Abstract
Exercise is an integral part of the rehabilitation of patients suffering a variety of chronic musculoskeletal conditions, such as fibromyalgia, chronic low back pain and myofascial pain. Regular physical activity is recommended for treatment of chronic pain and its effectiveness has been established in clinical trials for people with a variety of pain conditions. However, exercise can also increase pain making participation in rehabilitation challenging for the person with pain. Animal models of exercise‐induced pain have been developed and point to central mechanisms underlying this phenomena, such as increased activation of NMDA receptors in pain‐modulating areas. Meanwhile, a variety of basic science studies testing different exercise protocols, show exercise‐induced analgesia involves activation of central inhibitory pathways. Opioid, serotonin and NMDA mechanisms acting in rostral ventromedial medulla promote analgesia associated with exercise. This review explores and discusses current evidence on central mechanisms underlying exercised‐induced pain and analgesia.
Keywords: animal, central nervous system, exercise, glutamate, hyperalgesia, opioid, pain, physical activity, serotonin
Abbreviations
- 5‐HT
serotonin
- NMDA
N‐methyl‐d‐aspartate
- NRM
nucleus raphe magnus
- NRO
nucleus raphe obscurus
- NRP
nucleus raphe pallidus
- PAG
periaqueductal grey
- RVM
rostral ventromedial medulla
Introduction
Exercise not only reduces pain perception, but also has effects on mental health, such as mood elevation and reduction of stress and depression, which are often associated with chronic pain conditions (Bement & Sluka, 2016). Exercise is a powerful tool in the management of those conditions, especially considering the Centers for Disease Control and Prevention's new opioid‐prescribing guidelines, recommending a focus toward non‐opioid and non‐pharmacological treatments (Dowell et al. 2016). In healthy subjects, exercise increases thresholds for experimentally induced pain (Bement & Sluka, 2016). In clinical populations, exercise promotes analgesia in conditions such as low back pain, osteoarthritis, myofascial pain, chronic fatigue syndrome and fibromyalgia (Bement & Sluka, 2016). However, exercise has also been shown to increase pain in experimental and clinical settings, especially when a musculoskeletal pain condition is already established (Staud et al. 2005). Patients with fibromyalgia show greater increases in pain and perceived fatigue after performing a physically fatiguing task when compared to healthy subjects (Dailey et al. 2015). This increased pain to exercise in chronic pain patients is often a barrier to regular exercise, leading to a sedentary lifestyle that worsens the painful conditions and makes treatment even more difficult (Damsgard et al. 2010). Interestingly, contraction of painful muscles fails to activate pain inhibitory mechanisms in myalgia and fibromyalgia patients while it increases pressure pain thresholds in healthy subjects (Lannersten & Kosek, 2010). Exercise is, in most cases, one of the best approaches for managing chronic pain conditions, so understanding the mechanisms of both pain and analgesia induced by exercise is important to better define physical activity‐related treatment protocols for people with pain.
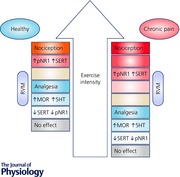
Centrally, the rostral ventromedial medulla (RVM) is a key relay for pain modulation, playing a major role in exercise‐induced pain and analgesia (Sluka & Rasmussen, 2010; Stagg et al. 2011; Sluka et al. 2012, 2013). Within the caudal brainstem, the nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO) and nucleus raphe pallidus (NRP) are involved in modulation of both pain and motor outputs (Fields et al. 1995; Porreca et al. 2002; Zhuo et al. 2002; Da Silva et al. 2010a), making these nuclei potential links between physical activity and pain perception. Other pain‐processing areas such as the periaqueductal grey (PAG) (Mathes & Kanarek, 2006; Stagg et al. 2011) and cortical areas (de Oliveira et al. 2010) have been implicated in exercise‐induced pain and analgesia. N‐Methyl‐d‐aspartate (NMDA) glutamate receptors in the RVM also play a key role in chronic muscle pain, including exercise‐induced pain (Da Silva et al. 2010a; Sluka et al. 2012). Phosphorylation of the NR1 subunits of NMDA receptors in the caudal brainstem mediates the hyperalgesia in animal models of chronic musculoskeletal pain and exercise‐induced pain (Sluka et al. 2012). On the other hand, opioidergic and serotonergic neurons are both expressed in the RVM (Basbaum & Fields, 1984) and there is recent evidence for the involvement of these systems in the analgesia induced by exercise (Stagg et al. 2011; Bobinski et al. 2015). Figure 1 illustrates the known mechanisms of exercise‐induced pain and analgesia.
Figure 1. Overview of the underlying mechanisms of exercise‐induced pain and analgesia.
Known neurotransmitters and receptors that have been shown to be involved at different areas of the central nervous system are listed. The majority of studies have focused on the PAG and the RVM. Increases in serotonin and opioids, and activation of μ‐opioid (MOR) and cannabinoid‐1 (CB1) receptors are implicated in the exercise‐induced analgesia. Further, the normally increased phosphorylation of the NR1 subunit of the NMDA receptor and the increased expression of serotonin transporter (SERT) that is increased by acute exercise are reduced by regular physical activity. +, increase; −, decrease; 5‐HT, serotonin; CB1, cannabinoid receptor 1; DH, dorsal horn; MOR, μ‐opioid receptor; PAG, periaqueductal grey; p‐NR1, phosphorylated NR1; RVM, rostral ventromedial medulla.
This review discusses animal studies that explore the underlying central mechanisms of both exercise induced pain and analgesia from different exercise protocols. We discuss the evidence with respect to type, duration, and frequency of exercise using different pain models.
Fatiguing exercise enhances pain
Pain and fatigue interactions
Clinically, physical fatigue is a common complaint in chronic musculoskeletal pain conditions, while chronic pain is common in chronic fatigue conditions (Vierck et al. 2001; Whiteside et al. 2004; Staud et al. 2005; Kadetoff & Kosek, 2007). The overlap between muscle fatigue and pain syndromes suggests an interaction between fatigue and pain such that fatigue may enhance pain. Pain may be a factor in reducing adherence to regular exercise and rehabilitation, leading the patient to a sedentary life (Damsgard et al. 2010). It is proposed that muscle fatigue promotes changes in central nervous system function that cannot be explained only in the muscle itself (Davis & Bailey, 1997).
Fatiguing exercise‐induced pain models
Several animal models were developed to better understand the interaction between muscle fatigue and pain. For example, when an acute bout of running wheel activity (2 h) was combined with intramuscular doses of saline of different pH (pH 4.0, 5.0, 6.0 or 7.2), enhanced hyperalgesia developed bilaterally when the pH 5.0 injections were combined with the fatigue task – no cutaneous hyperalgesia developed with pH 5.0 injections without fatigue (Yokoyama et al. 2007). In the initial studies, two 2 h runs prior to the first intramuscular pH 5.0 injection, and two 2 h runs prior to the second intramuscular pH 5.0 injection of acid saline produced an enhanced muscle hyperalgesia. Subsequently it was shown that a single 2 h or 30 min run prior to the subthreshold muscle insult produced the same widespread hyperalgesia (Sluka et al. 2012). Despite a 10% reduction in grip force after the 2 h fatiguing exercise, there were no changes in muscle ,, lactate, creatinine kinase MB and phosphate suggesting minimal fatigue metabolites were released during the fatiguing task. These results show that muscle fatigue enhances the probability of the development of mechanical hyperalgesia in mice in response to intramuscular acid saline without muscle histological changes.
Similarly, combining an acute bout of running wheel exercise with a low dose of intramuscular carrageenan injection (0.03%) produced widespread mechanical hyperalgesia. Interestingly, injection of carrageenan either 2 h before or 2 h after the fatigue task produced the same degree of mechanical hyperalgesia of the paw, but not the muscle (Sluka & Rasmussen, 2010). There was also an enhanced hyperalgesia in female mice that was eliminated by ovariectomy, suggesting oestradiol contributed to the development of exercise‐induced hyperalgesia in this model.
To test if localized fatigue of the injected muscle was sufficient to induce the hyperalgesia, electrical stimulation of the muscle replaced the whole‐body fatiguing task. When combining this electrically induced isometric contraction with pH 5.0 injections there was a significant hyperalgesia that developed in the ipsilateral muscle of male mice and bilaterally in the female mice (Gregory et al. 2013). Interestingly, the hyperalgesia was longer lasting and easier to induce in female mice. Hyperalgesia lasted for 2 weeks in males and over 1 month in females. Temporally separating the fatigue task and the muscle insult by 24 h resulted in bilateral hyperalgesia only in female mice, which suggests that the attenuation in response to muscle fatigue does not occur in females. Spatially separating the fatigue task and muscle insult by giving the fatigue task in the muscle contralateral to the injection also resulted in bilateral hyperalgesia only in female mice. In this case, ovariectomy had no effect on the sex differences suggesting oestradiol was not involved in the development of exercise‐induced hyperalgesia in this model. It may be that the isometric fatiguing task favours a peripheral mechanism that results in release of fatigue metabolites like acidic pH in muscle that subsequently activate acid sensing ion channels (ASICs). Indeed, we showed that blockade of ASIC3 prevents and ASIC3 knockout mice do not develop hyperalgesia in this localized fatigue‐induced pain model (Gregory et al. 2016).
In summary, the studies described above show that fatiguing exercise can enhance hyperalgesia in both male and female mice, and this enhancement is greater in females. Interestingly, whole body exercise produced the female phenotype through oestradiol while the localized fatigue exercise task produces the enhancement in an oestradiol‐independent manner. This highlights the complicated nature of nociceptive processing in males and females and suggests that there are task‐dependent mechanisms involved in the enhancement of hyperalgesia by exercise.
Central mechanisms of fatiguing exercise‐induced hyperalgesia
To examine potential brain sites that underlie exercise‐induced hyperalgesia, C‐fos immunostaining, as a marker of neuron activation, in the caudal brainstem was investigated. C‐fos immunoreactivity showed an increase in the number of cells in the NRM, NRO and NRP after a 2 h running‐wheel task, suggesting the caudal raphe might be involved in the development of exercise‐induced hyperalgesia (Sluka et al. 2012). Since NMDA receptors in the RVM are involved in pain facilitation (Sluka & Rasmussen, 2010), NMDA receptors were blocked in the NRO/NRP during the fatiguing task when combined with 0.03% carrageenan. NMDA receptor blockade during the fatiguing task prevented the development of exercise‐induced hyperalgesia. On the other hand, over‐expression of the NR1 subunit of the NMDA receptor in the RVM, using a feline immunodeficiency virus expressing the complementary DNA to NR1, produced bilateral mechanical hyperalgesia of the paw and muscle (Da Silva et al. 2010b), supporting a role for NR1 in development of hyperalgesia. Since phosphorylation of NMDA receptors can enhance neuron excitability (Chen & Roche, 2007), the expression of the phosphorylated NR1 subunit was investigated. In the exercise‐induced pain model induced by whole‐body running wheel activity combined with 0.03% carrageenan or pH 5.0 injections, there was an increase in the number of cells stained for phosphorylated NR1 in the NRO, NRM, and NRP (Sluka et al. 2012; Lima et al. 2016). However, there were no differences in the number of p‐NR1 labelled cells in the electrically stimulated fatigue task combined with two pH 5.0 injections (Gregory et al. 2013), suggesting different mechanisms in this model. Thus, NMDA receptor activation and phosphorylation of NMDA receptors underlies the development of hyperalgesia from a whole‐body fatiguing task, but not from a localized fatigue task.
Exercise‐induced analgesia
Mechanistic studies in human subjects
Exercise‐induced analgesia and the underlying mechanisms have been investigated in several studies using healthy control human subjects and more recently in patient populations. Early studies show that high intensity running, or bicycle ergometry produced analgesia that was reversed by systemic naloxone, suggesting the involvement of opioids in exercise‐induced analgesia (Janal et al. 1984; Olausson et al. 1986). Using a fatiguing isometric contraction, there were decreases in pain thresholds that were accompanied by a reduction in cortical excitability and motor evoked potentials assessed by transcranial magnetic stimulation (Bement et al. 2009). High levels of physical activity correlate with greater conditioned pain modulation, which is thought to measure central inhibition, in healthy controls (Geva & Defrin, 2013). Conditioned pain modulation is higher in athletes (Flood et al. 2017), and predicts exercise‐induced analgesia in healthy subjects (Ellingson et al. 2014; Lemley et al. 2015; Stolzman & Bement, 2016). In people with osteoarthritis, there were significant increases in pressure pain thresholds in those with normal conditioned pain modulation, and decreases in pressure pain thresholds in those with reduced conditioned pain modulation, suggesting exercise and conditioned pain modulation use similar mechanisms (Fingleton et al. 2017). Further, both conditioned pain modulation and exercise‐induced hypoalgesia predict greater pain relief 6 months after total knee replacement (Vaegter et al. 2017). Lastly, several studies show a reduction in temporal summation, a measure of central excitability, in healthy subjects and patient populations following aerobic and isometric exercise protocols (Koltyn et al. 2013; Henriksen et al. 2014; Naugle & Riley, 2014; Lemley et al. 2015; Stolzman & Bement, 2016; Vaegter et al. 2017). Thus, in human subjects there is evidence to support modulation of central nervous system function with enhanced inhibition and reduced excitation. A number of chronic pain conditions are associated with a loss of conditioned pain modulation and increased temporal summation, and thus lack of immediate effects of exercise, or even increases in pain with acute exercise, could be explained by this lack of inhibition and enhanced excitability. It is further likely that repeated regular exercise could restore the loss of conditioned pain modulation.
Animal models of exercise‐induced analgesia
The first evidence of centrally mediated mechanisms came from animal studies using swimming as the exercise stimulus in healthy, non‐injured rodents (Cooper & Carmody, 1982; Girardot & Holloway, 1984; Koltyn, 2000). Different protocols have been tested, testing different water temperatures and exercise durations (3–10 min). Although longer exercise protocols and colder water temperatures seemed to produce a stronger analgesic effect (Cooper & Carmody, 1982; O'Connor & Chipkin, 1984), swimming interventions as short as 15 s and in warm water promoted increases in pain thresholds that were at least partially reversed by the opioid antagonist naloxone (Cooper & Carmody, 1982). These studies in healthy animals performed a single bout of the exercise task to produce analgesia. Similar results were found in the formalin model, where as little as 3 min of swimming with a single bout of exercise produced a reduction of pain behaviours that was reversed by naloxone (Carmody & Cooper, 1987; Kuphal et al. 2007). Since most studies showed that opioid antagonists only partially reversed exercise‐induced analgesia, especially when lower temperatures and longer exercise times were used (Cooper & Carmody, 1982; Girardot & Holloway, 1984; Terman et al. 1986), it seems that other mechanisms could be involved, but also conditions other than exercise itself might have influenced the results, like changes in body temperature and stress (Koltyn, 2000).
Forced treadmill running in rodents has also been studied as an exercise stimulus and it excludes the temperature bias from swimming protocols. In a neuropathic pain model, 5 weeks of treadmill running with different frequencies (3 or 5 days week−1) and intensities (10 or 16 m min−1 speeds) reversed the injury‐induced hyperalgesia in an intensity‐ but not frequency‐dependent manner (Stagg et al. 2011). A 5‐day treadmill (15–30 min day−1) protocol found similar results in a chronic muscle pain model, with reduction in bilateral mechanical hyperalgesia occurring as soon as immediately after the first session (Bement & Sluka, 2005). In both studies, the effects of exercise were reversed by administration of opioid antagonists, showing evidence of opioid mechanisms underlying the observed exercise‐induced analgesia.
While treadmill running allows one to control the degree of physical activity each animal performs, it can produce a stress component (Contarteze et al. 2008), which itself could produce analgesia through activation of endogenous opioid and serotonergic systems (Yesilyurt et al. 2015), and thus confound interpretation of the results. One way to avoid this is by using running wheels placed in the animals’ home cages. Rodents voluntarily exercise in running wheels in a consistent manner (Sherwin, 1998). Recent studies used running wheels to investigate exercise‐induced analgesia (Smith & Yancey, 2003; Sluka et al. 2013; Grace et al. 2016; Leung et al. 2016) to isolate the effects of exercise from the influence of other stimuli. Different durations of running wheel activity, ranging from 5 consecutive days to 8 weeks and performed before or after the insult have been tested in different models, such as non‐inflammatory chronic muscle pain (Sluka et al. 2013), exercise‐induced pain (Sluka et al. 2013), acute inflammatory muscle pain (Sluka et al. 2013), neuropathic pain (Grace et al. 2016) and healthy control animals (Kanarek et al. 1998; Mathes & Kanarek, 2006). These studies showed the efficacy of running wheel activity in producing analgesia in healthy non‐injured animals, but more importantly, in preventing and reversing hyperalgesia in different pain models. There is a duration‐dependent effect. Importantly, in the studies investigating different pain models, the running wheels were removed from the cages at the time of induction of the model, and thus these studies compared physically active animals to physically inactive animals. Five days of wheel running prevents secondary, but not primary hyperalgesia, in the exercised‐induced pain model and has no effect on hyperalgesia in a chronic non‐inflammatory muscle pain model. On the other hand, 6–8 weeks of physical activity prevents both primary and secondary hyperalgesia in an exercise‐induced pain model, a chronic non‐inflammatory muscle pain model and a neuropathic pain model (Sluka et al. 2013; Grace et al. 2016), but not in an acute inflammatory pain model (Sluka et al. 2013). Further, 2 weeks of voluntary wheel running was unable to reverse hyperalgesia in mouse models of neuropathic pain and formalin‐induced acute pain (Sheahan et al. 2015), but longer duration wheel running (6 weeks) successfully prevented and reversed hyperalgesia from a neuropathic pain model (Grace et al. 2016). Table 1 summarizes the exercise protocols used in animal studies. Thus, multiple different protocols have been used to produce analgesia in uninjured animals and in multiple pain models. These include swimming, treadmill exercise, and wheel running with a single bout of exercise producing analgesia to multiple days and weeks. The analgesic effects depend on duration (days or weeks), with longer training protocols producing more significant results. Further, while protocols applied after the injury can reverse the hyperalgesia, intriguingly making animals physically active prior to the insult prevents the development of the hyperalgesia in both neuropathic pain and muscle pain models.
Table 1.
Summary of studies examining exercise‐induced analgesia
| Pain model | Exercise intervention | Duration | Effect | Study |
|---|---|---|---|---|
| Healthy animals | Swimming (15 s to 7.5 min) | Single bout | Reduction in thermal hyperalgesia | Cooper & Carmody (1982) |
| Cold water swim 3.5 min, 2°C | Single bout | Reduction in thermal hyperalgesia | Girardot & Holloway (1984) | |
| Swimming 3 min warm water, 2 min cold water | Single bout | Reduction in thermal hyperalgesia | O'Connor & Chipkin (1984) | |
| Voluntary wheel running | 6 weeks | Reduction in thermal hyperalgesia | Smith & Yancey (2003) | |
| 20 days | Increase in thermal hyperalgesia | Kanarek et al. (1998) | ||
| 24 h | No effect | |||
| 3 weeks | Reduction in thermal hyperalgesia | Mathes & Kanarek (2006) | ||
| Resistance exercise, 3 sets of 10 repetitions, 3 times week−1 | 12 weeks | No effect | Galdino et al. (2010) | |
| Resistance exercise, 15 sets of 15 repetitions | 1 day | Reduction in mechanical hyperalgesia | Galdino et al. (2014a) | |
| Treadmill running, 20 m min−1 speed, until fatigue (average of 49.06 ± 3 min) | Single bout | Reduction in thermal and mechanical hyperalgesia | Galdino et al. (2014b) | |
| Cold water swimming, 5 min, 1°C | Single bout | Reduction in thermal hyperalgesia | Vaswani et al. (1988) | |
| Formalin test | Voluntary wheel running | 2 weeks | No effect | Sheahan et al. (2015) |
| Cold water swimming | Single bout | Reduction in pain scores and thermal hyperalgesia | Terman et al. (1986) | |
| Swimming 3 min warm water | Single bout | Reduction in pain scores | Carmody & Cooper (1987) | |
| Swimming 37°C water for 90 min day−1 | 9 days | Reduction in pain scores | Kuphal et al. (2007) | |
| Neuropathic pain | Swimming | 18–25 days | Reduction in pain ratings, cold allodynia and thermal hyperalgesia | |
| Treadmill running, 3 or 5 days week−1, 16 m min−1 speed | 5 weeks | Reduction in mechanical hyperalgesia | Stagg et al. (2011) | |
| Voluntary wheel running | 6 weeks | Prevention of allodynia | Grace et al. (2016) | |
| Treadmill running, 30 min, 5 days week−1, 10 m min−1 speed | 2 weeks | Reduction in mechanical hyperalgesia | Bobinski et al. (2015) | |
| Voluntary wheel running | 2 weeks | No effect | Sheahan et al. (2015) | |
| 30 min day−1, 5 days a week, 8–20 m min−1 speed | 4 weeks | Reduction in thermal hyperalgesia and mechanical allodynia | Kim et al. (2015) | |
| Treadmill running, 20–60 min day−1, 9 m min−1 speed | 4 weeks | Reduction in mechanical allodynia | Korb et al. (2010) | |
| Chronic muscle | Treadmill running, 15–30 min day−1, 6–10 m min−1 speed | 5 days | Reduction in mechanical hyperalgesia | Bement & Sluka (2005) |
| pain model | Voluntary wheel running | 8 weeks | Prevention of primary and secondary hyperalgesia | Sluka et al. (2013) |
| 5 days | No effect | |||
| 8 weeks | Prevention of primary and secondary hyperalgesia | Leung et al. (2016) | ||
| Exercise‐enhanced | Voluntary wheel running | 5 days | Prevention of secondary hyperalgesia | Lima et al. (2016) |
| pain | 8 weeks | Prevention of secondary hyperalgesia | Sluka et al. (2013) | |
| 5 days | ||||
| Acute muscle | Voluntary wheel running | 8 weeks | No effect | Sluka et al. (2013) |
| inflammation | 5 days | |||
| Chemically induced nociception | Swimming, 30 min day−1 | 5 days | Reduction in pain behaviours (abdominal constrictions) | Mazzardo‐Martins et al. (2010) |
| Swimming, 10–30 min day−1 | 2 weeks | Reduction in pain behaviours (paw‐licking) and mechanical hyperalgesia | Martins et al. (2017) |
Central mechanisms involved in exercise‐induced analgesia
The RVM comprises, with the PAG and dorsal horn, a descending pain inhibitory system that both facilitates and inhibits noxious stimuli (Porreca et al. 2002). Within the RVM, NRM, NRO and NRP are nuclei known to be involved in pain modulation but are also involved in modulation of motor responses, making them potential key areas involved in exercise‐induced analgesia mechanisms (Fields et al. 2006). Three types of cells exist in the RVM: ON‐cells promote nociception when activated, OFF‐cells inhibit nociception when activated, and neutral cells do not respond to noxious stimuli (Fields et al. 2006). We propose that a shift in the balance between ON‐ and OFF‐cell activation defines hyperalgesia or analgesia from an exercise task. As discussed previously, NMDA receptors in the RVM play a role in facilitation of nociception with an increase in phosphorylation of the NR1 subunit playing a critical role (Da Silva et al. 2010a, b; Sluka et al. 2012). Exercise‐induced analgesia promotes the opposite response. Either 5 days or 8 weeks of wheel running prevented the increase in phosphorylation of NR1 in the RVM of mice induced with chronic non‐inflammatory muscle pain or exercise‐enhanced pain when compared to induced sedentary mice (Sluka et al. 2013). These data suggest that regular physical activity reduces facilitation in the caudal brainstem by modulating NMDA receptor function.
There is strong evidence that opioid mechanisms mediate exercise‐induced analgesia in both human and animal studies (Koltyn, 2000). Several studies showed that the opioid antagonist naloxone, given systemically, blocks the analgesic effects of swimming and resistance exercise in healthy, uninjured animals (Cooper & Carmody, 1982; O'Connor & Chipkin, 1984; Galdino et al. 2010; Mazzardo‐Martins et al. 2010; Martins et al. 2017), and treadmill running in chronic muscle pain (5‐day running) and neuropathic pain models (5‐weeks running) (Bement & Sluka, 2005; Stagg et al. 2011). Subsequent studies show that supraspinal naloxone blocks the analgesia produced by 5 weeks of treadmill running in a neuropathic pain model (Stagg et al. 2011). Further, there are increased concentrations of endogenous opioids systemically in both human subjects and in animals (Wildmann et al. 1986; Vaswani et al. 1988; Debruille et al. 1999; Stagg et al. 2011; Bidari et al. 2016), in the PAG and RVM in animals (Commons, 2003; Stagg et al. 2011; Kim et al. 2015), and increased μ‐opioid receptor expression in the hippocampus of rats after both acute (7 days) and chronic (45 days) treadmill or wheel running (de Oliveira et al. 2010). Further, 4–6 weeks of voluntary wheel running produces cross‐tolerance to μ‐opioid agonists and physical dependence, effects similar to those resulting from chronic use of opioids (Kanarek et al. 1998; Smith & Yancey, 2003) and 3 weeks of wheel running attenuates the analgesia from morphine injected into the PAG of rats (Mathes & Kanarek, 2006). Thus, regular physical activity and exercise use central opioid receptors to produce analgesia.
Serotonin (5‐HT) has also been implicated in exercise‐induced analgesia. One hour of swimming increases 5‐HT levels in the brainstem and hypothalamus, while 4 weeks of swimming extended this increase to the cerebral cortex (Dey et al. 1992). Similarly, 8 weeks of treadmill running showed increased levels of 5‐HT in the midbrain and cortex (Brown et al. 1979), and 4 weeks of treadmill running increases 5‐HT expression in the RVM (Korb et al. 2010). More recently, we extended these studies by examining the role of serotonin in a neuropathic pain model. We show that 2 weeks of low‐intensity treadmill running in a neuropathic pain model increased 5‐HT levels in the caudal brainstem, decreased expression of the serotonin transporter in the NRM, NRO and NRP, and altered serotonin receptor expression in the brainstem (Bobinski et al. 2015). Importantly, in neuropathic pain models there is an increase in serotonin transporter expression and a decrease in 5‐HT in the brainstem; 2 weeks of treadmill running reversed these injury‐induced changes. Further, systemic depletion of serotonin prevents the analgesia produced by treadmill running in neuropathic pain (Bobinski et al. 2015) and by high intensity swimming (30 min to 5 days) in the acetic acid writhing test (Mazzardo‐Martins et al. 2010). Thus, there is emerging evidence that increases in supraspinal serotonin release, along with reductions in the serotonin transporter, play a significant role in the analgesia produced by regular exercise.
There are reasons to believe that the opioid and serotonergic mechanisms are not independently activated by exercise, but rather they interact to promote analgesia. Serotonergic neurons receive input from endogenous opioid peptides and both coexist in RVM neurons (Fields et al. 2006). Further evidence of this interaction is shown by blockade of analgesia from systemic or RVM‐injected morphine following systemic depletion of serotonin, or blockade of serotonin receptors in the RVM (Schul & Frenk, 1991; Carruba et al. 1992). We recently tested this hypothesis by performing immunohistochemistry for serotonin transporter in μ‐opioid receptor knockout mice induced with exercise‐induced pain and comparing these to wild‐type mice (Lima et al. 2016). μ‐Opioid receptor knockout and wild‐type mice were exposed to 5 days of wheel‐running prior to induction the exercise‐induced pain model, and compared to sedentary mice. Wheel running prevented the increase in the serotonin transporter in the RVM induced by the muscle insult in wild‐type mice. However, in μ‐opioid receptor knockout mice, wheel running had no effect on the increased serotonin transporter expression induced by muscle insult. Thus, these data suggest that μ‐opioid receptor activation by exercise reduces expression of the serotonin transporter in the caudal brainstem to promote analgesia.
Endocannabinoids in the central nervous system also play a role in exercise‐induced analgesia (Dietrich & McDaniel, 2004). Endocannabinoid receptors are present in pain‐modulating areas of the brain and spinal cord (Herkenham et al. 1991) and activation of endocannabinoid receptors produces analgesia (Dietrich & McDaniel, 2004). Further, exercise increases circulating levels of the endocannabinoid N‐arachidonylethanolamine in healthy human subjects (Koltyn et al. 2014). After both aerobic and resistance exercise tasks, there is an increased expression of the cannabinoid receptor CB1 in the brain, including the PAG, in healthy uninjured animals. This effect is prevented by systemic and central blockade with cannabinoid receptor antagonists (AM251 and AM630) (Galdino et al. 2014a, b). Since endocannabinoids have synergistic interactions with opioids to produce antinociception (Navarro et al. 1998), one could speculate that the same interaction occurs during exercise‐induced analgesia. Thus, there is emerging evidence that endogenous endocannabinoids in the central nervous system contribute to the analgesia produced by regular exercise.
Conclusion
A single bout of fatiguing exercise in the presence of a chronic pain condition can exacerbate pain that is characterized by increased phosphorylation of NMDA receptors in the RVM, suggesting enhanced central facilitation. On the other hand, regular exercise promotes pain relief and is characterized by reduced NMDA receptor phosphorylation, suggesting reduced central facilitation. Further regular exercise reduces serotonin transporter expression, increases serotonin levels, and increases opioids in central inhibitory pathways including the PAG and RVM, suggesting exercise utilizes our endogenous inhibitory systems to reduce pain (Fig. 1). We propose that there is a balance between inhibition and excitation in the central nervous system that determines whether exercise will promote analgesia or promote pain. Several factors, such as fitness level, physical activity levels, and state of the injury or pain condition influence this balance. The great majority of the animal studies examining pain mechanisms are performed in physically inactive animals, and nearly all the exercise studies are focused on aerobic exercise. Further, there is no consistency regarding intensity, duration, frequency or exercise type making interpretation difficult. Understanding the mechanisms underlying different forms of exercise, as well as the different intensities and duration of exercise that produce analgesia, will be critically important to translate animal studies to human subjects, particularly those with acute and chronic pain.
Additional information
Competing interests
All authors declare no conflict of interest
Author contributions
L.V.L: designed, wrote and reviewed the manuscript; T.S.S.A.: wrote and reviewed the manuscript; K.A.S.: designed, wrote and reviewed the manuscript. All authors contributed to the writing of the manuscript and approved with the final version. All designated authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Funded by NIH grants R01 AR061371 and UM1 AR06338.
Biographies
Lucas Lima and Thiago Abner are graduate students in Physical Therapy at the Federal University of Sergipe, and did a research fellowship with Kathleen Sluka at the University of Iowa.

Kathleen Sluka is a professor in the Department of Physical Therapy and Rehabilitation Science at the University of Iowa. She received a physical therapy degree from Georgia State University and a PhD in Anatomy from the University of Texas Medical Branch in Galveston. After a postdoctoral fellowship with Dr William D. Willis, she joined the faculty at the University of Iowa. Her research focuses on the neurobiology of musculoskeletal pain as well as the mechanisms and effectiveness of non‐pharmacological pain treatments commonly used by physical therapists. She has published over 180 peer‐reviewed manuscripts, numerous book chapters, and a textbook on Pain Mechanisms and Management for the Physical Therapist.
This review was presented at the symposium “Top‐down control of pain”, which took place at Physiology 2016, Dublin, Ireland, 29‐31 July 2016.
References
- Basbaum AI & Fields HL (1984). Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neuroscience 7, 309–338. [DOI] [PubMed] [Google Scholar]
- Bement MKH & Sluka KA (2005). Low‐intensity exercise reverses chronic muscle pain in the rat in a naloxone‐dependent manner. Arch Phys Med Rehabil 86, 1736–1740. [DOI] [PubMed] [Google Scholar]
- Bement MKH & Sluka KA (2016). Exercise‐induced analgesia: an evidence‐based review In Mechanisms and Management of Pain for the Physical Therapist, 2nd edn, ed. Sluka KA, Ch. 10. pp. 177–201. Wolters Kuwer, IASP Press, Seattle. [Google Scholar]
- Bement MKH, Weyer A, Hartley S, Yoon T & Hunter SK (2009). Fatiguing exercise attenuates pain‐induced corticomotor excitability. Neurosci Lett 452, 209–213. [DOI] [PubMed] [Google Scholar]
- Bidari A, Ghavidel‐Parsa B, Rajabi S, Sanaei O & Toutounchi M (2016). The acute effect of maximal exercise on plasma beta‐endorphin levels in fibromyalgia patients. Korean J Pain 29, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinski F, Ferreira TA, Córdova MM, Dombrowski PA, da Cunha C, do Espírito Santo CC, Poli A, Pires RG, Martins‐Silva C & Sluka KA (2015). Role of brainstem serotonin in analgesia produced by low‐intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 156, 2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BS, Payne T, Kim C, Moore G, Krebs P & Martin W (1979). Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol 46, 19–23. [DOI] [PubMed] [Google Scholar]
- Carmody J & Cooper K (1987). Swim stress reduces chronic pain in mice through an opioid mechanism. Neurosci Lett 74, 358–363. [DOI] [PubMed] [Google Scholar]
- Carruba MO, Nisoli E, Garosi V, Sacerdote P, Panerai AE & Da Prada M (1992). Catecholamine and serotonin depletion from rat spinal cord: effects on morphine and footshock induced analgesia. Pharmacol Res 25, 187–194. [DOI] [PubMed] [Google Scholar]
- Chen B‐S & Roche KW (2007). Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG (2003). Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol 464, 197–207. [DOI] [PubMed] [Google Scholar]
- Contarteze RVL, Manchado FDB, Gobatto CA & De Mello MAR (2008). Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol 151, 415–422. [DOI] [PubMed] [Google Scholar]
- Cooper K & Carmody J (1982). The characteristics of the opioid‐related analgesia induced by the stress of swimming in the mouse. Neurosci Lett 31, 165–170. [DOI] [PubMed] [Google Scholar]
- Dailey DL, Keffala VJ & Sluka KA (2015). Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res 67, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgard E, Thrane G, Anke A, Fors T & Røe C (2010). Activity‐related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil 32, 1428–1437. [DOI] [PubMed] [Google Scholar]
- Da Silva LF, DeSantana JM & Sluka KA (2010a). Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain 11, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva LFS, Walder RY, Davidson BL, Wilson SP & Sluka KA (2010b). Changes in expression of NMDA‐NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain 151, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM & Bailey SP (1997). Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 29, 45–57. [DOI] [PubMed] [Google Scholar]
- Debruille C, Luyckx M, Ballester L, Brunet C, Odou P, Dine T, Gressier B, Cayin M & Cayin J (1999). Serum opioid activity after physical exercise in rats. Physiol Res 48, 129–134. [PubMed] [Google Scholar]
- de Oliveira MSR, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA & Arida RM (2010). Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull 83, 278–283. [DOI] [PubMed] [Google Scholar]
- Dey S, Singh R & Dey P (1992). Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav 52, 1095–1099. [DOI] [PubMed] [Google Scholar]
- Dietrich A & McDaniel W (2004). Endocannabinoids and exercise. Br J Sports Med 38, 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM & Chou R (2016). CDC guideline for prescribing opioids for chronic pain—United States, 2016. J Am Med Assoc 315, 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson LD, Koltyn KF, Kim JS & Cook DB (2014). Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 51, 267–276. [DOI] [PubMed] [Google Scholar]
- Fields H, Malick A & Burstein R (1995). Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol 74, 1742–1759. [DOI] [PubMed] [Google Scholar]
- Fingleton C, Smart K, Doody C & Dip T (2017). Exercise‐induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain 33, 395–404. [DOI] [PubMed] [Google Scholar]
- Flood A, Waddington G, Thompson K & Cathcart S (2017). Increased conditioned pain modulation in athletes. J Sports Sci 35, 1066–1072. [DOI] [PubMed] [Google Scholar]
- Galdino G, Duarte I & Perez A (2010). Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res 43, 906–909. [DOI] [PubMed] [Google Scholar]
- Galdino G, Romero T, da Silva JFP, Aguiar D, de Paula AM, Cruz J, Parrella C, Piscitelli F, Duarte I & Di Marzo V (2014a). Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg 119, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdino G, Romero TR, Silva JFP, Aguiar DC, de Paula AM, Cruz JS, Parrella C, Piscitelli F, Duarte ID & Di Marzo V (2014b). The endocannabinoid system mediates aerobic exercise‐induced antinociception in rats. Neuropharmacology 77, 313–324. [DOI] [PubMed] [Google Scholar]
- Geva N & Defrin R (2013). Enhanced pain modulation among triathletes: A possible explanation for their exceptional capabilities. Pain 154, 2317–2323. [DOI] [PubMed] [Google Scholar]
- Girardot M‐N & Holloway FA (1984). Cold water stress analgesia in rats: Differential effects of naltrexone. Physiol Behav 32, 547–555. [DOI] [PubMed] [Google Scholar]
- Grace PM, Fabisiak TJ, Green‐Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, Galer EL, Walker FR, Greenwood BN & Maier SF (2016). Prior voluntary wheel running attenuates neuropathic pain. Pain 157, 2012–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Brito RG, Fusaro MC, Sluka KA (2016). ASIC3 is required for development of fatigue‐induced hyperalgesia. Mol Neurobiol 53, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Gibson‐Corley K, Frey‐Law L & Sluka KA (2013). Fatigue‐enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex‐dependent manner. Pain 154, 2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen M, Klokker L, Graven‐Nielsen T, Bartholdy C, Schjødt Jørgensen T, Bandak E, Danneskiold‐Samsøe B, Christensen R & Bliddal H (2014). Association of exercise therapy and reduction of pain sensitivity in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res 66, 1836–1843. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR & Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11, 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janal MN, Colt EW, Clark WC & Glusman M (1984). Pain sensitivity, mood and plasma endocrine levels in man following long‐distance running: effects of naloxone. Pain 19, 13–25. [DOI] [PubMed] [Google Scholar]
- Kadetoff D & Kosek E (2007). The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain 11, 39–47. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Gerstein AV, Wildman RP, Mathes WF & D'Anci KE (1998). Chronic running‐wheel activity decreases sensitivity to morphine‐induced analgesia in male and female rats. Pharmacol Biochem Behav 61, 19–27. [DOI] [PubMed] [Google Scholar]
- Kim Y‐J, Byun J‐H & Choi I‐S (2015). Effect of exercise on μ‐opioid receptor expression in the rostral ventromedial medulla in neuropathic pain rat model. Ann Rehabil Med 39, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn KF (2000). Analgesia following exercise. Sports Med 29, 85–98. [DOI] [PubMed] [Google Scholar]
- Koltyn KF, Brellenthin AG, Cook DB, Sehgal N & Hillard C (2014). Mechanisms of exercise‐induced hypoalgesia. J Pain 15, 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn KF, Knauf MT & Brellenthin AG (2013). Temporal summation of heat pain modulated by isometric exercise. Eur J Pain 17, 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb A, Bonetti LV, Da Silva SA, Marcuzzo S, Ilha J, Bertagnolli M, Partata WA & Faccioni‐Heuser MC (2010). Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem Res 35, 380–389. [DOI] [PubMed] [Google Scholar]
- Kuphal KE, Fibuch EE & Taylor BK (2007). Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain 8, 989–997. [DOI] [PubMed] [Google Scholar]
- Lannersten L & Kosek E (2010). Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 151, 77–86. [DOI] [PubMed] [Google Scholar]
- Lemley KJ, Hunter SK & Bement M (2015). Conditioned pain modulation predicts exercise‐induced hypoalgesia in healthy adults. Med Sci Sports Exerc 47, 176–184. [DOI] [PubMed] [Google Scholar]
- Leung A, Gregory NS, Allen L‐AH & Sluka KA (2016). Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin‐10 in mice. Pain 157, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L, DeSantana J, Rasmussen L & Sluka K (2016). Short‐duration physical activity prevents the development of exercise‐enhanced hyperalgesia through opioid mechanisms. J Pain 17, S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins DF, Siteneski A, Ludtke DD, Dal‐Secco D & Santos AR (2017). High‐intensity swimming exercise decreases glutamate‐induced nociception by activation of G‐protein‐coupled receptors inhibiting phosphorylated protein kinase A. Mol Neurobiol (in press; https://doi.org/10.1007/s12035-016-0095-9). [DOI] [PubMed] [Google Scholar]
- Mathes WF & Kanarek RB (2006). Chronic running wheel activity attenuates the antinociceptive actions of morphine and morphine‐6‐glucouronide administration into the periaqueductal gray in rats. Pharmacol Biochem Behav 83, 578–584. [DOI] [PubMed] [Google Scholar]
- Mazzardo‐Martins L, Martins DF, Marcon R, dos Santos UD, Speckhann B, Gadotti VM, Sigwalt AR, Guglielmo LGA & Santos ARS (2010). High‐intensity extended swimming exercise reduces pain‐related behavior in mice: involvement of endogenous opioids and the serotonergic system. J Pain 11, 1384–1393. [DOI] [PubMed] [Google Scholar]
- Naugle KM & Riley JL (2014). Self‐reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc 46, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Chowen J, Carrera MRA, del Arco I, Villanúa MA, Martin Y, Roberts AJ, Koob GF & de Fonseca FR (1998). CB1 cannabinoid receptor antagonist‐induced opiate withdrawal in morphine‐dependent rats. Neuroreport 9, 3397–3402. [DOI] [PubMed] [Google Scholar]
- O'Connor P & Chipkin RE (1984). Comparisons between warm and cold water swim stress in mice. Life Sci 35, 631–639. [DOI] [PubMed] [Google Scholar]
- Olausson B, Eriksson E, Ellmarker L, Rydenhag B, Shyu BC & Andersson S (1986). Effects of naloxone on dental pain threshold following muscle exercise and low frequency transcutaneous nerve stimulation: a comparative study in man. Acta Physiol Scand 126, 299–305. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH & Gebhart G (2002). Chronic pain and medullary descending facilitation. Trends Neurosci 25, 319–325. [DOI] [PubMed] [Google Scholar]
- Schul R & Frenk H (1991). The role of serotonin in analgesia elicited by morphine in the periaqueductal gray matter (PAG). Brain Res 553, 353–357. [DOI] [PubMed] [Google Scholar]
- Sheahan TD, Copits BA, Golden JP & IV Gereau RW (2015). Voluntary exercise training: analysis of mice in uninjured, inflammatory, and nerve‐injured pain states. PLoS One 10, e0133191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin C (1998). Voluntary wheel running: a review and novel interpretation. Anim Behav 56, 11–27. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Danielson J, Rasmussen L & Dasilva LF (2012). Exercise‐induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc 44, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, O'Donnell JM, Danielson J & Rasmussen LA (2013). Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol 114, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA & Rasmussen LA (2010). Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain 148, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA & Yancey DL (2003). Sensitivity to the effects of opioids in rats with free access to exercise wheels: μ‐opioid tolerance and physical dependence. Psychopharmacology (Berl) 168, 426–434. [DOI] [PubMed] [Google Scholar]
- Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW & Malan TP (2011). Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain modelrole of endogenous opioids. Anesthesiol 114, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Robinson ME & Price DD (2005). Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain 118, 176–184. [DOI] [PubMed] [Google Scholar]
- Stolzman S & Bement MH (2016). Does exercise decrease pain via conditioned pain modulation in adolescents? Pediatr Phys Ther 28, 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Morgan MJ & Liebeskind JC (1986). Opioid and non‐opioid stress analgesia from cold water swim: importance of stress severity. Brain Res 372, 167–171. [DOI] [PubMed] [Google Scholar]
- Vaegter HB, Handberg G, Emmeluth C & Graven‐Nielsen T (2017). Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief six months after total knee replacement. Clin J Pain (in press; https://doi.org/10.1097/AJP.0000000000000428). [DOI] [PubMed] [Google Scholar]
- Vaswani KK, Richard CW & Tejwani GA (1988). Cold swim stress‐induced changes in the levels of opioid peptides in the rat CNS and peripheral tissues. Pharmacol Biochem Behav 29, 163–168. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP & Martin AD (2001). The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain 2, 334–344. [DOI] [PubMed] [Google Scholar]
- Whiteside A, Hansen S & Chaudhuri A (2004). Exercise lowers pain threshold in chronic fatigue syndrome. Pain 109, 497–499. [DOI] [PubMed] [Google Scholar]
- Wildmann J, Krüger A, Schmole M, Niemann J & Matthaei H (1986). Increase of circulating beta‐endorphin‐like immunoreactivity correlates with the change in feeling of pleasantness after running. Life Sci 38, 997–1003. [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Seyrek M, Tasdemir S, Kahraman S, Deveci MS, Karakus E, Halici Z & Dogrul A (2015). The critical role of spinal 5‐HT 7 receptors in opioid and non‐opioid type stress‐induced analgesia. Eur J Pharmacol 762, 402–410. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Lisi TL, Moore SA & Sluka KA (2007). Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain 8, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Sengupta J & Gebhart G (2002). Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol 87, 2225–2236. [DOI] [PubMed] [Google Scholar]


