‐sections (10 μm) were exposed to DHE (2 μm) and incubated in the dark at 37°C for 20 min. Samples were then washed with PBS and images of plantaris muscle cross‐sections were taken with a charge coupled device camera and a DeltaVision Elite inverted microscope (U‐Plan S‐Apo 20X/0.75; excitation 557 nm, emission 576 nm; Olympus, Tokyo, Japan). Background fluorescence was subtracted (± 2 SD of mean fluorescence) and the maximum fluorescence intensity was standardized using Image J, version 1.48v (National Institutes of Health; Bethesda, MD, USA).
In vitro analysis of ROS production
Skeletal muscle ROS production was assessed using in vitro DHE fluorescence and lucigenin chemiluminescent assays as adapted from methods described previously (Ohara et al. 1993; Griendling et al. 1994; Rajagopalan et al. 1996; Zou et al. 2001; Zhao et al. 2006). Lucigenin is a chemiluminescent reagent that releases a photon upon exposure to superoxide and can be detected with a luminometer (Dikalov et al. 2007). In decerebrate ‘ligated’ and ‘freely perfused’ rats, resting triceps surae and plantaris muscles were quickly excised and snap frozen in liquid nitrogen. The tissue was then immediately pulverized under liquid nitrogen and stored at −80°C until later use.
DHE fluorescence assay
All procedures were performed under reduced ambient light conditions while the reagents and samples were kept on ice. Pulverized tissue was homogenized in Hepes buffer (25 mmol L−1 Hepes, 1 mmol L−1 EDTA and a cOmplete, mini, EDTA‐free protease inhibitor cocktail) and was centrifuged at 6000 g for 5 min at 4°C. The supernatant was collected and protein concentration was measured using the method of Bradford (1976). Tissue homogenates (100 μg) were then incubated in a 96‐well plate (Thermo Fisher Scientific, Waltham, MA, USA) with 10 μm L−1 DHE in the presence of salmon testes DNA (0.5 mg ml−1; Sigma‐Aldrich) and NADH (0.1 mmol L−1) at 37°C for 45 min. Ethidium fluorescence was measured at excitation and emission wavelengths of 405 nm and 605 nm, respectively, to minimize interference by non‐specific DHE oxidation products (Griendling et al. 2016). NADH was used as a substrate for NAD(P)H oxidase‐dependent ROS production, and salmon testes DNA was added to stabilize 2‐hydroxyethidium and amplify the fluorescence signal. Samples were run in duplicate, with and without the anti‐oxidant Tiron (20 mg ml−1). Tiron alone, in the absence of tissue samples, had no effect on ethidium fluorescence. A standard curve was constructed with ethidium bromide in the presence of salmon testes DNA (0.5 mg ml−1). Ethidium fluorescence is expressed as the change in fluorescence following the total 45 min incubation period in nanomoles normalized to mg of protein.
Lucigenin chemiluminescent assay
All procedures were performed under reduced ambient light conditions while the samples were kept on ice. Pulverized tissue was homogenized in a monobasic potassium phosphate buffer (20 mmol L−1, pH 7.0; 1 mmol L−1 EGTA and a cOmplete, mini, EDTA‐free protease inhibitor cocktail) and centrifuged at 6000 g for 5 min at 4°C (Griendling et al. 1994). The supernatant was collected and protein concentration was determined using the method of Bradford (1976). Basal luminescence was measured in a 96‐well white opaque microplate (Thermo Fisher Scientific) in the absence of tissue homogenates in which each well contained 125 μl of assay buffer (50 mmol L−1 KH2PO4, pH 7.0; 1 mmol L−1 EGTA and 150 mm L−1 sucrose), 50 μl of lucigenin (5 μm), 25 μl of NADH (0.1 mmol L−1) and 25 μl of either additional assay buffer or tiron (20 mg ml−1). Tissue homogenates (15 μg) were then added and luminescence for each sample was measured in triplicate for a 30 s integration period, every 1–2 min for 50 min. Basal luminescence was subtracted and the slope of a linear curve of best fit for each sample was determined (∆ chemiluminescence/time). The slopes for the ‘ligated’, ‘freely perfused’ + tiron and ‘ligated’ + tiron samples were normalized to the ‘freely perfused’ control samples. Previous studies have shown that the change in lucigenin‐derived chemiluminescence is indicative of superoxide production using a standard curve constructed from xanthine and xanthine oxidase (Ohara et al. 1993). The normalized values were therefore expressed as the relative change in superoxide production.
Western immunoblotting
Western immunoblotting was used to determine the amount of the NAD(P)H‐oxidase subunits, Nox2 and p67phox in skeletal muscle and dorsal root ganglia. Pulverized skeletal muscle tissue was homogenized in Hepes buffer (25 mmol L−1 Hepes, 1 mmol L−1 EDTA, 1% Triton‐X‐100 and a cOmplete, mini, EDTA‐free protease inhibitor cocktail) and was centrifuged at 10 000 g for 15 min at 4°C. The supernatant was collected and protein concentration was measured using the method of Bradford (1976). Dorsal root ganglia (L4 and L5) were homogenized with plastic tissue grinders in 1.5 ml microcentrifuge tubes and protein was isolated using a NucleoSpin RNA and protein purification kit (Macherey‐Nagel, Düren, Germany), in accordance with the manufacturer's instructions. The isolated, soluble dorsal root ganglion (DRG) protein concentration was determined using a Qubit® protein assay kit (Invitrogen, Carlsbad, CA, USA).
For polyacrylamide gel electrophoresis, skeletal muscle protein homogenates were resuspended in LDS sample buffer + 10% DTT, DRG protein homogenates were resuspended in bromophenol blue, and both were heated for 10 min at 70°C. Protein samples (20–80 μg) were then loaded onto 4–12% Bis‐Tris mini gels and separated in an XCell Surelock™ system (Invitrogen) with Mops SDS running buffer (200 V for 1 h). Gels were transferred (30 V for 60–75 min) to nitrocellulose membranes and stained with a 0.1% Ponceau red solution in 5% acetic acid to confirm equal loading. Ponceau red stained membranes were digitized and washed in Tris buffer saline + 0.1% Tween‐20 (TTBS) for stain removal. Membranes were blocked in 5% non‐fat dry milk in TTBS and then probed using mouse anti‐gp91phox (dilution 1:200, #611415; BD Biosciences, San Jose, CA, USA) and anti‐p67phox (dilution 1:2500, #610912; BD Biosciences), rabbit anti‐gp91phox (dilution 1:500, ab129068; Abcam, Cambridge, MA, USA) and anti‐vinculin (dilution 1:10,000, ab73412; Abcam), as well as species‐specific secondary antibodies (dilution 1:2500 to 1:5000). All membranes were stained with chemiluminescent agents for film development, and films were digitized for subsequent analysis using Image J, version 1.48. Skeletal muscle protein quantity is expressed as relative units normalized to Ponceau red, involving densitometry analysis of all discriminable protein bands stained with Ponceau red in each respective electrophoresis lane, and the same ‘freely perfused’ control sample loaded onto each gel. Ponceau red was used as a loading control because some traditional loading controls may be sensitive to redox status (Butterfield et al. 2010; Sasser et al. 2012). DRG protein quantity is expressed as relative units normalized to vinculin and ‘freely perfused’ control samples.
Data analysis
Pulsatile and mean arterial blood pressure (MAP), heart rate (HR) and muscle tension were displayed continuously in real‐time using a Spike 2 data acquisition system (Cambridge Electronic Design). In addition, data were recorded and stored on a computer hard drive (Dell Technologies, Round Rock, TX, USA) for offline analysis. Baseline MAP and HR values were determined from the 30 s baseline period that preceded contraction. The ‘peak’ pressor (increases in MAP) and cardioaccelerator (increases in HR) responses to static contraction were calculated as the differences between the maximum MAP and HR values obtained during contraction and the baseline values. In addition, the pressor responses to static contraction were calculated over the duration of the 30 s contraction period. This was accomplished by subtracting the integrated area under the mean arterial pressure trace for the 30 s baseline period from the integrated area under the mean arterial pressure trace for the 30 s contraction period. Likewise, the tension‐time index for static contraction was calculated by subtracting the area under the tension trace for the 30 s baseline period from the area under the tension trace for the 30 s contraction period.
Statistical analysis
All data are expressed as the mean ± SE. Statistical comparisons were performed using Prism, version 6 software (GraphPad, La Jolla, CA, USA) using two‐way ANOVAs with repeated measures for between (‘freely perfused’ vs. ‘ligated’) and within (control vs. tiron vs. washout) group comparisons. For control experiments and gp91ds‐tat, one‐way ANOVAs were used for within‐group comparison. Individual means were compared with Holm–Sidak post hoc tests if the appropriate main effect was found to be significant. P < 0.05 was considered statistically significant.
Results
Ligated rats
In twelve rats, tiron infused into the superficial epigastric artery while the hindlimb muscles were statically contracted markedly decreased the exercise pressor reflex. This was the case both when the exercise pressor reflex was analysed for its peak and for its integrated area over the 30 s contraction period. The infusion of the anti‐oxidant was started 10 min before the onset of contraction and was maintained throughout the contraction period (Fig. 1). The tiron‐induced attenuation of the exercise pressor reflex was partially restored when saline replaced tiron as the perfusate (i.e. washout) (Figs 1 and 2). As shown previously by Tsuchimochi et al. (2010), the exercise pressor reflex evoked in rats with ligated femoral arteries was significantly larger than that evoked in rats with freely perfused femoral arteries (Fig. 2). The exaggerated pressor evoked in rats with ligated femoral arteries was not a result of increased contractile force because the tension‐time indices of ‘ligated’ rats infused with saline were slightly less than those of the ‘freely perfused’ rats infused with saline (P = 0.047) (Fig. 2 D). Infusion of tiron into the superficial epigastric artery of ‘ligated’ rats produced noticeable but not significant increases in baseline MAP (P = 0.41 vs. saline) (Fig. 2 A) and HR (P = 0.83 vs. saline) (Fig. 2 C).
Figure 1. Traces showing the pressor (BP) and cardioaccelerator (HR) responses to static contraction for two ‘ligated’ rats (A,B) during consecutive infusions of saline (control, left), tiron (centre) and saline (washout, right) into the superficial epigastric artery.
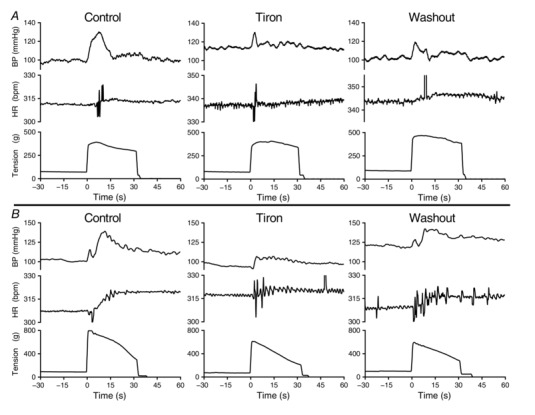
Tiron reduced the peak pressor and cardioaccelerator responses to contraction, as well as the duration of the pressor responses, compared to the responses of either of the saline infusion controls. Reinfusion of saline following tiron contraction (washout) partially restored the pressor and cardioaccelerator responses to contraction.
Figure 2. Summary data for ‘freely perfused’ (grey, n = 9) and ‘ligated’ (black, n = 12) rats undergoing three consecutive, static contractions during infusion of saline (Ctrl), tiron (Trn) and saline washout (Wash).
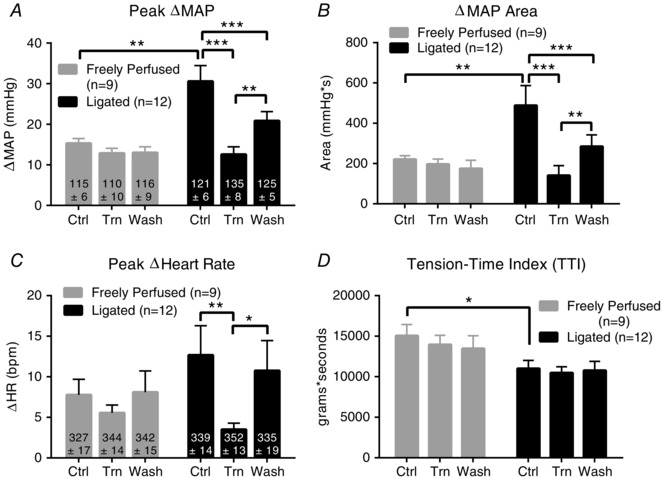
A and B, ‘ligated’ rats show a greater increase in mean arterial pressure (ΔMAP) during saline infusion than do ‘freely perfused’ rats. This exaggeration is abolished by tiron infusion, but partially restored during saline washout. C, tiron reduced the cardioaccelerator response (ΔHR) to contraction in ‘ligated’ rats, which was restored following saline washout. Tiron had no significant effect on the cardioaccelerator response to contraction in ‘freely perfused’ rats. D, rats undergoing femoral artery ligation showed a main‐effect reduction in the tension‐time index (TTI) compared to ‘freely perfused’ rats. * P < 0.05, ** P < 0.01 and *** P < 0.001 for bracketed comparison. The baseline mean ± SE is shown within each vertical bar.
In seven ‘ligated’ rats, gp91ds‐tat, infused into the superficial epigastric artery while the hindlimb muscles were statically contracted, significantly decreased the exercise pressor reflex. This was the case both when the reflex was analysed for its peak, as well as for its integrated area of the 30 s contraction period. The attenuation of the exercise pressor reflex induced by gp91ds‐tat remained when saline replaced it as the perfusate (Fig. 3). The lack of a restored reflex during ‘washout’ is not surprising because gp91ds‐tat penetrates the myocytes and binds to intracellular p47phox.
Figure 3. Summary data for seven ‘ligated’ rats undergoing three consecutive, static contractions during infusion of saline (Ctrl), gp91ds‐tat and saline washout (Wash).
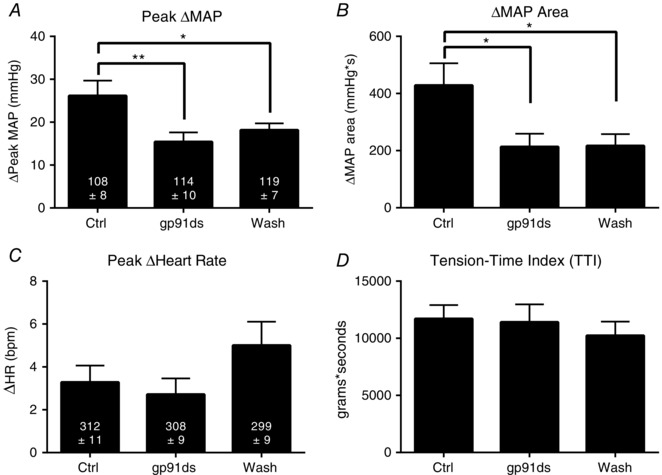
A and B, infusion of gp91ds‐tat abolished the exaggerated increase in mean arterial pressure (ΔMAP) in ‘ligated’ rats. The cardioaccelerator response to contraction (ΔHR, C), and tension‐time index of contraction (TTI, D) did not differ significantly between groups. * P < 0.05, ** P < 0.01 and *** P < 0.001 for bracketed comparison. The baseline mean ± SE is shown within each vertical bar.
Freely perfused rats
In nine rats, tiron infused into the superficial epigastric artery when the hindlimb muscles were statically contracted had no effect on the exercise pressor reflex (Fig. 2). The infusion of this anti‐oxidant was started 10 min before the onset of contraction and was maintained throughout the contraction period. Three consecutive contractions spaced 20 min apart evoked reflex pressor responses of very similar magnitudes, regardless of whether saline or tiron was infused into the superficial epigastric artery (Fig. 2). In three additional ‘freely perfused’ rats, we examined the effect of tiron infused into the superficial epigastric artery when the left iliac artery and vein were snared. We performed this manoeuver to partially trap tiron in the hindlimb circulation. Tiron infusion in these three rats had no effect on the exercise pressor reflex (Fig. 4 A).
Figure 4. Control experiments.
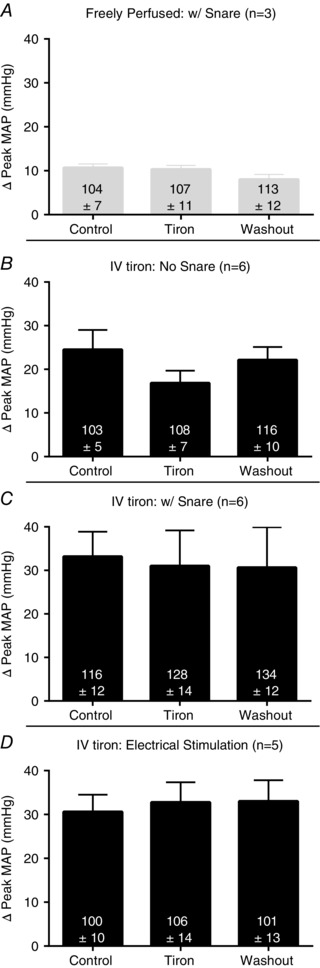
A, in three ‘freely perfused’ rats, we infused tiron into the superficial epigastric artery while acutely restricting blood flow through the iliac artery and vein to trap tiron in the hindlimb circulation; note that tiron still had no effect on the pressor response. B, infusion of tiron into the jugular vein (IV) in six, ‘ligated’ rats slightly and insignificantly reduced the pressor response to contraction (P = 0.32). C, in another six, ‘ligated’ rats, acutely restricting blood flow through the iliac artery and vein while infusing tiron i.v. mitigated (P = 0.97) the effect seen in (B). D, i.v. tiron infusion did not change the pressor response to electrical stimulation of the tibial nerve at 10–20 times the muscle twitch threshold in five ‘ligated’ rats that underwent neuromuscular blockade (P = 0.93).
Control experiments
In six ‘ligated’ rats, tiron, infused i.v., had no statistically significant effect (P = 0.32) on the magnitude of the exercise pressor reflex. Nevertheless, the magnitude of the reflex when tiron was infused i.v. tended to be marginally lower than that when saline was infused i.v. (Fig. 4 B). This finding prompted us to examine, in another six ‘ligated’ rats, the effect of i.v. tiron on the exercise pressor reflex when we snared the right iliac artery and vein. During snare of the iliac artery and vein, i.v. tiron had no effect (P = 0.97) on the magnitude of the exercise pressor reflex (Fig. 4 C). This finding led us to conclude that the small non‐significant attenuation of the exercise pressor reflex by i.v. tiron in the ‘ligated’ rats whose iliac artery and vein was not snared was caused by circulation of this anti‐oxidant to the hindlimb muscles via their collateral circulation. Finally, in another five ‘ligated’ rats, we infused tiron i.v. while electrically stimulating the tibial nerve at 10–20 times the motor twitch threshold. These stimulations evoked pressor responses similar to static contraction in ‘ligated’ rats, and were unaffected by i.v. tiron infusion (P = 0.93) (Fig. 4 D).
In every ‘freely perfused’ and ‘ligated’ rat described above, we found that electrical stimulation of the tibial nerve at two times the motor threshold in rats where neuromuscular blockade was achieved with pancuronium bromide did not increase arterial pressure and heart rate, whereas electrical stimulation before they underwent neuromuscular blockade increased both arterial pressure and heart rate. Likewise, in every rat in which we infused tiron into the superficial epigastric artery, infusion of Evan's blue dye into the superficial epigastric artery stained the triceps surae muscles. We interpreted the former finding as evidence indicating that the pressor and cardioaccelerator responses to electrical stimulation of the tibial nerve in rats without neuromuscular blockade were caused by contraction of the triceps surae muscles and were not caused by electrical stimulation of group III and IV afferent fibres in the tibial nerve. We interpreted the latter finding as evidence that infusion of tiron into the superficial epigastric artery entered the circulation of the triceps surae muscles.
Analysis of ROS production
The presence of ethidium‐positive nuclei was more evident in ‘ligated’ rat skeletal muscle compared to ‘freely perfused’ controls (Fig. 5 A), suggesting increased ROS production, which was effectively sequestered following intra‐ arterial tiron infusion (Fig. 5 A). In a separate in vitro fluorescence assay, NADH, a substrate for NAD(P)H‐oxidase, was used to drive the production of ROS, an effect that was measured by DHE fluorescence. We found that production of ROS in homogenates of the triceps surae and plantaris muscles was significantly greater in ‘ligated’ rats (n = 6) compared to the production of ROS in homogenates of these muscles in ‘freely perfused’ rats (n = 6) (Fig. 5 B). Tiron significantly decreased the production of ROS in both ‘ligated’ and ‘freely perfused’ rats and the magnitude of the effect of tiron was greater in the former than in the latter (Fig. 5 D).
Figure 5. Skeletal muscle ROS production is increased in rats with ‘ligated’ femoral arteries.
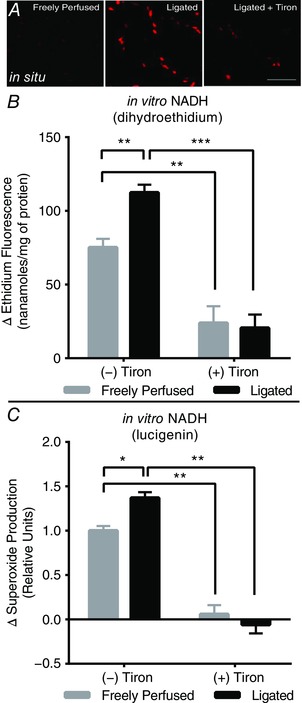
A, representative 20× images of pseudo‐coloured, red ethidium‐positive nuclei in plantaris muscle cross‐sections (scale bar = 100 μm; n = 2). The number of ethidium‐positive nuclei was increased in muscle from ‘ligated’ rats (middle) relative to ‘freely perfused’ controls (top) and was reduced following intra‐arterial tiron infusion (bottom). B, similarly, in vitro ethidium fluorescence in the presence of the Nox substrate, NADH, was quantitatively augmented in ‘ligated’ (n = 6) vs. ‘freely perfused’ rats (n = 6) and reduced with tiron administration in both groups, although more so in ‘ligated’ animals. C, lucigenin‐derived chemiluminescence in the presence of NADH was increased in ‘ligated’ (n = 8) vs. ‘freely perfused’ rats (n = 8) and was reduced similarly in both groups by tiron. * P < 0.05, ** P < 0.01 and *** P < 0.001 for bracketed comparison. [Color figure can be viewed at wileyonlinelibrary.com]
We also used a complementary chemiluminescent assay to assess NADH‐driven ROS production in homogenates of the triceps surae and plantaris muscles. We found that muscle homogenates from ‘ligated’ rats (n = 8) produced more ROS compared to muscle homogenates from their ‘freely produced’ counterparts (n = 8). Tiron significantly decreased the production of ROS in both groups of rats (Fig. 5 C).
Western immunoblotting
Expression of both Nox2 and p67phox (Fig. 6) was greater in muscle homogenates taken from rats with ligated femoral arteries compared to homogenates taken from rats with freely perfused arteries. By contrast, expression of Nox2 and p67phox (Fig. 6) was not different in DRG taken from rats with ligated femoral arteries compared to that in the L4‐L6 DRG taken from rats with freely perfused arteries.
Figure 6. Membrane bound Nox2 and cytosolic p67phox are increased in muscle but not in the DRG of rats with ligated femoral arteries.
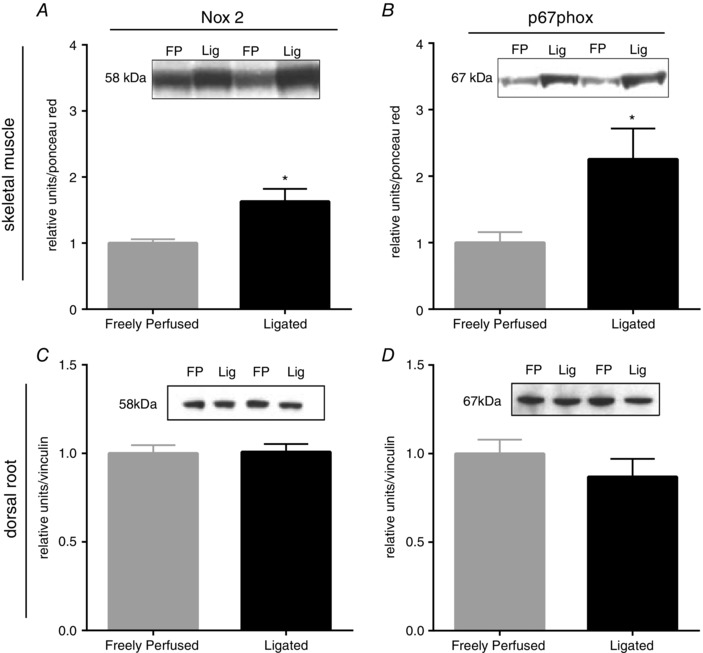
A and B, the increase in muscle ROS production with ligation was associated with increased immunoreactivity to Nox2 and p67phox. C and D, in contrast, the quantity of Nox2 and p67phox immunoreactivity was not increased in the DRG of rats with ligated femoral arteries. * P < 0.05 vs. freely perfused.
Discussion
In the present study, we found that infusion of tiron into the arterial circulation of the hindlimb muscles of ‘ligated’ rats attenuated the exercise pressor reflex, whereas infusion of tiron into the arterial circulation of the hindlimb muscles of ‘freely perfused’ rats had no effect on the reflex. Our present study demonstrating that tiron attenuated the exercise pressor reflex in ‘ligated’ rats contrasts with our previous reports that neither tiron nor tempol attenuated the reflex in ‘ligated’ rats (McCord et al. 2011; Yamauchi et al. 2012). We attribute these contrasting findings to differences in the methods used to administer the anti‐oxidants. In our present study, tiron was constantly infused while the hindlimb muscles were contracting, whereas, in our previous studies, tiron or tempol was injected as a bolus 15 min before contraction was initiated (McCord et al. 2011; Yamauchi et al. 2012). Moreover, during the last 5 min of that 15 min period, we released the snare functioning to trap the tiron within the arterial circulation of the hindlimb. Release of the snare may have increased resting blood flow to the hindlimb muscles, thereby flushing tiron or tempol from the circulation of the hindlimb muscles. Alternatively, or in combination, the anti‐oxidant activity of tiron or tempol may have diminished during this 15 min latent period.
Our present finding that tiron had no effect on the exercise pressor reflex in ‘freely perfused’ rats contrasts with that of Wang et al. (2009), who performed the same experiment as we did, except that they infused tempol rather than tiron into the arterial supply of the contracting muscles. Tempol is a superoxide dismutase mimetic, whereas tiron is a superoxide scavenger. We chose not to use tempol in our experiments because this compound opens KATP and BK channels on vascular smooth muscle cells (Xu et al. 2006; Chen et al. 2010). Consequently, any tempol‐induced attenuation of the exercise pressor reflex could be explained by its hyperpolarizing effect on vascular smooth muscle cells and not by its ability to decrease the concentration of superoxide ions in the contracting skeletal muscles. Tiron, in contrast, has no effect on either KATP or BK channels (Xu et al. 2006). In any event, our finding that tiron had no effect on the reflex in ‘freely perfused’ rats is consistent with a study by Muller et al. (2013) reporting that ascorbic acid, which also has anti‐oxidant actions, did not attenuate the reflex pressor response to plantar flexion in healthy humans.
The exercise pressor reflex is greater in ‘ligated’ rats than it is in ‘freely perfused’ rats (Tsuchimochi et al. 2010; McCord et al. 2011; Yamauchi et al. 2012). Our finding that tiron infusion attenuated the reflex in ‘ligated’ rats provides support for the hypothesis that the ligation‐induced exaggeration of the reflex is at least in part attributable to oxidative stress. Moreover, our finding that femoral artery ligation increased the concentration of two subunits of NAD(P)H oxidase in the triceps surae and plantaris muscles is consistent with this hypothesis. In particular, the increase in the expression of Nox2 could be viewed as especially supportive of our hypothesis because Nox2 is the catalytic subunit of the enzyme producing superoxide ions (Bedard & Krause, 2007). In a similar fashion, the increase in the concentration of p67phox is important because its translocation from the cytosol and binding to Nox2 at the cell membrane is required for Nox2 activation in contracting muscle (Sakellariou et al. 2014). This latter finding is consistent with a recent report of a two‐fold increase in p67phox levels in biopsied muscle tissue obtained from PAD patients compared to healthy control subjects (Walker et al. 2016). In addition, the binding of p67phox to Nox2 at the cell membrane is consistent with the view that during contraction NAD(P)H oxidase releases superoxide ions into the interstitial space (Sasser et al. 2012), which is the location of the endings of the thin fibre muscle afferents evoking the exercise pressor reflex.
Additional support for our hypothesis that oxidative stress contributed to the exaggeration of the exercise pressor reflex in ‘ligated’ rats came from our in vitro finding that ethidium fluorescence and lucigenin chemiluminescence, indices of superoxide production when driven by NADH, were greater in muscle homogenates taken from rats whose femoral arteries were ligated compared to muscle homogenates taken from rats whose femoral arteries were freely perfused. We also found that the addition of tiron to these homogenates markedly decreased this fluorescence and chemiluminescence in muscle homogenates taken from both ‘ligated’ and ‘freely perfused’ rats. We interpret these findings as being supportive of the concept that femoral artery ligation increased the capacity of skeletal muscles to produce superoxide ions and that tiron functioned in our experiments as a superoxide scavenger.
In a previous study, we reported that static contraction for 60 s increased 8‐isoprostane concentrations in the interstitial fluid of triceps surae muscles in both ‘freely perfused’ and ‘ligated’ rats (McCord et al. 2011). In this previous study, we were surprised to find that the magnitudes of the contraction‐induced increase in 8‐isoprostane concentrations were the same in both groups. Our inability to measure a difference in interstitial concentrations of 8‐isoprostane between ‘ligated’ and ‘freely perfused’ rats may have been attributable to limitations in the enzyme‐linked immunosorbent assay technique used to determine 8‐isoprostane concentrations. Specifically, the accuracy of this technique is dependent on the specificity of the antibody used to determine the concentrations of 8‐isoprostane, an eicosanoid that is assumed to be an index of cumulative oxidative damage rather than an index of short‐term ROS production (Griendling et al. 2016). Moreover, 8‐isoprostane concentrations as measured by gas chromatography with mass spectrometry detection correlated poorly with 8‐isoprostane concentrations as measured by the enzyme‐linked immunosorbent assay (Il'yasova et al. 2004). Consequently, within the short time frame of the 60 s static contraction used in our previous experiments (McCord et al. 2011), a difference in 8‐isoprostane concentrations between ‘freely perfused’ and ‘ligated’ rats may not have been either detectable or accurate. Despite this reservation, we cannot exclude the possibility that femoral artery ligation did not increase ROS production during the short duration static contractions used in our experiments. This possibility is probably not the case because we found that muscle homogenates taken from ‘ligated’ muscles had the capability to produce more ROS than did muscle homogenates taken from ‘freely perfused’ muscles. We also found that femoral arterial ligation increased the amount of two subunits of the enzyme responsible for the production of superoxide ions in contracting muscles.
We can only speculate about how contraction‐induced increases in ROS functioned to exaggerate the exercise pressor reflex evoked by static contraction in ‘ligated’ rats. One possibility is that the increase in the production of ROS sensitized metaboreceptors innervating the exercising muscles of ‘ligated’ rats. A second possibility is that the increase in ROS sensitized the mechanoreceptors innervating these muscles. The two possibilities are not mutually exclusive in that both may occur simultaneously. The ligation‐induced increase in the production of ROS during contraction may also interact with ligation‐induced increases in receptors on thin fibre muscle afferents responsive to metabolic and mechanical stimuli. In particular, ligation has been shown to increase receptors on dorsal root ganglion cells that innervate the hindlimb and are responsive to metabolic by‐products of muscle contraction. These receptors include ASIC 3, P2X3, EP4 and TRPV1 (Xing et al. 2008; Liu et al. 2010; Xing et al. 2013; Yamauchi et al. 2013). By contrast, ligation has been shown to have no effect on Piezo 2 protein concentrations found in dorsal root ganglion cells innervating the hindlimb. Piezo 2 channels are conisdered to comprise at least in part the mechanoreceptor channel on thin fibre muscle afferents (Copp et al. 2016a, b).
In our experiments, baseline arterial pressure increased by an average of 14 mmHg when we infused tiron into the superficial epigastric artery of ‘ligated’ rats. On the other hand, baseline arterial pressure did not increase when we infused this anti‐oxidant into the superficial epigastric artery of ‘freely perfused’ rats. Although we can offer no explanation as to why tiron infusion increased arterial pressure in “ligated’ rats, it could be argued that the increase in baseline might have functioned as a ceiling in our experiments to limit the magnitude of the exercise pressor reflex. We think that this is not the case because an even larger average increase in baseline arterial pressure of 18 mmHg (Fig. 3 C) had no effect on the magnitude of the exercise pressor reflex.
In summary, we have found that oxidative stress played a role in evoking the exercise pressor reflex in rats whose femoral arteries were ligated, although it had no role in evoking the reflex in rats whose femoral arteries were freely perfused. The significance of our latter finding is that we duplicated the infusion technique used by Wang et al. (2009) but could not duplicate their finding that oxidative stress plays a role in evoking the exercise pressor reflex in rats with patent femoral arteries. By contrast, our finding showing that oxidative stress played a role in evoking the exercise pressor reflex in simulated peripheral artery disease confirms and extends previous findings in humans showing that oxidative stress played a role in causing the exaggeration of the exercise pressor reflex (Muller et al. 2012).
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
JEH and MPK conceived and designed the contraction experiments. JMK, GDT and MPK conceived and designed the fluorescence and immunoblotting experiments. JEH and JSK acquired the contraction data, which was analysed by JEH and interpreted by JEH and MPK. JEH and JMK acquired the tissue for fluorescence, immunoblotting and lucigenin experiments, which was analysed by JMK and interpreted by JMK and GDT. All authors contributed to drafting the manuscript, are accountable for its scientific integrity, and approve of its submission.
Funding
This work was funded by National Institutes of Health grants R01 AR059397, R01 HL130987 and P01 HL096570. The NIH is thanked for their support of this work.
References
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K & Krause KH (2007). The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hardas SS & Lange ML (2010). Oxidatively modified glyceraldehyde‐3‐ phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J Alzheimers Dis 20, 369–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Patel K, Connors SG, Mendonca M, Welch WJ & Wilcox CS (2010). Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293, H3246–H3253. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez‐Gonzalez JF (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz‐Velasco V & Kaufman MP (2016a). The mechano‐gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol 594, 641–655, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Kim JS, Ruiz‐Velasco V & Kaufman MP (2016b). The mechano‐gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 310, H1233–H1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S, Griendling KK, Harrison DG (2007). Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A & American Heart Association Council on Basic Cardiovascular S (2016). Measurement of reactive oxygen species, reactive nitrogen species, and redox‐dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res 119, e39–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW (1994). Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74:1141–1148. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in the journal of physiology and experimental physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'yasova D, Morrow JD, Ivanova A & Wagenknecht LE (2004). Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3‐dinor‐5,6‐dihydro‐15‐F2t‐isoprostane. Ann Epidemiol 14, 793–797. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Koba S, Gao Z & Sinoway LI (2009). Oxidative stress and the muscle reflex in heart failure. J Physiol 587:5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Watanabe R, Kano N & Watanabe T (2013). Oxidative stress exaggerates skeletal muscle contraction‐evoked reflex sympathoexcitation in rats with hypertension induced by angiotensin II. Am J Physiol Heart Circ Physiol 304, H142–H153. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao Z & Li J (2010). Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299, H1357–H1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Yamauchi K, Leal A & Kaufman MP (2011). Tempol attenuates the exercise pressor reflex independently of neutralizing reactive oxygen species in femoral artery ligated rats. J Appl Physiol 111, 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA (1983). The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB & Sinoway LI (2012). Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590, 6237–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Cui J, Blaha CA, Mast JL & Sinoway LI (2013). Effect of oxidative stress on sympathetic and renal vascular responses to ischemic exercise. Physiol Rep 1, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS, Augustyniak RA, Ansorge EJ & Collins HL (1999). Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol 276, H1399–H1403. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Peterson TE & Harrison DG (1993). Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91, 2546–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS & Iadecola C (2008). Nox2‐derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A 105:1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK & Harrison DG (1996). Angiotensin II‐mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97:1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT & Pagano PJ (2001). Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2– and systolic blood pressure in mice. Circ Res 89, 408–414. [DOI] [PubMed] [Google Scholar]
- Rotto DM & Kaufman MP (1988). Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Jackson MJ & Vasilaki A (2014). Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic Res 48, 12–29. [DOI] [PubMed] [Google Scholar]
- Sasser JM, Moningka NC, Tsarova T & Baylis C (2012). Nebivolol does not protect against 5/6 ablation/infarction induced chronic kidney disease in rats – comparison with angiotensin II receptor blockade. Life Sci 91, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH & Garry MG (2001). Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JC, Li Z, Yang HT, Laughlin MH & Terjung RL (2008). Alpha‐adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586, 1649–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S & Kaufman MP (2010). Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299, H106–H113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y & Askew CD (2016). Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246, 98–105. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Pan YX, Wang WZ, Zucker IH & Wang W (2009). NADPH oxidase‐derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Gao Z, Lu J, Sinoway LI & Li J (2008). Femoral artery occlusion augments TRPV1‐mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295, H1262–H1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J & Li J (2013). Augmented P2X response and immunolabeling in dorsal root ganglion neurons innervating skeletal muscle following femoral artery occlusion. J Neurophysiol 109, 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jackson WF, Fink GD & Galligan JJ (2006). Activation of potassium channels by tempol in arterial smooth muscle cells from normotensive and deoxycorticosterone acetate‐salt hypertensive rats. Hypertension 48, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Kim JS, Stone AJ, Ruiz‐Velasco V & Kaufman MP (2013). Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591, 2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Stone AJ, Stocker SD & Kaufman MP (2012). Blockade of ATP‐sensitive potassium channels prevents the attenuation of the exercise pressor reflex by tempol in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol 303, H332–H340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W & Thomas GD (2006). Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II‐dependent hypertension. Hypertension 48, 637–643. [DOI] [PubMed] [Google Scholar]
- Zou AP, Li N & Cowley AW, Jr (2001). Production and actions of superoxide in the renal medulla. Hypertension 37, 547–553. [DOI] [PubMed] [Google Scholar]


