Abstract
The success of alpha interferon (IFN-α) monotherapy for the treatment of chronic hepatitis D is very limited. In this study, the efficacy of IFN-α and ribavirin combination therapy for chronic hepatitis D was investigated. Nineteen patients (15 males; mean age ± standard deviation, 36.8 ± 12.8 years) with chronic hepatitis D who were treated with IFN-α2b (10 million U, three times/week, subcutaneously) and ribavirin (1,000 to 1,200 mg/day, orally) for 24 months were studied. All patients had compensated liver disease (15 were precirrhotic), elevated transaminase levels, and hepatitis D virus RNA positivity at baseline. Genotypic analyses revealed hepatitis D virus genotype I and hepatitis B virus genotype D. All patients completed the 24 months of treatment and at least 6 months (7 to 19 months) of a follow-up period. Biochemical responses were observed in eight patients (42.1%) at the end of treatment and in seven patients (36.8%) at the end of follow-up. Eight patients (42.1%) at the end of treatment and four patients (21%) at the end of follow-up had virological responses. In conclusion, combination treatment of IFN-α and ribavirin for chronic hepatitis D is not able to induce virological responses at a sufficient rate, despite its partial effectiveness in improving biochemical responses, and is not superior to IFN-α monotherapy.
The hepatitis D virus (HDV) is a defective RNA virus that requires a helper function provided by hepatitis B virus (HBV) for viral assembly and propagation (16). The clinical course of chronic hepatitis D (CDH) is generally more severe than that of other forms of viral hepatitis. CDH is a serious and rapidly progressive liver disease that leads to cirrhosis in 70% of patients (17, 18). The only effective therapy for CDH is alpha interferon (IFN-α) (4). Normalization of aminotransferase levels, loss of HDV RNA, and improvement in liver histology are observed in some patients who are treated with IFN-α. However, after discontinuation of therapy, the majority of patients experience relapses. Recently, the idea of combining IFN-α with nucleoside analogues to increase the efficacy and decrease the recurrence rate became popular for treatment of chronic viral hepatitis. Unfortunately, lamivudine, one of the nucleoside analogues, did not show any increase in efficacy in the treatment of CDH (21, 22). Another nucleoside analogue, ribavirin, is effective against RNA viruses, and it has been shown experimentally to inhibit HDV genome replication in hepatocyte cultures (3). However, in a pilot study including patients with CDH, ribavirin monotherapy did not result in biochemical, virological, or histological improvements (6).
In the present study, we aimed to determine the efficacy of IFN-α and ribavirin in combination therapy for CDH.
MATERIALS AND METHODS
This study was planned as an open, one-armed, prospective trial. CDH patients who were admitted to the Department of Gastroenterohepatology, Istanbul Medical Faculty, during the period March 2000 to March 2001 were included in the trial. The study protocol was approved by the Local Ethics Committee, and informed consent was obtained from all patients.
All included patients (n = 19) were positive for HBV surface antigen (HBsAg), HDV total antibody (anti-HDV), and HDV RNA for a minimum of 6 months; demonstrated serum alanine aminotransferase (ALT) levels at least 1.3-fold higher than the upper limit of normal (normal range, 5 to 40 IU/liter) on two occasions during the preceding 3 months; had compensated liver disease with histologic evidence of chronic hepatitis; and were between 18 and 65 years old. The patients were excluded if they met any of the following criteria: presence of any other etiology of chronic liver disease or seropositivity for human immunodeficiency virus antibody (anti-HIV), associated serious medical illness, pregnancy or breastfeeding, hepatocellular carcinoma, decompensated liver disease, a white blood cell count lower than 3,000/mm3, or a platelet count lower than 50,000/mm3. The diagnosis of CDH was based on anti-HDV and HDV RNA positivities and histological findings of chronic hepatitis.
All patients were administered IFN-α2b (Intron-A; Schering-Plough, Cork City, Ireland) (10 million U, subcutaneously, three times weekly) and ribavirin (Rebetol; Schering-Plough) (1,000 to 1,200 mg/day, orally) for 24 months. All subjects were monitored for at least 6 months after therapy discontinuation. A liver biopsy specimen was taken prior to the beginning of therapy. All liver biopsy slides were assessed according to the scoring system of Knodell et al. (11).
Every patient underwent a detailed physical examination during visits, twice in the first month, once a month in the following 5 months, and once every 2 months during the remaining period. Serial hematological and biochemical studies were done during visits. Serum HDV RNA was tested at the beginning of treatment, at months 12 and 18, at the end of treatment, and at the end of follow-up. Compliance with therapy and adverse events were assessed at each visit.
HBsAg, hepatitis B “e” antigen (HBeAg), antibody to hepatitis B “e” antigen (anti-HBe) (Sanofi Pasteur Diagnostics, Marnes la Coquette, France), and anti-HDV (Abbott Laboratories) were determined by immunoenzymatic assays. Antibody to hepatitis C virus (anti-HCV) was tested with third-generation UBI HCV EIA 4.0 kits (Organon Teknika, RM Boxtel, The Netherlands), and anti-HIV was tested with a Vironostika HIV Uni-Form II Plus O kit (Organon Teknika) based on the technique called sandwich enzyme immunoassay. HBV DNA was quantitated by a hybridization technique (Hybride capture system; Digene Corp., Gaithersburg, Md.). The lower detection limit of this assay was 5 pg/ml. HBV DNA and HbsAg were analyzed before and after treatment.
For the diagnostic HDV RNA PCR and genotyping PCR, HDV RNA was extracted by a method described by Boom et al. (2). A 100-μl serum sample was used appropriately for the protocol. Then cDNA was purified by using 10 μl of extracted material, 100 ng of random primer (Roche; catalog no. 1034731), a 0.4 mM concentration of each deoxynucleoside triphosphate (dNTP), 20 U of RNase inhibitor (Sigma; R2520), 5 U of avian myeloblastosis virus reverse transcriptase (Roche; catalog no. 1 495 062), and 1× buffer (total volume, 25 μl of reverse transcription [RT] buffer). The cDNA mixture was incubated at 42°C for 1 h.
HDV RNA has been used for the diagnosis of patients by RT nested PCR (13). In the first stage of PCR, 5 μl of cDNA was added to a PCR mixture consisting of 10 mM Tris HCl, 1.5 mM MgCl2, 50 mM KCl, a 0.2 mM concentration of each dNTP, a 0.5 mM concentration of outer primers 5413 (5′-GCC CAG GTC GGA CCG CGA GGA GGT) and 8276 (5′-ACA AGG AGA GGC AGG ATC ACC GAC), and 2.5 U of Taq DNA polymerase enzyme (Sigma; D1806) (total volume, 50 μl). The reaction mixture was incubated for 35 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s. After incubation at 72°C for 10 min, the process was finalized. The same PCR cycle conditions and reagent concentrations were used in the second PCR stage with inner primers 5414 (5′-GAG ATG CCA TGC CGA CCC GAA GAG) and 5415 (5′-GAA GGA AGG CCC TCG AGA ACA AGA) and 5 μl of the first PCR product. PCR results were analyzed by performing electrophoresis with 1.5% agarose gels. According to the results of the serial dilutions performed, it was determined that the cutoff value of the PCR assay was 1,000 copies/ml.
The genotype analyses were done by using RT-PCR and restriction fragment length polymorphism methods (10). For the PCR stage, 10 μl of cDNA was added to the PCR mixture consisting of 10 mM Tris HCl, 1.5 mM MgCl2, 50 mM KCl, a 0.2 mM concentration of each dNTP, a 0.5 mM concentration of primers 900s (5′-GCC GAC CCG AAG AGG AAA G) and 1280as (5′-GAA GGA AGG CCC TSG AGA ACA AGA), and 2.5 U of Taq DNA polymerase enzyme (Sigma; D1806) (total volume, 50 μl). The reaction mixture was incubated at 94°C for 9 min and then for 40 cycles of 94°C for 45 s, 58°C for 30 s, and 72°C for 45 s. After incubation at 72°C for 10 min, the process was finalized. PCR results were analyzed by performing electrophoresis with 1.5% agarose gels. For HDV genotyping with restriction fragment length polymorphism analysis, digestion was performed with 5-μl samples containing 5 U of SmaI enzyme (Fermentas ER0662) and 1× buffer (total volume, 20 μl), and then the mixture was incubated overnight. Digested products were analyzed by electrophoresis in 2% agarose gels. The DNA patterns (227 and 178 bp) were determined to be related to genotype I in all samples.
The HBV genotype was also tested by single-strand conformation polymorphism analysis or by a research line probe assay (INNO-LiPA HBV genotyping; Innogenetics N.V., Cohent, Belgium), which contained probes specific for the six major genotypes (A to F).
A virological response was defined by the disappearance of HDV RNA in serum. Normalization of serum ALT level was accepted as proof of a biochemical response. These measures of both virological and biochemical responses were assessed at the end of treatment and at the end of the follow-up period. During the follow-up period, the reappearance of serum HDV RNA and an increase in serum ALT level from the normal range to more than 1.3 times the upper limit of normal were defined as indicating virological and biochemical relapses, respectively.
Statistical analysis.
All patients who completed the treatment and follow-up period were evaluated. The results are expressed as means ± standard deviations. The Mann-Whitney test was used to analyze quantitative data, and the chi-square test was used to analyze qualitative data. A P value of <0.05 was considered to indicate statistical significance. All calculations were made using SPSS 10.0 for Windows.
RESULTS
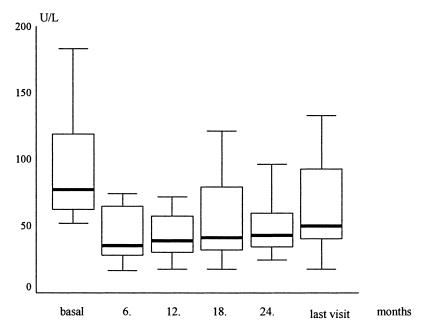
Nineteen patients were included in this study (15 males; mean age ± standard deviation, 36.8 ± 12.8 years). At the time of enrollment, all patients were positive for anti-HDV and HDV RNA. None of the patients showed the presence of anti-HCV or anti-HIV. All the patients were positive for HBsAg and anti-HBe but negative for HBeAg. HBV infection was nonreplicative, as shown by the negative hybridization assay for HBV DNA. Genotypic analyses revealed HDV genotype I and HBV genotype D in all patients. All subjects had histologically proven chronic hepatitis (15 were precirrhotic, and 4 were cirrhotic) and compensated liver disease. The mean histological activity index (HAI) score was 8.4 (range, 4 to 17) at the beginning of therapy. All patients had elevated ALT levels (mean ± standard deviation, 97.6 ± 47.3 IU/liter) (Fig. 1). Of the studied patients, eight were nonresponders to the previous IFN-α monotherapy. They had received IFN-α2a (9 MU, three times weekly, subcutaneously) for 12 months, and their aminotransferase levels were not normal at the end of the treatment period. These patients were included in the present study within 6 to 10 months after finishing the previous IFN-α therapy. The remaining 11 patients had not undergone treatment (Table 1). All patients completed the 24 months of treatment and at least 6 months (range, 7 to 19 months) of a follow-up period.
FIG. 1.
ALT levels at various times during therapy. Dark horizontal bars represent median level, and error bars represent standard deviations.
TABLE 1.
Baseline characteristics and virological response rates of the patients
| Patient | Age (yr) | Sexa | ALT level (IU/liter) | HAIb
|
Stageb
|
Previous IFN treatment? | ETR?c | EFUR?c | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||
| 1 | 19 | F | 102 | 6 | 1 | No | No | No | ||
| 2 | 38 | M | 119 | 5 | 2 | No | No | No | ||
| 3 | 29 | M | 75 | 12 | 15 | 3 | 3 | No | No | No |
| 4 | 45 | M | 246 | 4 | 2 | Yes | Yes | No | ||
| 5 | 40 | M | 62 | 4 | 4 | 1 | 1 | No | Yes | No |
| 6 | 34 | M | 183 | 9 | 5 | 1 | 1 | Yes | Yes | Yes |
| 7 | 35 | F | 67 | 8 | 3 | No | No | No | ||
| 8 | 38 | M | 92 | 13 | 1 | No | No | No | ||
| 9 | 19 | M | 63 | 4 | 4 | 1 | 1 | Yes | No | No |
| 10 | 23 | F | 115 | 17 | 3 | Yes | No | No | ||
| 11 | 23 | M | 52 | 7 | 1 | No | Yes | No | ||
| 12 | 44 | M | 103 | 4 | 9 | 1 | 3 | No | No | No |
| 13 | 49 | M | 103 | 12 | 4 | Yes | No | No | ||
| 14 | 63 | M | 152 | 12 | 9 | 4 | 4 | Yes | Yes | Yes |
| 15 | 40 | F | 53 | 13 | 4 | No | Yes | Yes | ||
| 16 | 20 | M | 67 | 4 | 5 | 1 | 2 | No | No | No |
| 17 | 51 | M | 60 | 12 | 4 | Yes | Yes | No | ||
| 18 | 57 | M | 80 | 8 | 4 | 3 | 2 | Yes | Yes | Yes |
| 19 | 34 | M | 61 | 6 | 12 | 3 | 3 | No | No | No |
F, female; M, male.
HAI and stage scores were determined before and after treatment.
ETR, end-of-treatment virological response; EFUR, end-of-follow-up virological response.
Biochemical responses were observed in 11 patients (57.9%) at month 12, in 9 patients (47.3%) at month 18, and in 8 patients (42.1%) at month 24. Tests for HDV RNA became negative in five patients (26.3%) at month 12 and in six (31.6%) at month 18. Eight patients (42.1%) were negative for HDV RNA at the end of treatment (Table 1). Every patient with a virological response also had a biochemical response. After discontinuation of therapy, the patients were monitored for a mean period of 14 months (range, 7 to 19 months). During the follow-up period, virological responses were maintained in four patients (21%), and biochemical responses were maintained in seven (36.8%). There were no significant differences in biochemical and virological responses between IFN-α-naive and nonresponder subgroups (P > 0.05). After treatment, patients tested positive for HBsAg and negative for HBV DNA. According to the univariant analyses, age, gender, initial aminotransferase level, response to previous IFN-α therapy, staging score (histological stage of chronic hepatitis), and HAI had no effects on biochemical or virological responses.
Ten patients underwent a second liver biopsy. These control liver biopsies were performed 6 months after termination of therapy. Of the patients who underwent control liver biopsies, six were unresponsive to the therapy and four were responsive both virologically and biochemically. The results for the patients who had both pretreatment and posttreatment liver biopsies are shown in Table 1. A histologic response was defined as a reduction in HAI of 2 points or more or a reduction in staging score after treatment of 1 point or more in comparison to those scores before treatment. Among the virological and biochemical nonresponders, there was not a single patient with a histological response. Of the four responsive patients who were responsive both virologically and biochemically and who underwent control biopsies, three (75%) had histological improvements. During the long-term follow-up, serum HBsAg and anti-HDV markers remained positive for all patients.
The flulike symptoms attributed to IFN-α therapy were observed in most of the patients. They were generally mild or moderate and were easily tolerated. Two patients required a short-term dose reduction from 10 to 5 MU, one patient at 2 months and one at 4 months after beginning therapy, because of leukopenia and thrombocytopenia. Additionally, decreases in hemoglobin concentrations occurred in two patients, and these effects were managed with a reduction in the ribavirin dosage to 600 mg/day. Combination treatment was not withdrawn from any patient due to side effects.
DISCUSSION
HDV is an etiologic agent of acute and chronic liver disease. Although the prevalence of HDV infection has declined in the Mediterranean basin in the last decade, it is still an important public health problem (19). Chronic HDV infection is usually associated with severe histological changes in the liver and a rapidly progressive course that can lead to cirrhosis, liver failure, and finally death (15). Since CDH patients have a high risk of developing liver cirrhosis and its consequences, they need an effective antiviral therapy to prevent the progression of liver disease. Until now, the only drug that has been shown to be effective for the treatment of CDH is IFN-α. Several studies have shown that IFN-α treatment results in an improvement in CDH. The efficacy of IFN-α is correlated with the dosage and length of the treatment period (7, 8, 20). The patients treated with high IFN-α doses for at least 1 year experienced both biochemical and histological responses at considerable rates by the end of treatment. But virological responses have occurred in only a minority of these patients. Furthermore, after therapy was stopped, high rates of virological recurrences were observed in almost all the studies. Although virological responses could not be obtained, biochemical responses were maintained in the majority of patients during a long-term period after treatment. In these patients, the long-term clinical and histological outcomes of CDH were significantly improved (5). Despite the encouraging results, the efficacy of IFN-α monotherapy for CDH is not satisfactory because of low response rates and high relapse rates. The need for new therapeutic agents for CDH requires continued investigation. Several antiviral agents, including acyclovir (1), lamivudine (21), and famciclovir (23), have not been found to be effective for CDH. To prevent virological recurrences, prolonged courses of IFN-α treatment are usually needed (18). In our study, while the virological response rate was 26.3% at month 12, it increased to 42.1% at month 24. It has even been suggested that IFN-α treatment be continued until HbsAg is eliminated (12).
Experimentally, ribavirin, which is effective against RNA viruses, has been reported to inhibit replication of the HDV genome in hepatocyte cultures (3). But in clinical practice, ribavirin alone has not proven to be efficient in treatment of CDH (6). Although the pathogenesis of HDV-induced liver disease is still unknown, immune mechanisms may play a role (14). Ribavirin has been demonstrated to promote CD4+ T helper 1 cytokine-mediated immune responses (9), indicating that ribavirin may enhance the immunomodulatory properties of IFN-α.
This study was designed to investigate whether ribavirin combined with IFN-α enhances the efficacy of IFN-α in the treatment of CDH. The study clearly shows that the virological and biochemical response rates we obtained were parallel to those resulting from IFN-α monotherapies (7, 8, 20). In addition, ribavirin certainly increases the treatment cost, which is an important disadvantage.
The present study clearly shows that in comparison to IFN-α monotherapy, the addition of ribavirin to IFN-α does not increase the response rate in patients with CDH.
REFERENCES
- 1.Berk, L., R. A. de Man, C. Housset, P. Berthelot, and S. W. Schalm. 1991. Alpha lymphoblastoid interferon and acyclovir for chronic hepatitis delta. Prog. Clin. Biol. Res. 364:411-420. [PubMed] [Google Scholar]
- 2.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Bisceglie, A. M. 1997. Hepatitis D virus, p. 217-238. In R. A. Wilson (ed.), Viral hepatitis. Marcel Dekker, Inc., New York, N.Y.
- 4.Farci, P., A. Mandas, A. Coiana, M. E. Lai, V. Desmet, P. Van Eyken, Y. Gibo, L. Caruso, S. Scaccabarozzi, D. Criscuolo, J. C. Ryff, and A. Balestrieri. 1994. Treatment of chronic hepatitis D with interferon alfa-2a. N. Engl. J. Med. 330:88-94. [DOI] [PubMed] [Google Scholar]
- 5.Farci, P., L. Chessa, G. Peddis, R. Strazzera, E. Pascariello, R. Scioscia, M. E. Lai, and A. P. Mazzoleni. 2000. Influence of alfa-interferon on the natural history of chronic hepatitis D: dissociation of histologic and virologic response. Hepatology 32:222A. [Google Scholar]
- 6.Garipoli, A., V. Di Marco, R. Cozzolongo, C. Costa, A. Smedile, A. Fabiano, F. Bonino, M. Rizzetto, G. Verme, and A. Craxi. 1994. Ribavirin treatment for chronic hepatitis D: a pilot study. Liver 14:154-157. [DOI] [PubMed] [Google Scholar]
- 7.Gaudin, J. L., P. Faure, H. Godinot, F. Gerard, and C. Trepo. 1995. The French experience of treatment of chronic type D hepatitis with a 12-month course of interferon alpha-2b. Results of a randomized controlled trial. Liver 15:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis, S. J. 1991. Use of alpha-interferon in the treatment of chronic delta hepatitis. J. Hepatol. 13(Suppl. 1):21-26. [DOI] [PubMed] [Google Scholar]
- 9.Hultgren, C., D. R. Milich, O. Weiland, and M. Sallberg. 1998. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus specific immune responses. J. Gen. Virol. 79:2381-2391. [DOI] [PubMed] [Google Scholar]
- 10.Ivaniushina, V., N. Radjef, M. Alexeeva, E. Gault, S. Semenov, M. Salhi, O. Kiselev, and P. Deny. 2001. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 82:2707-2718. [DOI] [PubMed] [Google Scholar]
- 11.Knodell, R. G., K. G. Ishak, W. C. Black, T. S. Chen, R. Craig, N. Kaplowitz, T. W. Kiernan, and J. Wollman. 1981. Formulation and application of numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431-435. [DOI] [PubMed] [Google Scholar]
- 12.Lau, D. T.-Y., D. E. Kleiner, Y. Park, A. M. Di Bisceglie, and J. H. Hoofnagle. 1999. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology 117:1229-1233. [DOI] [PubMed] [Google Scholar]
- 13.Niro, G. A., A. Smedile, A. Andriulli, M. Rizzetto, J. L. Gerin, and J. L. Cassey. 1997. The predominance of hepatitis delta virus genotype I among chronically infected Italian patients. Hepatology 25:728-734 [DOI] [PubMed] [Google Scholar]
- 14.Nisini, R., M. Paroli, D. Accapezzato, F. Bonino, F. Rosina, T. Santantonio, F. Sallusto, A. Amoroso, M. Houghton, and V. Barnaba. 1997. Human CD4 T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J. Virol. 71:2241-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purcell, R. H., M. Rizzetto, and J. L. Gerin. 1984. Hepatitis delta virus infection of the liver. Semin. Liver Dis. 4:340-346. [DOI] [PubMed] [Google Scholar]
- 16.Rizzetto, M., M. G. Canese, S. Arico, O. Crivelli, C. Trepo, F. Bonino, and G. Verme. 1977. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and serum of HBsAg carriers. Gut 18:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzetto, M., B. Hoyer, R. Purcell, and J. Gerin. 1984. Hepatitis delta virus infection, p. 371-377. In G. Vyas, J. Dienstag, and J. Hoofnagle (ed.), Viral hepatitis and liver disease. Grune & Stratton, New York, N.Y.
- 18.Rizzetto, M., G. Verme, S. Recchia, F. Bonino, P. Farci, S. Arico, R. Calzia, A. Picciotto, M. Colombo, and H. Popper. 1983. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of delta antigen: an active and progressive disease unresponsive to immunosuppressive treatment. Ann. Intern. Med. 98:437-441. [DOI] [PubMed] [Google Scholar]
- 19.Rosina, F., P. Conoscitore, R. Cuppone, G. Rocca, A. Giuliani, R. Cozzolongo, G. Niro, A. Smedile, G. Saracco, A. Andriulli, O. G. Manghisi, and M. Rizzetto. 1999. Changing pattern of chronic hepatitis D in southern Europe. Gastroenterology 117:161-166. [DOI] [PubMed] [Google Scholar]
- 20.Rosina, F., C. Pintus, C. Meschievitz, and M. Rizzetto. 1991. A randomized controlled trial of a 12-month course of recombinant human interferon-alpha in chronic delta (type D) hepatitis: a multicenter Italian study. Hepatology 13:1052-1056. [PubMed] [Google Scholar]
- 21.Wolters, L. M., A. B. van Nunen, P. Honkoop, A. C. T. M. Vossen, H. G. M. Niesters, P. E. Zondervan, and R. A. de Man. 2000. Lamivudine-high dose interferon combination therapy for chronic hepatitis B patients co-infected with the hepatitis D virus. J. Viral Hepatol. 7:428-434. [DOI] [PubMed] [Google Scholar]
- 22.Yurdaydin, C., H. Bozkaya, H. Senturk, M. W. Fried, M. Akdogan, F. R. Schinazi, H. Cetinkaya, E. Erden, M. Bozdayi, H. Degertekin, and O. Uzunalimoglu. 2000. Treatment of chronic hepatitis D with lamivudine versus lamivudine + interferon versus interferon: a randomized controlled clinical trial. J. Hepatol. 32(Suppl. 2):113A.10728799 [Google Scholar]
- 23.Yurdaydin, C., H. Bozkaya, S. Gurel, H. L. Tillmann, N. Aslan, A. Okcu-Heper, E. Erden, K. Yalcin, N. Iliman, O. Uzunalimoglu, M. P. Manns, and M. Bozdayi. 2002. Famciclovir treatment of chronic delta hepatitis. J. Hepatol. 37:266-271. [DOI] [PubMed] [Google Scholar]