Abstract
Key points
Golgi tendon organs (GTOs) and associated Ib reflexes contribute to standing balance, but the potential impacts of threats to standing balance on Ib reflexes are unknown.
Tendon electrical stimulation to the Achilles’ tendon was used to probe changes in Ib inhibition in medial gastrocnemius with postural orientation (lying prone vs. upright standing; experiment 1) and height‐induced postural threat (standing at low and high surface heights; experiment 2).
Ib inhibition was reduced while participants stood upright, compared to lying prone (42.2%); and further reduced when standing in the high, compared to low, threat condition (32.4%).
These experiments will impact future research because they demonstrate that tendon electrical stimulation can be used to probe Ib reflexes in muscles engaged in standing balance.
These results provide novel evidence that human short‐latency GTO‐Ib reflexes are dependent upon both task, as evidenced by changes with postural orientation, and context, such as height‐induced postural threat during standing.
Abstract
Golgi tendon organ Ib reflexes are thought to contribute to standing balance control, but it is unknown if they are modulated when people are exposed to a postural threat. We used a novel application of tendon electrical stimulation (TStim) to elicit Ib inhibitory reflexes in the medial gastrocnemius, while actively engaged in upright standing balance, to examine (a) how Ib reflexes to TStim are influenced by upright stance, and (b) the effects of height‐induced postural threat on Ib reflexes during standing. TStim evoked short‐latency (<47 ms) inhibition apparent in trigger‐averaged rectified EMG, which was quantified in terms of area, duration and mean amplitude of inhibition. In order to validate the use of TStim in a standing model, TStim‐Ib inhibition was compared from conditions where participants were lying prone vs. standing upright. TStim evoked Ib inhibition in both conditions; however, significant reductions in Ib inhibition area (42.2%) and duration (32.9%) were observed during stance. Postural threat, manipulated by having participants stand at LOW (0.8 m high, 0.6 m from edge) and HIGH (3.2 m, at edge) elevated surfaces, significantly reduced Ib inhibition area (32.4%), duration (16.4%) and amplitude (24.8%) in the HIGH, compared to LOW, threat condition. These results demonstrate TStim is a viable technique for investigating Ib reflexes in standing, and confirm Ib reflexes are modulated with postural orientation. The novel observation of reduced Ib inhibition with elevated postural threat reveals that human Ib reflexes are context dependent, and the human Ib reflex pathways are modulated by threat or emotional processing centres of the CNS.
Keywords: ib reflexes, golgi tendon organ, postural threat
Key points
Golgi tendon organs (GTOs) and associated Ib reflexes contribute to standing balance, but the potential impacts of threats to standing balance on Ib reflexes are unknown.
Tendon electrical stimulation to the Achilles’ tendon was used to probe changes in Ib inhibition in medial gastrocnemius with postural orientation (lying prone vs. upright standing; experiment 1) and height‐induced postural threat (standing at low and high surface heights; experiment 2).
Ib inhibition was reduced while participants stood upright, compared to lying prone (42.2%); and further reduced when standing in the high, compared to low, threat condition (32.4%).
These experiments will impact future research because they demonstrate that tendon electrical stimulation can be used to probe Ib reflexes in muscles engaged in standing balance.
These results provide novel evidence that human short‐latency GTO‐Ib reflexes are dependent upon both task, as evidenced by changes with postural orientation, and context, such as height‐induced postural threat during standing.
Abbreviations
- η2
Eta squared effect size
- EDA
electrodermal activity
- GTO
Golgi tendon organ
- H‐reflex
Hoffmann reflex
- HIGH
high surface height postural threat condition
- LOW
low surface height postural threat condition
- MGas
medial gastrocnemius muscle
- MTJ
musculo‐tendinous junction
- PT
perceptual threshold
- RMS
root mean square
- TA
tibialis anterior muscle
- TStim
tendon electrical stimulation
Introduction
Golgi tendon organs (GTOs) are muscle mechanoreceptors located at the musculo‐tendinous junction (MTJ) and are arranged in series with contractile muscle fibres (Jami, 1992; Pearson & Gordon, 2000). GTOs are sensitive to tensile load applied to the tendon, particularly from active muscle contraction (Houk & Henneman, 1967). Ib afferents, arising from GTOs, project to a wide variety of CNS targets. While Ib homonymous inhibition is the shortest‐latency reflex evoked by GTOs, Ib afferents contribute to a broad array of excitatory and inhibitory spinal and supra‐spinal reflexes (Jami, 1992; Jankowska, 1992). GTOs and associated Ib reflexes are thought to contribute to balance control by evaluating body loading (i.e. gravity effects) and contributing to the setting of anti‐gravity muscle activity (Duysens et al. 2000; Van Doornik et al. 2011). Net GTO‐based Ib reflexes are largely inhibitory while people sit or lie prone, yet inhibition is reduced when standing (Faist et al. 2006), and duration of inhibition is reduced while net reflexes can become excitatory when walking (Stephens & Yang, 1996; Faist et al. 2006). Short‐latency (<50 ms) plantar flexor GTO reflexes are thought to promote triceps surae muscle activity during quiet standing (Van Doornik et al. 2011) and in the stance phase of gait (Sinkjær et al. 2000; Grey et al. 2007), and have been suggested to contribute to the scaling of reactive responses to balance disturbances (Dietz et al. 1992).
One important context for balance control is postural threat. Postural threats are environmental factors which impose a challenge to standing balance by increasing either the likelihood (e.g. threat of perturbation: Horslen et al. 2013; Lim et al. 2016) or consequence of falling (e.g. height‐induced threat: Carpenter et al. 1999; Cleworth et al. 2012), yet do not alter the essential balance task at the time of measurement (e.g. stand in place on a stable surface). Height‐induced postural threats increase fear of falling and anxiety, increase sympathetic arousal (Carpenter et al. 2006), and affect balance behaviours. Typically, postural sway is reduced and people withdraw from the edge while standing quietly (Carpenter et al. 2001) and move less toward the edge when perturbed (Carpenter et al. 2004). Postural threats have also been shown to influence multiple balance‐relevant sensory systems, including vestibular‐evoked muscle and balance responses (Horslen et al. 2014; Naranjo et al. 2015; Lim et al. 2016) and Ia monosynaptic reflexes (Llewellyn et al. 1990; Sibley et al. 2007; Davis et al. 2011; Horslen et al. 2013). However, it is currently unknown to what extent postural threat influences GTOs and associated Ib reflexes while engaged in balancing.
Ib reflexes have traditionally been probed with either Hoffmann (H‐) reflex conditioning (Pierrot‐Deseilligny et al. 1979; Stephens & Yang, 1996; Faist et al. 2006) or muscle unloading (Dietz et al. 1992; Sinkjær et al. 2000; Grey et al. 2007; Van Doornik et al. 2011). However, these techniques may be confounded in a postural threat scenario because H‐reflexes may be altered (Llewellyn et al. 1990; Sibley et al. 2007) and the perturbations used to unload muscles may trigger balance‐correcting responses, which are affected by threat (Carpenter et al. 2004). Tendon electrical stimulation (TStim) has emerged as an alternative, more direct, method of evoking Ib reflexes (Burne & Lippold, 1996). TStim involves square‐wave electrical stimulation of a tendon at the MTJ and evokes short latency reflexive inhibition in the stimulated muscle (see Fig. 1 C). The reflex has been demonstrated in several upper‐ and lower‐limb muscles (Burne & Lippold, 1996; Priori et al. 1998), but has most extensively been studied in the gastrocnemii muscles (Khan & Burne, 2007, 2009, 2010; Rogasch et al. 2011, 2012).
Figure 1. Tendon imaging, stimulating electrode placement and TStim inhibition analyses.
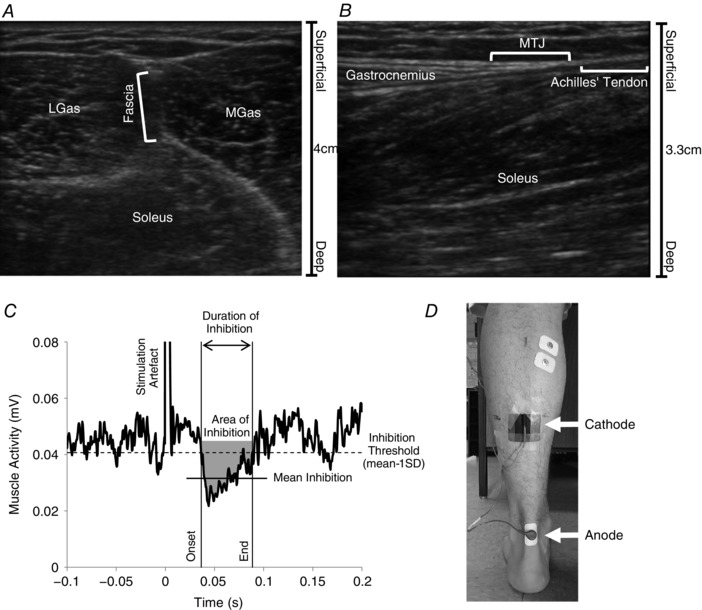
Ultrasound images were used to locate the musculo‐tendinous junction (MTJ) for placement of the cathode. First, transverse plane ultrasound was used to locate the fascial band between the heads of the gastrocnemii (A), which was used to mark the centre of the MTJ in the medio‐lateral line. Based on the technique of Maganaris & Paul (1999), a sagittal view of the muscle along the marked medio‐lateral line was used to identify where muscle fibres terminated and the Achilles’ tendon began (B) and the skin was marked accordingly. C, representative trace of TStim‐evoked inhibition in a 100‐pulse waveform average of rectified MGas EMG, aligned in time to stimulation. A mean − 1 SD threshold (dashed horizontal line) was used to determine the onset and end of the response (continuous vertical lines); the threshold for detection of both onset and end was the signal remaining below or above threshold for at least 7 ms in a 10 ms period, only onsets occurring between 35 and 65 ms post‐stimulation were accepted. The area of inhibition referenced to mean background activity (grey shaded area), duration of inhibition, and mean amplitude of inhibition (continuous horizontal line) were calculated in this window. D, locations of the cathode (over the MTJ) and anode electrode placements.
Percutaneous electrical stimulation of the Achilles’ tendon near the gastrocnemii MTJ (i.e. over the proximal gastrocnemius aponeurosis; Fig. 1 D) evokes a short latency (<50 ms), multiphasic pattern of negative and positive peaks in rectified surface EMG in both medial and lateral gastrocnemii (Khan & Burne, 2007, 2009, 2010). Despite apparent increases in EMG activity at approximately 140 ms and 240 ms post‐stimulation (Khan & Burne, 2007), the response is thought to be a, possibly singular, inhibitory reflex. This is because tendon stretch (Ia) reflexes and transcranial magnetic stimulation‐evoked twitches are both inhibited when triggered during the TStim reflex (Khan & Burne, 2010), and single motor unit peristimulus frequencygrams do not demonstrate periods of excitation (Rogasch et al. 2011). This reflex is thought to be mediated by Ib spinal pathways because of the short latency (<50 ms in gastrocnemii), which is slightly longer than Ia monosynaptic tendon‐tap reflex latencies from the same muscle group (mean: ∼40 ms, range: 36–50 ms; Türker et al. 1997), and the polarity of the response is consistent with Ib autogenic inhibition. Nonetheless, contributions from alternative sources, such as muscle or tendon type III afferents, to TStim‐evoked inhibition cannot be completely ruled out at this time (cf. Priori et al. 1998). TStim inhibition has not yet been demonstrated in standing participants. Ib inhibition is thought to be reduced in standing, compared to sitting or lying (Faist et al. 2006), yet H‐reflexes, which were used in the previous studies, are also affected by standing (Cattagni et al. 2014). Therefore, it would be beneficial to replicate standing‐related changes to Ib inhibition with a complementary method to ensure Ib reflexes are indeed subject to postural orientation prior to attempting to characterize the effects of postural threat on Ib reflexes.
This paper is intended to address two general aims: first, to determine if TStim is a suitable technique for probing Ib reflexes in standing, with responses mediated in a manner consistent with known changes to Ib reflexes in standing (Expt 1); and second, to understand the effects of height‐induced postural threat on GTO‐based reflexes (Expt 2). In Expt 1, TStim‐evoked reflexes in the medial gastrocnemius were compared between upright standing and lying prone conditions. It was hypothesized that TStim‐evoked inhibition would be observed in both conditions, but the response in standing would be reduced in amplitude and duration, consistent with observations of decreased Ib inhibition in standing from H‐reflex conditioning studies (Faist et al. 2006). In Expt 2, TStim‐evoked inhibition was compared between conditions of low (LOW) and high (HIGH) postural threat induced by standing at different heights. Based on previous observations of threat‐related facilitation of plantar flexor reflexes during standing balance (Davis et al. 2011; Horslen et al. 2013; Naranjo et al. 2015; Lim et al. 2016), we hypothesized TStim‐evoked inhibitory responses would be diminished in amplitude and duration when standing at a HIGH, compared to a LOW, threat condition.
Methods
Ethical approval
All participants were young healthy adults recruited from the university community who gave written informed consent. All methods were approved by the University of British Columbia Clinical Research Ethics Board and conformed to the Declaration of Helsinki. Participants were recruited for both experiments (Expt 1 and 2), which took place in a single session (usually less than 2 h). Of the 41 participants recruited (22 females and 19 males), 29 participants completed the experiments and 12 were excluded from both experiments because they found the stimulus painful or unpleasant (n = 2), the stimulus evoked a sural nerve paraesthesia or no reflex was observed (n = 9), or due to equipment malfunction (n = 1); 2 participants withdrew consent specifically for Expt 2, and therefore were only included in Expt 1. Other participants were excluded post hoc from one or both experiments due to observations of non‐physiological noise (n = 3), or a lack of measurable TStim response (i.e. response did not return to baseline or no response was evoked; n = 8). As such, 24 people were included in the final Expt 1 sample (12 females, 12 males; age: (mean ± SEM) 23.7 ± 1.0 years; height: 171.5 ± 2.1 cm; weight: 67.8 ± 2.8 kg) and 21 people were included in the final Expt 2 sample (9 females, 12 males; age: 24.1 ± 1.1 years; height: 171.4 ± 2.2 cm; weight: 68.8 ± 3.0 kg).
Imaging
Ultrasound imaging (MicroMaxx; SonoSite, Inc., Bothell, WA, USA) was used to locate the MTJ in all subjects as a guide for positioning the cathode stimulating electrode (see Fig. 1 for details). This site was targeted because GTOs are precisely located at the junction between contractile muscle fibres and non‐contractile tendinous fibres, and not in the tendon proper (Jami, 1992), and gastrocnemius TStim effects are optimal when the cathode is positioned near the MTJ (Khan & Burne, 2009).
Muscle activity recordings
Muscle activity was recorded in both experiments with surface EMG from the medial gastrocnemius (MGas) and tibialis anterior (TA) muscles on the left side in all subjects. EMG were pre‐amplified ×1000, bandpass filtered online 10–1000 Hz (P55 A.C. Pre‐amplifier, Grass, West Warwick, RI, USA) and sampled at 2000 Hz (Power 1401 with Spike2 software, Cambridge Electronics Design (CED), Cambridge, UK). These experiments focused primarily on MGas as the muscle of interest because it is: (a) an anti‐gravity ankle extensor involved in quiet standing; (b) accessible and inserts into the Achilles’ tendon (i.e. soleus is deep and lies between cathode and anode, and therefore EMG contained significant artefacts); and (c), is active in unperturbed quiet standing, where lateral gastrocnemius is often not (Héroux et al. 2014).
Muscle activity matching
Participants performed a 90 s quiet standing trial prior to any stimulation, which was used to calculate a baseline mean ± 1 SD of MGas EMG root mean square (RMS) activity. Since muscle activity must be present to observe inhibition, and some people do not tonically activate MGas while standing quietly (Héroux et al. 2014), some participants were asked to adopt a slight forward lean in standing trials to ensure MGas was engaged. Muscle activity levels were matched between conditions within an experiment (i.e. between prone and standing for Expt 1, and across heights for Expt 2). Muscle activity levels were monitored online by an experimenter and verbal feedback was given in all trials (even if participant was successfully maintaining target activity) to ensure participants remained in the target mean ± 1 SD range.
EMG offline processing and reflex measurement
Raw EMG data were baseline corrected and rectified offline (Spike2, CED) and trigger‐averaged to stimulus onset. Two separate techniques were used to build trigger averages: first, averages were constructed from all stimuli in a trial to capture the natural effect of the manipulation. However, the amplitude of TStim‐evoked inhibition has been shown to be negatively correlated with background muscle activity level while participants lay on their side (Khan & Burne, 2007) and changes in muscle activity might be expected in both experiments, even with feedback. Therefore, trigger averages were also re‐constructed using a more conservative approach where individual stimuli were excluded if they were preceded by background muscle activity outside a pre‐specified range, as described below (screened stimuli; Figs 2 C and 3 B; based on Davis et al. 2011). Custom software (Spike2, CED) was used to calculate RMS amplitude of unrectified MGas EMG over a 100 ms period ending 10 ms before stimulation for each stimulus and a mean and SD were calculated from these values for each condition. The condition with the smallest SD was used to set the mean ± 1 SD thresholds for screening. Each trial was then re‐evaluated and individual stimuli from either condition falling outside the threshold were omitted (i.e. prone or standing trial for Expt 1, between heights for Expt 2).
Figure 2. Experiment 1: comparison of prone and standing conditions.
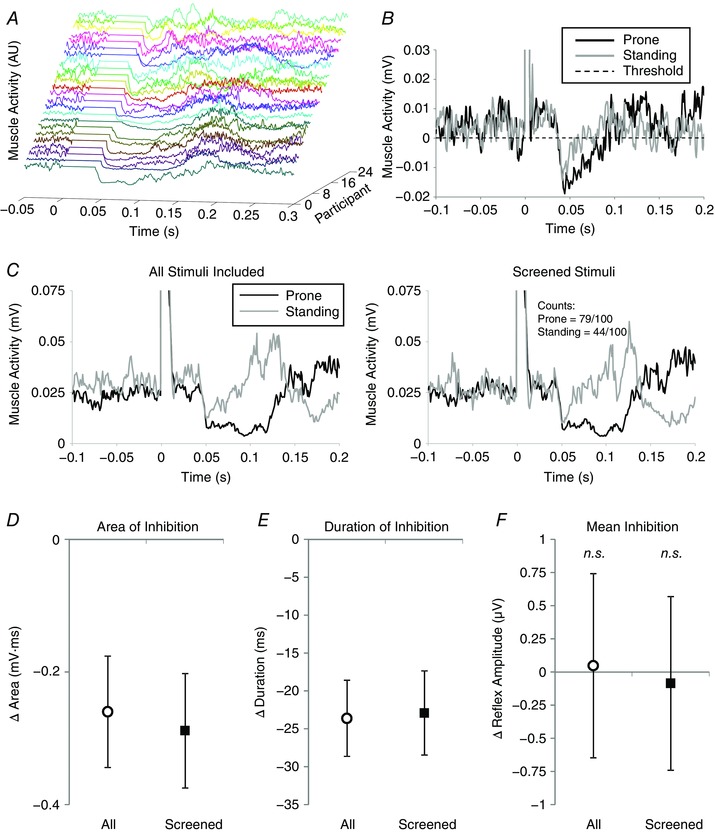
A, individual traces demonstrating TStim‐evoked inhibition for all participants (different colours) in the prone condition from Expt 1. Traces have been staggered to separate individuals and amplitude normalized to highlight the inhibition period. The artefact has been removed and the period between stimulation (time zero) and the algorithm‐detected onset of inhibition has been zeroed to highlight differences in onset latencies across participants. Cases are presented from longest to shortest duration of inhibition. B, prone (black) and standing (grey) 100‐pulse waveform averages from a representative participant are contrasted. The waveforms have been shifted for display purposes so that their respective inhibition detection thresholds (dashed horizontal line) are aligned at zero. The inhibition begins at approximately the same latency in both prone and standing conditions, but the duration and area of inhibition are longer and larger, respectively, in the prone condition. C, the effects of screening for changes in background muscle activity are demonstrated for a second representative participant. The traces in the left column were constructed using all 100 stimuli from each condition, whereas the waveform averages on the right were constructed after individual stimuli outside the screening range were excluded (21 removed from prone, 56 removed from standing condition). D–F, group‐wide mean differences in area, duration and mean amplitude of inhibition. In each panel, open circles represent the average effect when all stimuli are included in the analysis and filled squares represent effects after screening for changes in background muscle activity; error bars indicate standard error about the mean and n.s. indicates effect is not statistically different between postural orientation conditions.
Figure 3. Experiment 2: comparison of LOW and HIGH postural threat conditions.
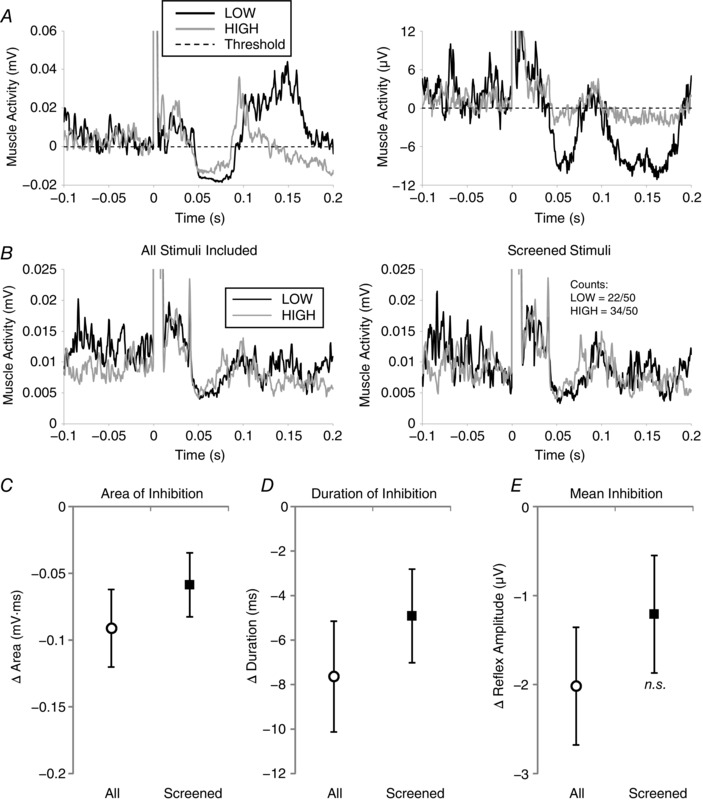
A, 50‐pulse waveform averages from two representative participants (left and right panels) from the LOW (black) and HIGH (grey) postural threat conditions, with amplitude aligned to inhibition detection thresholds (horizontal dashed line) for display purposes. B, similar to Fig. 2, the effects of background muscle activity screening on TStim waveform averages are shown for a third representative participant. Here, 28 of 50 stimuli were removed in the LOW threat condition, and 16 of 50 removed from the HIGH condition trace in the muscle activity screening process. C–E, mean differences in area, duration and amplitude of inhibition across threat conditions. Similar to Fig. 2, open circles (n = 21) and filled squares (n = 18, 3 participants excluded because too few stimuli remained after screening) represent analyses based on all and screened stimuli, respectively. Error bars indicate standard error, and n.s. indicates differences between threat conditions are not statistically significant.
Four measures of TStim inhibition were calculated offline using custom algorithms (Spike2, CED). A mean − 1 SD threshold (calculated over a 100 ms period ending 10 ms before stimulation onset) was used to calculate onset and duration of inhibition (Fig. 1 C). Each period of inhibition was then used to calculate a mean level (mean over duration of response, referenced to background) and area of inhibition (based on trapezoid integration) relative to pre‐stimulus background EMG activity (see Fig. 1 C for details).
Height‐specific measures
In Expt 2, participants’ psychological and autonomic responses to threat were also assessed. Self‐reported balance confidence (100‐point scale where higher values indicate greater confidence) was recorded prior to each trial, and self‐reports of anxiety (16‐item 1–9 rating questionnaire where higher scores indicate more anxiety; maximum score: 144), fear of falling (100‐point scale where higher scores indicate greater fear), and perceived stability (100‐point scale where lower scores indicate the participant felt less stable) were recorded after each trial. These questionnaires have previously been demonstrated to have moderate to high reliability in a height‐induced postural threat protocol (Hauck et al. 2008). Electrodermal activity (EDA) was measured with galvanic skin conductance (model 2501, CED) over the course of each trial (sampling: 100 Hz; Power 1401, CED) to quantify sympathetic autonomic arousal (Boucsein et al. 2012). EDA data were low‐pass filtered offline (5 Hz) and averaged over the duration of the trial in each condition (Spike2, CED); mean conductance levels are reported in microsiemens (μS).
Stimulation
TStim stimuli were delivered to the left Achilles’ tendon with single 0.5 ms square‐wave pulses (DS7A Constant Current Stimulator, Digitimer, Welwyn Garden City, UK; V max set to 300 V) with cathode (9 cm² carbon‐rubber pad coated with conductive gel; Spectra 360 Gel, Parker Labs, Fairfield, NJ, USA) positioned at the MTJ and anode (Kendall H59P Cloth Electrode, Medtronic, Dublin, Ireland) over the tendon approximately 2 cm proximal to the calcaneus (Fig. 1 D). Stimulation protocols began with detection of perceptual threshold (PT), defined as the lowest current intensity where participants could detect a non‐specific tingling or tapping sensation on both ascending and descending incremental current changes (mean PT was 4.41 ± 0.36 mA). Participants were then presented with individual pulses at multiples of ×2 PT (×2, ×4, ×6, ×8 and ×10) and asked to describe the sensation evoked. Participants typically described a muscular sensation, generally near ×4–6 PT, such as a tugging or pulling sensation at/near the insertion of the Achilles’ tendon onto the calcaneus, a deep paraesthesia or tingling near the cathode or on a line between cathode and anode, or felt a muscle twitch. Participants were excluded at this stage if they felt a paraesthesia along the lateral border of the foot, consistent with sural nerve stimulation. Qualitative descriptions were not further analysed.
Next, participants were given 25‐pulse trials (random 1–4 s interstimulus interval) at a fixed intensity while lying prone with ankle at approximately 90 deg and contracting against manual resistance provided by an experimenter to activate MGas to determine if a reflex was present. Stimulation intensity was adjusted in ×0.5 PT increments between trials to find the lowest intensity where a response could be observed (group mean = ×4.9 ± 0.3 PT). The criteria used to identify the presence of a response to TStim was MGas rectified EMG dropping below background levels for at least 10 ms (width of algorithm detection window, see Fig. 1 legend) starting approximately 45 ms post‐stimulation, determined by visual inspection of a waveform average of each 25‐pulse trial.
Participants then did a practice 100‐pulse prone trial at the lowest intensity at which a reflex could be evoked. During the trial, the participant plantar flexed against manual resistance and verbal feedback was used to guide contraction to previously determined baseline quiet standing levels. Immediately following the practice prone trial, the participant stood up and performed a 100‐pulse practice standing trial. During the standing trial the participant faced a blank wall and an experimenter occasionally gave verbal feedback about participant's forward lean to maintain target activation levels. The reflexes from both practice standing and prone trials were assessed online and durations of inhibition (if present) were noted. In most cases, the inhibitory reflex during standing was decreased and shorter in duration than in the practice prone trial; in some cases, no inhibition was initially observed in standing. Subsequently, stimulation intensity was adjusted (×0.5 PT increments) to a level where reflexes comparable to those evoked in the practice prone trial could be evoked in standing (assessed in 25‐pulse trials). The purpose of the adjustment was to ensure that a reflex similar to that achievable while lying prone could be achieved in standing, enabling comparison of like responses between conditions. The new stimulation intensity (group mean = ×6.8 ± 0.3 PT) was used for all subsequent trials.
Protocol
Participants performed two trials in each experiment. In Expt 1, trials consisted of 100 stimuli presented with a 1–4 s interstimulus interval, and the standing trial was always performed first. During the standing trial participants stood facing a blank wall and, where necessary, maintained a forward lean to activate MGas; verbal feedback about muscle activity levels was given to all participants (e.g. lean forward/backward/maintain), even if they successfully maintained target levels. In the prone trial, participants lay prone on a padded table and made an isometric plantar flexor contraction with ankle at approximately 90 deg against manual resistance. Similar to the standing trial, in all cases an experimenter gave verbal feedback about contraction intensity (e.g. push harder/less/maintain).
After completion of Expt 1, participants were transferred to a hydraulic lift (M419‐207B01H01D, Pentalift, Guelph, Canada) for Expt 2. Participants stood at the edge of the lift first in the LOW condition, where the lift was set to the lowest level (0.8 m) and a 0.6 m‐wide stable support surface was placed directly in front of the participant; this setting is comparable to standing on the ground (Carpenter et al. 1999). The support surface extension was removed and the lift elevated to 3.2 m for the HIGH condition. Since there is a known order effect for this postural threat manipulation (Adkin et al. 2000), and we wished to maximize contrast between conditions, the LOW condition was always presented first. Participants wore a safety harness attached to a safety line and an experimenter was within arm's reach at all times in case the participant lost balance. No participants lost their balance in either experiment. The number of stimuli was reduced to 50 per condition in Expt 2 to limit the amount of time participants had to stand at the edge in the HIGH condition, and because 50 pulses have been shown to be sufficient to evoke TStim inhibition (Miller & Burne, 2014). Otherwise, all methods, including analyses and provision of feedback, were similar to Expt 1.
Since non‐GTO origins of TStim inhibition, including skin overlying the tendon, sural nerve, or stimulation of other muscle or tendon afferents cannot yet be completely ruled out, we performed a post hoc experiment to compare reflexes evoked by percutaneous and direct subcutaneous tendon stimulation in a single 40‐year‐old male subject. Methods were drawn from a similar two‐person pilot study by Rogasch et al. (2012). The MTJ was identified and marked (see ‘Imaging’), and percutaneous stimulation intensity adjusted to a level where the participant felt a tendinous sensation (e.g. tugging, pulling or tapping). Surface EMG was recorded from the left MGas and TA (Neurolog NL 824 pre‐amplified with NL 820 optical isolator, Digitimer, UK) and sampled at 2000 Hz (Power 1401 with Spike2, CED). After percutaneous stimulation, the site was cleansed and two sterile tungsten microneurography needle electrodes insulated to the tip (impedance, 10 MΩ on insertion; UNA47F0U; FHC, Bowdoin, ME, USA) were inserted into the tendon (cathode ∼1 cm distal to the marked MTJ and anode 2 cm distal to the cathode). Placement of both electrodes into the tendon was confirmed by having the participant make a gentle isometric plantarflexion contraction, which caused deflection of both needles toward the foot (i.e. imbedded tips were pulled toward knee). Stimulation intensity was set to a level subjectively similar to that evoked by percutaneous TStim; stimulation intensity was not the same across conditions (×4 PT with percutaneous and ×10 PT with indwelling TStim). Waveform averages for both percutaneous and indwelling stimulation conditions were constructed from 100‐pulse trials. Both percutaneous and indwelling stimulation conditions were performed with the participant lying prone.
Statistics
It was hypothesized in Expt 1 that TStim‐evoked inhibition would be reduced in amplitude and duration in standing, compared to lying prone. The effects of postural orientation on TStim inhibition were explored with pre‐planned paired‐samples Student's t tests between prone and standing conditions for reflex area, duration and mean inhibition levels (IBM SPSS v23, IBM Corp., Armonk, NY, USA); paired‐samples t tests were also used to characterize changes in MGas and TA background muscle activity between conditions. In Expt 2, it was hypothesized that TStim‐evoked inhibition would be reduced in amplitude and duration when standing at the HIGH, compared to a LOW, threat condition. The effects of height‐induced postural threat on TStim inhibition were also explored with pre‐planned paired‐samples t tests on area, duration and mean level of inhibition between LOW and HIGH threat conditions. The effects of threat on psychological state (balance confidence, fear of falling, anxiety and perceived stability), sympathetic arousal (EDA), and background muscle activity (MGas and TA) were also explored with paired‐samples t tests. α was set to 0.05 for all statistical tests. Calculated effect sizes are reported as Eta squared (η2).
Results
Experiment 1: prone vs. standing
As shown in Fig. 2 A, TStim‐evoked inhibition was observed in all participants included in the study. Inhibitory responses to TStim were different in the prone and standing conditions (Fig. 2 B). The effects of changing postural orientation from prone to standing are detailed in Table 1. Irrespective of whether data were screened or not, there were no significant differences in MGas background muscle activity found between conditions; however, TA background activity was significantly increased in standing, compared to lying prone. The effects of screening for changes in MGas background EMG on TStim reflexes are demonstrated with data from a representative participant plotted in Fig. 2 C. Based on screened data, there were significant reductions in area (45.7%) and duration (32.7%) of inhibition observed in standing, compared to lying prone (Fig. 2 D and E). Mean amplitude of inhibition was not affected (Fig. 2 F) and there was a small but statistically significant change in onset latencies between prone and standing conditions. Likewise, when all stimuli were included in the analysis (Fig. 2 B), both area (42.2%) and duration (32.9%) of TStim‐evoked inhibition were significantly reduced when standing, compared to lying prone (Fig. 2 D and E). There was no significant change in mean inhibition, or onset latency between prone and standing conditions when all stimuli were assessed (Fig. 2 F), suggesting the change in area is related to reduced duration of inhibition.
Table 1.
Summary of TStim inhibition effects across postural orientations (Expt 1)
| Prone | Standing | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Sample | Mean (SEM) | Range | Mean (SEM) | Range | %Change | t (d.f.) | P | η² |
| Latency (ms) | Screened | 44.3 (0.8) | 37–56.5 | 45.1 (0.8) | 37–54.5 | 1.9% | −2.49 (23) | 0.021 | 0.212 |
| Full | 44.0 (0.8) | 36.5–56.5 | 44.3 (0.7) | 37–50.5 | 1.0% | −0.75 (23) | 0.462 | 0.024 | |
| Area of Inhib. (mV·ms) | Screened | 0.63 (0.16) | 0.12–3.92 | 0.34 (0.08) | 0.11–2.04 | −45.7% | 3.34 (23) | 0.003 | 0.326 |
| Full | 0.62 (0.15) | 0.12–3.78 | 0.36 (0.07) | 0.11–1.87 | −42.2% | 3.1 (23) | 0.005 | 0.294 | |
| Duration (ms) | Screened | 70.1 (6.5) | 17.5–133 | 47.2 (6.2) | 15–112 | −32.7% | 4.14 (23) | <0.001 | 0.426 |
| Full | 71.9 (6.4) | 21.5–133.5 | 48.3 (5.1) | 16–117.5 | −32.9% | 4.7 (23) | <0.001 | 0.490 | |
| Mean Inhib. (μV) | Screened | −9.4 (1.4) | −1.4 to −29.5 | −9.48 (1.73) | −1.2 to −40.8 | 0.9% | 0.13 (23) | 0.898 | 0.001 |
| Full | −8.9 (1.3) | −1.4 to −28.3 | −8.81 (1.59) | −1.5 to −39.4 | −0.5% | −0.07 (23) | 0.946 | <0.001 | |
| MGas BGA (μV) | Screened | 21.2 (3.3) | 3.1–62.8 | 21.6 (3.4) | 3.8–64.2 | 3.8% | −1.20 (23) | 0.242 | 0.059 |
| Full | 21.1 (3.2) | 3.2–58.2 | 21.9 (3.2) | 5.3–62.1 | 11.2% | −1.08 (23) | 0.290 | 0.049 | |
| TA BGA (μV) | Screened | 4.7 (0.6) | 1.3–12.6 | 6.6 (0.8) | 2.0–18.0 | 59.4% | −3.69 (20) | 0.001 | 0.405 |
| Full | 4.7 (0.6) | 1.3–12.5 | 6.6 (0.8) | 2.0–17.8 | 58.0% | −3.62 (20) | 0.002 | 0.396 | |
Note: Inhib. refers to inhibition and BGA refers to background muscle activity. Values in italics indicate significant difference at P < 0.05.
Experiment 2: LOW vs. HIGH threat
Participants were more aroused and had significant psychological responses to standing at the edge of the elevated platform. Sympathetic arousal, as indicated by EDA, was significantly higher in the HIGH, compared to LOW, threat condition (LOW: 18.1 ± 1.8 μS; HIGH: 27.7 ± 2.3 μS; t (18) = −5.71, P < 0.001, η2 = 0.644). Prior to starting the trial, participants were less confident in their ability to maintain balance in the HIGH, compared to LOW, condition (LOW: 95.5 ± 1.7%; HIGH: 68.9 ± 4.1%; t (20) = 6.69, P < 0.001, η2 = 0.691). Participants were more anxious at height (LOW: 31.0 ± 2.3/144; HIGH: 54.8 ± 6.2/144; t (20) = −4.01, P = 0.001, η2 = 0.446), more afraid of falling (LOW: 7.1 ± 2.6%; HIGH: 45.0 ± 6.3%; t (19) = −6.69, P < 0.001, η2 = 0.701), and also felt less stable (LOW: 85.5 ± 2.5%; HIGH: 55.0 ± 5.3%; t (19) = 7.14, P < 0.001, η2 = 0.728).
The effects of height‐induced postural threat on TStim inhibition are detailed in Table 2. When only stimuli passing the MGas screening criteria are analysed, there were significant reductions in area (21.1%) and duration of inhibition (11.4%), as well as a trend to a decrease in mean inhibition (14.1%) in the HIGH, compared to LOW, threat condition (Fig. 3 B–E). The effects of screening for changes in background muscle activity on reflex waveform averages in Expt 2 are demonstrated with data from a representative participant plotted in Fig. 3 B. There were no significant differences in latency of inhibition, or MGas or TA background muscle activity levels across threat conditions. When all stimuli were included there was a small but statistically significant 1.3 ms increase in onset latency of inhibition in the HIGH, compared to LOW, threat condition. There were also significant reductions in area (32.4%), duration (16.4%) and mean amplitude of inhibition (24.8%) in the HIGH, compared to LOW, condition (Fig. 3 A and C–E). However, there was also a significant decrease in MGas background muscle activity and trend to increase in antagonistic TA muscle activity between height conditions when all stimuli were analysed.
Table 2.
Summary of TStim inhibition effects across threat conditions (Expt 2)
| LOW threat | HIGH threat | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Sample | Mean (SEM) | Range | Mean (SEM) | Range | %Change | t (d.f.) | P | η² |
| Latency (ms) | Screened | 45.8 (1.0) | 39.5–56.0 | 46.9 (1.1) | 40.5–60.5 | 2.5% | −1.65 (17) | 0.116 | 0.139 |
| Full | 45.2 (0.7) | 40.0–52.0 | 46.5 (1.0) | 40.5–59.5 | 2.9% | −2.12 (20) | 0.047 | 0.183 | |
| Area of Inhib. (mV·ms) | Screened | 0.28 (0.06) | 0.02–1.0 | 0.22 (0.04) | 0.04–0.72 | −21.1% | 2.45 (17) | 0.026 | 0.26 |
| Full | 0.28 (0.04) | 0.05–0.94 | 0.19 (0.03) | 0.05–0.61 | −32.4% | 3.1 (20) | 0.005 | 0.330 | |
| Duration (ms) | Screened | 43.1 (7.0) | 7.0–111.5 | 38.2 (6.46) | 6.5–102.5 | −11.4% | 2.34 (17) | 0.032 | 0.243 |
| Full | 46.5 (6.4) | 7.0–116 | 38.9 (5.7) | 8.5–104.5 | −16.4% | 3.07 (20) | 0.006 | 0.320 | |
| Mean Inhib. (μV) | Screened | −8.56 (1.55) | −0.7 to −23.3 | −7.35 (1.26) | −1.2 to −17.9 | −14.1% | −1.83 (17) | 0.085 | 0.164 |
| Full | −8.12 (1.2) | −0.9 to −20.6 | −6.13 (0.98) | −1.4 to −13.9 | −24.8% | −3.05 (20) | 0.006 | 0.317 | |
| MGas BGA (μV) | Screened | 18.5 (3.39) | 2.8–55.9 | 17.2 (3.3) | 3.3–58.5 | −5.0% | 1.74 (17) | 0.099 | 0.152 |
| Full | 18.5 (2.81) | 3.2–51.7 | 16.0 (2.8) | 3.8–57.1 | −12.3% | 2.65 (20) | 0.015 | 0.260 | |
| TA BGA (μV) | Screened | 4.9 (0.89) | 1.8–16.2 | 6.3 (1.0) | 1.5–14.0 | 59.2% | −1.38 (17) | 0.185 | 0.101 |
| Full | 4.95 (0.80) | 1.8–16.7 | 7.13 (1.12) | 1.5–17.4 | 93.4% | −1.81 (20) | 0.086 | 0.140 | |
Note: Inhib. refers to inhibition and BGA refers to background muscle activity. Values in italics indicate significant difference at P < 0.05.
Indwelling direct tendon stimulation
As shown in Fig. 4, direct electrical stimulation of the tendon with indwelling TStim evoked an inhibitory response with similar shape and timing to that evoked with percutaneous stimulation. The onsets of inhibition to percutaneous and indwelling TStim were 49.4 ms and 47.7 ms, respectively. The duration of inhibition was longer with percutaneous stimulation; the inhibitory period in this participant lasted 28.4 ms with percutaneous stimulation and 17.8 ms with indwelling TStim.
Figure 4. Comparison of indwelling and percutaneous tendon stimulation.
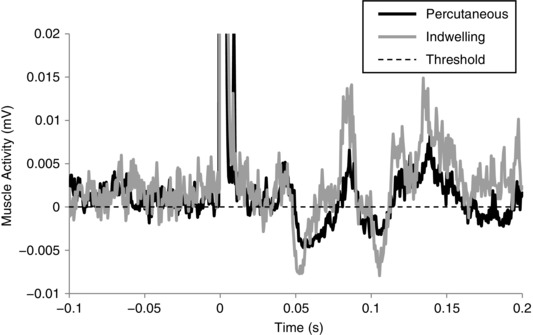
Figure shows 100‐pulse waveform averages from the percutaneous (black) and indwelling stimulation (grey) techniques in a 40‐year‐old healthy male lying prone and plantar flexing. The waveforms have been baseline‐corrected to align their respective inhibition detection thresholds (dashed horizontal line) to zero for display purposes. Both techniques evoke inhibition at approximately 48 ms post‐stimulation, and the patterns of inhibition are similar; however, the duration of inhibition is shorter in this participant with indwelling stimulation.
Discussion
The purposes of these experiments were: (a) to determine if TStim is a suitable technique for probing Ib inhibition in a posturally engaged muscle in standing (Expt 1), and (b), to characterize the effects of height‐induced postural threat on Ib reflexes (Expt 2). In accordance with our hypothesis for Expt 1, TStim‐evoked inhibitory reflexes were significantly shorter in duration, and reduced in area, but were not different in latency or mean amplitude in standing compared to lying postures; these effects occurred despite similar levels of MGas muscle activity between postural orientation conditions. We interpret this result as evidence of reduced Ib inhibition in a posturally engaged muscle during standing, compared to a voluntary contraction to a comparable level while lying prone. In agreement with our hypothesis for Expt 2, TStim‐evoked inhibition was reduced in area, duration and amplitude when standing in the HIGH, compared to LOW, threat condition; while this effect was observed in conjunction with a change in background MGas activity across threat conditions when all stimuli were considered, the reflex changes persisted after controlling for changes in background muscle activity with the screening protocol. This result is interpreted as evidence of context‐dependent modulation of Ib inhibition in a posturally engaged muscle in response to a threat to standing balance.
To date, TStim studies have fixed postural orientation (seated or lying) and task as methodological controls. Expt 1 is novel because we used TStim in upright standing and showed changes in TStim‐evoked inhibitory reflexes with changes in postural orientation and/or engagement in upright balancing. These results are in line with indirect evidence of decreased Ib inhibition in standing, compared to lying supine or sitting (Faist et al. 2006), as indicated by H‐reflex conditioning from heteronymous muscles. It is thought the reduction in inhibition reflects a shift from predominantly inhibitory toward excitatory reflexes in standing, which may be important for weight‐bearing (Van Doornik et al. 2011) or walking (Duysens et al. 2000). The shorter duration of TStim‐evoked inhibition during standing could reflect this shift, as the motor neuron pool took less time to return to activation in Expt 1. Similar changes to Ib reflexes have been observed between sitting and walking with MGas conditioning of soleus H‐reflexes; Ib reflexes are inhibitory (and similar in amplitude) in both sitting and walking at short conditioning intervals (1–3 ms); however, Ib inhibition disappears, and may become excitatory, at longer conditioning intervals (>4 ms) in walking (Stephens & Yang, 1996). We note that the standing posture used in this study differs from free, unconstrained standing in that we had participants adopt a voluntary forward lean. This was a necessary methodological concession made to ensure sufficient background MGas activity was present to observe Ib‐induced inhibition. The voluntary lean may have caused participants to adopt a more conscious control of posture than is usual, and may have made motor control in the standing and prone conditions in Expt 1 more similar than would normally be the case.
While TStim inhibition is thought to arise from Ib afferents or GTOs, other origins, including stimulation of skin overlying the tendon, sural nerve, or stimulation of other muscle or tendon afferents should be considered. In a replication of a two‐person pilot study by Rogasch et al. (2012), we compared reflexes evoked by percutaneous and direct subcutaneous tendon stimulation in a single pilot subject (40‐year‐old male) in a controlled setting in order to rule out contributions from skin or muscle afferents. Direct electrical stimulation of the tendon with indwelling TStim evoked an inhibitory response with similar shape and timing to that evoked with percutaneous stimulation in the same participant (Fig. 4). The current results are similar to those of Rogasch et al. (2012) in that both studies found similar patterns of inhibition, which occurred at similar latencies across modalities. The studies differ in that the stimulation intensity used to evoke the reflex was higher, and the duration of the inhibitory period was shorter, with indwelling than with percutaneous TStim in the present study. These discrepancies might be due to methodological differences in terms of stimulation site and how it was located (ultrasound vs. palpation), criteria for setting stimulation intensity (based on sensation vs. highest intensity that did not cause a twitch), and small sample sizes used in both cases. Furthermore, stimulation of the skin lying over the tendon is not likely to be the cause of TStim inhibition because the response is abolished when the skin is stretched so that the electrodes no longer lie over the tendon (Burne & Lippold, 1996). Likewise, sural nerve stimulation cannot explain TStim inhibition because it evokes qualitatively different reflex responses from TStim (Khan & Burne, 2009, 2010; Rogasch et al. 2012), and sural nerve conditioning effects on other reflexes differ from TStim in pattern and duration (Khan & Burne, 2010). Furthermore, the TStim‐evoked reflex disappears when the tibial nerve is blocked with anaesthetic (which supplies the gastrocnemii as well as part of the sural nerve) but is not affected by blocking the sural nerve (Khan & Burne, 2009). Therefore, the combined observations of reduced inhibition in standing, compared to lying prone (Expt 1; Fig. 2), and similar patterns of inhibition from percutaneous and direct tendon stimulation (Fig. 4) suggest a Ib reflex of tendinous origin. The exact point of stimulation and origin of the response, be it GTO or peripheral afferent, cannot be determined with these data.
To our knowledge, this is the first example of context‐dependent modulation of Ib reflexes without changing the essential postural task (e.g. lying to standing) in humans. The effect of reduced Ib inhibition with threat would be to limit the quiescent period in a posturally engaged anti‐gravity muscle. In the context of the height‐induced postural threat, inhibition of MGas would cause the body to fall forward, toward the edge of the platform; therefore, reducing the amount of inhibition would be protective because the person would not sway as far forward. It is not clear from the current data if the observed effects reflect a generalized response to a postural threat (e.g. do not fall down) or a direction‐specific response to the forward edge of the platform. However, evidence from other sensory systems suggests these effects are more likely to reflect a generalized response to the threat than a directional response to the edge. Threat of unpredictable balance perturbation, where there is no clear direction of threat, has previously been shown to affect muscle spindle stretch (Horslen et al. 2013) and electrical vestibular stimulation‐evoked balance reflexes (Lim et al. 2016). Likewise, vestibular‐evoked balance responses are larger in the medio‐lateral plane (orthogonal to the direction of threat) when standing facing the edge in HIGH, compared to LOW, surface heights (Horslen et al. 2014), and plantar flexor vestibular‐evoked myogenic potentials to auditory clicks are larger when participants stand with their side to the edge (again, response changed in a muscle acting orthogonal to the direction of threat; Naranjo et al. 2015). This issue might be resolved with a different, non‐directionally specific threat, such as threat of whole‐body perturbation (Horslen et al. 2013; Lim et al. 2016), or standing with the direction of threat to either side (Tersteeg et al. 2012; Osler et al. 2013), or behind the subject, in which cases a forward lean due to inhibition of plantar flexors could be protective and thus facilitated with greater Ib inhibition.
Reduced TStim‐evoked inhibition might be achieved by either reducing the potency of inhibitory effects (e.g. disinhibition), or by countering inhibition with excitatory influences. Ib reflexes are subject to many spinal (Pierrot‐Deseilligny et al. 1979; Jankowska, 1992) and supraspinal modulatory influences (Jami, 1992; Jankowska, 1992). Of particular interest are reticulospinal modulatory projections onto Ib reflex pathways (Jankowska, 1992; McCrea, 2001), which are known to inhibit non‐reciprocal inhibition of motor‐neurons (i.e. disinhibition; Jami, 1992), and are known to be modulated by fear and anxiety networks (Balaban & Thayer, 2001; Staab et al. 2013). Alternatively, the threat‐effects might reflect more central Ib excitation. Ib reflexes in anti‐gravity muscles are thought to help excite the motor neuron pool in standing to help resist gravity and changes in muscle loading (Dietz et al. 1992; Sinkjær et al. 2000; Grey et al. 2007; Van Doornik et al. 2011). While Faist et al. (2006) distinguished between diminished Ib inhibition in standing and activation of Ib excitatory effects with gait, Van Doornik et al. (2011) argued Ib afferent activity contributes to plantar flexor excitation in quiet standing because sudden muscle unloading causes short latency decreases in muscle activity. Unfortunately, the data from the current study do not reveal how the reduction in inhibition was achieved. While surface EMG cannot dissociate between these effects, examining changes in individual motor‐unit discharge rates may reveal how threat is reducing Ib inhibition (Rogasch et al. 2011).
Further study is required to understand how changes in Ib reflexes might contribute to altered balance behaviours observed with height‐induced postural threat. Typically, people demonstrate smaller amplitude and higher frequency centre‐of‐pressure (Carpenter et al. 1999) and centre‐of‐mass oscillations (Carpenter et al. 2001), as well as less tonic plantar flexor and more dorsi‐flexor background muscle activity when standing quietly at height (Carpenter et al. 2001). They also permit less forward sway in response to whole‐body postural perturbations (Carpenter et al. 2004) and adopt a more cautious gait (Tersteeg et al. 2012). Taken together with known changes in muscle spindle sensitivity (Davis et al. 2011; Horslen et al. 2013) and vestibular reflexes (Horslen et al. 2014; Naranjo et al. 2015, 2016) with height‐induced postural threat, the observations from the current study point to a broad, multi‐sensory adaptation process to threat geared to limiting body movement, reminiscent of, but to a lesser degree than, ‘freezing’ behaviours observed in fearful animals (reviewed by Lang et al. 2000). Recent mouse model evidence suggests freezing behaviour is linked to activation of the ventro‐lateral periaqueductal grey (Koutsikou et al. 2014, 2015), and involves spinocerebellar gaiting, where noxious stimuli are suppressed and movement‐relevant muscle afferent information is augmented (Koutsikou et al. 2015), in order to limit self‐motion (Balaban, 2002). Likewise, in humans it has been hypothesized that threat‐induced changes to sensory function might evoke larger myogenic responses to balance disturbances, as well as permit reductions in postural sway without compromising the fidelity of balance‐relevant sensory feedback (Horslen et al. 2013, 2014). The changes in Ib reflexes observed here could lead to more tonic plantar flexor muscle activity, and may contribute to altered scaling of responses to postural disturbances with threat.
There are several limitations to acknowledge for this study. Upright standing may have led to changes in muscle activation between prone and standing conditions, over the course of a standing condition, or between threat conditions. The amplitude of TStim‐evoked inhibition is known to scale negatively with background muscle activation (Khan & Burne, 2007). We used verbal feedback and post hoc screening to control for background muscle activation levels, therefore it is unlikely the changes observed in either Expt 1 or 2 can be explained by changes in muscle activation levels. It is also unlikely the changes in TStim inhibition can be explained by changes in antagonist (TA) background muscle activation (Expt 1). TA activation might be expected to influence MGas TStim inhibition through reciprocal connections, where TA to MGas reciprocal inhibition may summate with TStim inhibition and lead to greater evoked MGas inhibition while standing (cf. Kasai et al. 1998). However, there was less evoked inhibition in standing compared to lying prone, which is opposite to the expected effect of added reciprocal inhibition. Nonetheless, the effects of antagonist activation on MGas TStim inhibition remain to be explored. Participants may have also adopted different ankle angles between threat or postural orientation conditions, and changes in ankle angle can affect the TStim response (Khan & Burne, 2009). Verbal feedback about muscle activity was used as a proxy for feedback about ankle angle in the standing conditions, as participants were instructed to lean forward or backward to compensate for changes in activation levels. Similarly, manual manipulation of ankle angle by an experimenter was used to approximately match prone ankle angle with standing angle. Furthermore, loading the plantar flexors in standing may have shifted the location of the MTJ, compared with the voluntary contraction used to load the muscle in the prone condition of Expt 1. The location of the cathode with respect to the MTJ is important for evoking the TStim response (Khan & Burne, 2009). While we cannot completely rule out a change in MTJ location between standing and lying trials, it is unlikely the MTJ moved out of the 9 cm2 (3 cm long) cathode stimulation area. While, to our knowledge, changes in Achilles’ tendon length between standing and lying prone while contracting isometrically have not been investigated, ultrasound imaging of the Achilles’ tendon reveals less than 1 cm longitudinal displacement of the lateral gastrocnemius MTJ over the course of the gait cycle (Franz et al. 2015). Furthermore, this would not explain changes observed with height, in which subjects maintained a similar forward lean between height conditions. Finally, TStim at the MTJ is likely to only affect a sub‐population of all MGas GTOs. Approximately half of all MGas GTOs attach to the aponeurosis of insertion vs. origin (Swett & Eldred, 1960; Jami, 1992), and due to the pennate orientation of MGas muscle fibres, GTOs on the insertion are distributed from approximately mid‐length to distal end of the aponeurosis (Swett & Eldred, 1960). Assuming TStim is stimulating GTOs, then the sample here is limited to the most distal sub‐set of MGas GTOs, and the results may not reflect the whole population response.
These experiments make two significant contributions to the study of the role of Ib reflexes in standing balance control. First, the results demonstrate that TStim can be used in standing participants to evoke Ib inhibition, and confirms that Ib inhibition is reduced when standing, compared to lying prone. The study also provides novel evidence of context‐dependent modulation of Ib reflexes within a single task in humans, without modulation of muscle state (e.g. muscle cramp; Khan & Burne, 2007; Miller & Burne, 2014). Finally, these data further support sensory adaptation processes as a likely contributor to altered balance behaviours with threats to standing balance. Future studies should endeavour to reveal how changes in sensory function, or possibly changes in sensory integration, contribute to changes in balance behaviours when humans experience threat.
Additional information
Competing interests
None declared.
Author contributions
These experiments were performed in either the Neural Control of Posture and Movement Lab (Expts 1 and 2) or the Human Neurophysiology Lab (single‐subject indwelling data) at the University of British Columbia. All authors contributed to conception and design of the work, as well as interpretation of the data. B.C.H. collected and analysed the data, and drafted the manuscript and figures under the supervision of M.G.C. J.T.I., J.‐S.B. and M.G.C. all contributed critical revisions of the intellectual content. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work, and qualify for authorship.
Funding
The authors wish to thank the Natural Sciences and Engineering Research Council of Canada, and Canada Foundation for Innovation for project funding, and for salary support provided by the Natural Sciences and Engineering Research Council of Canada to B.C.H., and the Canadian Chiropractic Research Foundation to J.‐S.B.
Acknowledgements
The authors wish to acknowledge Dr Ryan M. Peters and Mr Diego Orcioli da Silva for assistance with data collection.
Linked articles This article is highlighted by a Perspective by Doumas. To read this Perspective, visit https://doi.org/10.1113/JP274367.
This is an Editor's Choice article from the 1 July 2017 issue.
References
- Adkin AL, Frank JS, Carpenter MG & Peysar GW (2000). Postural control is scaled to level of postural threat. Gait Posture 12, 87–93. [DOI] [PubMed] [Google Scholar]
- Balaban CD (2002). Neural substrates linking balance control and anxiety. Physiol Behav 77, 469–475. [DOI] [PubMed] [Google Scholar]
- Balaban CD & Thayer JF (2001). Neurological bases for balance–anxiety links. J Anxiety Disord 15, 53–79. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben‐Shakhar G, Roth WT, Dawson ME, Filion DL & Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures (2012). Publication recommendations for electrodermal measurements. Psychophysiology 49, 1017–1034. [DOI] [PubMed] [Google Scholar]
- Burne JA & Lippold OCJ (1996). Reflex inhibition following electrical stimulation over muscle tendons in man. Brain 119, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Adkin AL, Brawley LR & Frank JS (2006). Postural, physiological and psychological reactions to challenging balance: does age make a difference? Age Ageing 35, 298–303. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A & Allum JHJ (2004). Influence of postural anxiety on postural reactions to multi‐directional surface rotations. J Neurophysiol 92, 3255–3265. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS & Silcher CP (1999). Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res 9, 277–286. [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP & Peysar GW (2001). The influence of postural threat on the control of upright stance. Exp Brain Res 138, 210–218. [DOI] [PubMed] [Google Scholar]
- Cattagni T, Martin A & Scaglioni G (2014). Is spinal excitability of the triceps surae mainly affected by muscle activity or body position? J Neurophysiol 111, 2525–2532. [DOI] [PubMed] [Google Scholar]
- Cleworth TW, Horslen BC & Carpenter MG (2012). Influence of real and virtual heights on standing balance. Gait Posture 36, 172–176. [DOI] [PubMed] [Google Scholar]
- Davis JR, Horslen BC, Nishikawa K, Fukushima K, Chua R, Inglis JT & Carpenter MG (2011). Human proprioceptive adaptations during states of height‐induced fear and anxiety. J Neurophysiol 106, 3082–3090. [DOI] [PubMed] [Google Scholar]
- Dietz V, Gollhofer A, Kleiber M & Trippel M (1992). Regulation of bipedal stance: dependency on ‘load’ receptors. Exp Brain Res 89, 229–231. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F & Cruse H (2000). Load‐regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80, 83–133. [DOI] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W & Duysens J (2006). In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076, 87–92. [DOI] [PubMed] [Google Scholar]
- Franz JR, Slane LC, Rasske K & Thelen DG (2015). Non‐uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture 41, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N & Sinkjær T (2007). Positive force feedback in human walking. J Physiol 581, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck LJ, Carpenter MG & Frank JS (2008). Task‐specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture 27, 676–682. [DOI] [PubMed] [Google Scholar]
- Héroux ME, Dakin CJ, Luu BL, Inglis JT & Blouin JS (2014). Absence of lateral gastrocnemius activity and differential motor unit behavior in soleus and medial gastrocnemius during standing balance. J Appl Physiol (1985) 116, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Dakin CJ, Inglis JT, Blouin JS & Carpenter MG (2014). Modulation of human vestibular reflexes with increased postural threat. J Physiol 592, 3671–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R & Carpenter MG (2013). Effects of postural threat on spinal stretch reflexes: evidence for increased muscle spindle sensitivity? J Neurophysiol 110, 899–906. [DOI] [PubMed] [Google Scholar]
- Houk J & Henneman E (1967). Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30, 466–481. [DOI] [PubMed] [Google Scholar]
- Jami L (1992). Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72, 623–666. [DOI] [PubMed] [Google Scholar]
- Jankowska E (1992). Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38, 335–378. [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawanishi M & Yahagi S (1998). Posture‐dependent modulation of reciprocal inhibition of ankle dorsiflexion in man. Brain Res 792, 159–163. [DOI] [PubMed] [Google Scholar]
- Khan SI & Burne JA (2007). Reflex inhibition of normal cramp following electrical stimulation of the muscle tendon. J Neurophysiol 98, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Khan SI & Burne JA (2009). Afferents contributing to autogenic inhibition of gastrocnemius following electrical stimulation of its tendon. Brain Res 1282, 28–37. [DOI] [PubMed] [Google Scholar]
- Khan SI & Burne JA (2010). Inhibitory mechanisms following electrical stimulation of tendon and cutaneous afferents in the lower limb. Brain Res 1308, 47–57. [DOI] [PubMed] [Google Scholar]
- Koutsikou S, Crook JJ, Earl EV, Leith JL, Watson TC, Lumb BM & Apps R (2014). Neural substrates underlying fear‐evoked freezing: the periaqueductal grey‐cerebellar link. J Physiol 592, 2197–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsikou S, Watson TC, Crook JJ, Leith JL, Lawrenson CL, Apps R & Lumb BM (2015). The periaqueductal gray orchestrates sensory and motor circuits at multiple levels of the neuraxis. J Neurosci 35, 14132–14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M & Öhman A (2000). Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord 61, 137–159. [DOI] [PubMed] [Google Scholar]
- Lim SB, Cleworth TW, Horslen BC, Blouin JS, Inglis JT & Carpenter MG (2016). Postural threat influences vestibular‐evoked muscular responses. J Neurophysiol 117, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF & Prochazka A (1990). Human H‐reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83, 22–28. [DOI] [PubMed] [Google Scholar]
- Maganaris CN & Paul JP (1999). In vivo human tendon mechanical properties. J Physiol 521, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA (2001). Spinal circuitry of sensorimotor control of locomotion. J Physiol 533, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KC & Burne JA (2014). Golgi tendon organ reflex inhibition following manually applied acute static stretching. J Sports Sci 32, 1491–1497. [DOI] [PubMed] [Google Scholar]
- Naranjo EN, Allum JH, Inglis JT & Carpenter MG (2015). Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height‐induced postural threat. Neuroscience 293, 45–54. [DOI] [PubMed] [Google Scholar]
- Naranjo EN, Cleworth TW, Allum JH, Inglis JT, Lea J, Westerberg BD & Carpenter MG (2016). Vestibulo‐spinal and vestibulo‐ocular reflexes are modulated when standing with increased postural threat. J Neurophysiol 115, 833–842. [DOI] [PubMed] [Google Scholar]
- Osler CJ, Tersteeg MC, Reynolds RF & Loram ID (2013). Postural threat differentially affects the feedforward and feedback components of the vestibular‐evoked balance response. Eur J Neurosci 38, 3239–3247. [DOI] [PubMed] [Google Scholar]
- Pearson KG & Gordon J (2000). Spinal reflexes In Principles of Neural Science, 4th edn, ed. Kandel ER, Schwartz JH. & Jessell TM, pp. 713–736. McGraw‐Hill, New York, NY, USA. [Google Scholar]
- Pierrot‐Deseilligny E, Katz R & Morin C (1979). Evidence for lb inhibition in human subjects. Brain Res 166, 176–179. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Pedace F, Giovannelli M & Manfredi M (1998). Electrical stimulation over muscle tendons in humans. Evidence favouring presynaptic inhibition of Ia fibres due to the activation of group III tendon afferents. Brain 121, 373–380. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Burne JA, Binboğa E & Türker KS (2011). Synaptic potentials contributing to reflex inhibition in gastrocnemius following tendon electrical stimulation. Clin Neurophysiol 122, 1190–1196. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Burne JA & Türker KS (2012). Comparison of the inhibitory response to tendon and cutaneous afferent stimulation in the human lower limb. J Neurophysiol 107, 564–572. [DOI] [PubMed] [Google Scholar]
- Sibley KM, Carpenter MG, Perry JC & Frank JS (2007). Effects of postural anxiety on the soleus H‐reflex. Hum Mov Sci 26, 103–112. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Ladouceur M, Christensen LO & Nielsen JB (2000). Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523, 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JP, Balaban CD & Furman JM (2013). Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33, 297–306. [DOI] [PubMed] [Google Scholar]
- Stephens MJ & Yang JF (1996). Short latency, non‐reciprocal group I inhibition is reduced during the stance phase of walking in humans. Brain Res 743, 24–31. [DOI] [PubMed] [Google Scholar]
- Swett JE & Eldred E (1960). Distribution and numbers of stretch receptors in medial gastrocnemius and soleus muscles of the cat. Anat Rec 137, 453–460. [DOI] [PubMed] [Google Scholar]
- Tersteeg MCA, Marple‐Horvat DE & Loram ID (2012). Cautious gait in relation to knowledge and vision of height: is altered visual information the dominant influence? J Neurophysiol 107, 2686–2691. [DOI] [PubMed] [Google Scholar]
- Türker KS, Yang J & Scutter SD (1997). Tendon tap induces a single long‐lasting excitatory reflex in the motoneurons of human soleus muscle. Exp Brain Res 115, 169–173. [DOI] [PubMed] [Google Scholar]
- Van Doornik J, Azevedo Coste C, Ushiba J & Sinkjær T (2011). Positive afferent feedback to the human soleus muscle during quiet standing. Muscle Nerve 43, 726–732. [DOI] [PubMed] [Google Scholar]


