Figure 1.
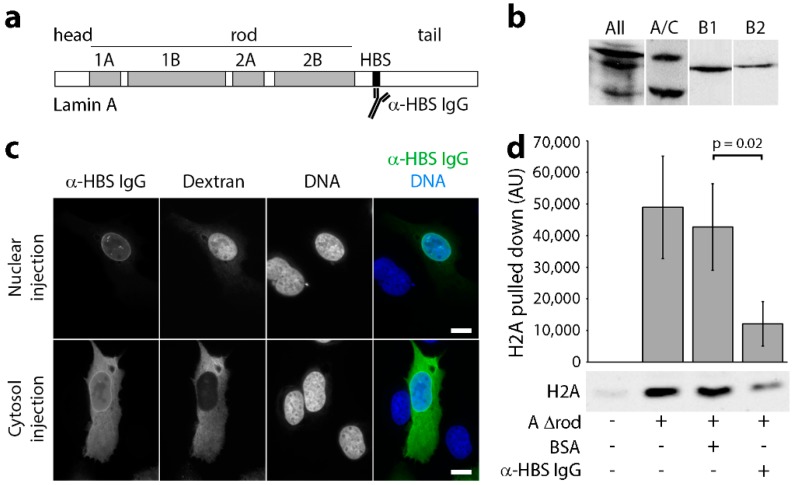
Lamin A/C histone binding site antibodies are specific, target in vivo and block H2A binding in vitro. (a) Schematic of lamin A domain structure highlighting the head, rod and tail domains, as well as the mapped chromatin binding site (HBS; Taniura et al., 1995). (b) Western blot on total HeLa cell lysates using a pan-lamin antibody and each histone-binding site antibody (A/C, B1 and B2) revealed that all antibodies prepared against the chromatin binding sites of lamins were each highly specific for each subtype. (c) The affinity-purified antibodies were conjugated to an SV40 NLS peptide and microinjected into either the nuclei or cytoplasm of U2OS cells. In both cases, subsequent fixation and visualization with fluorophore-conjugated secondary antibodies revealed nuclear rim staining, consistent with their binding the expected target on lamin A in the polymer underlying the inner nuclear membrane. (d) To test for antibody blocking of histone binding, lamin A∆rod protein was coupled to beads, incubated with either no antibodies (Lane 2), antibodies generated against BSA (Lane 3) or the lamin A histone-binding site antibodies (Lane 4). Uncoupled beads were also tested as a control for background binding to the beads because histones tend to be sticky (Lane 1). After washing, each was incubated with histones, eluted with SDS and analysed for the amount of bound histones by Western blot. Three technical replicates were quantified for fluorescence intensity using a LiCor Odyssey and plotted (n = 3). Standard deviations are shown, paired t-test shows a significant reduction in H2A bound by lamin A∆rod in the presence of the histone-binding site antibody compared to the BSA control. Scale bars, 10 µm.