Abstract
We compared the in vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium species with a combination of two non-culture-based techniques: the tetrazolium salt 2,3-bis-(2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide) (XTT) colorimetric reduction assay, and fluorescent microscopy with the cellular morbidity dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) to directly visualize hyphal damage. Amphotericin B exhibited species-specific concentration-dependent activity, with 50% effective concentrations (EC50s) ranging from 0.10 to 0.12 mg/ml for A. fumigatus, 0.36 to 0.53 mg/ml for A. terreus, 0.27 to ≥32 mg/ml for F. solani, 0.41 to 0.55 mg/ml for F. oxysporum, and 0.97 and 0.65 mg/ml for S. apiospermum and S. prolificans, respectively. Similarly, itraconazole inhibited the growth of A. fumigatus and A. terreus isolates with MICs of <1 mg/ml (EC50 0.03 to 0.85 mg/ml) and S. apiospermum, but was not active against Fusarium species or S. prolificans. Voriconazole effectively inhibited the growth of Aspergillus, Fusarium, and S. apiospermum (EC50 0.10 to 3.3 mg/ml) but had minimal activity against a multidrug-resistant isolate of F. solani or S. prolificans. Hyphal damage visualized by DiBAC staining was observed more frequently with voriconazole and amphotericin B versus itraconazole. These data highlight the species-specific differences in antifungal pharmacodynamics between mold-active agents that could be relevant for the development of in vitro susceptibility breakpoints and antifungal dosing in vivo.
Infections caused by the opportunistic mold genera Aspergillus, Fusarium, and Scedosporium have increased over the last decade and are associated with significant morbidity and mortality in severely immunocompromised patients (10, 12). In vitro studies have revealed that some Aspergillus species, particularly A. terreus, and most Fusarium and Scedosporium isolates are only moderately susceptible or resistant to amphotericin B (18). Similarly, itraconazole appears to have negligible activity against Fusarium and Scedosporium species (18), which have become increasingly common causes of breakthrough infections in immunosuppressed patients (7). More recently, voriconazole has been shown to be effective as primary therapy in the treatment of invasive aspergillosis and is an effective salvage therapy for refractory infections caused by Fusarium species and Scedosporium apiospermum (17). However, no comparative studies have directly compared the pharmacodynamics of amphotericin B, itraconazole, and voriconazole for Aspergillus, Fusarium, and Scedosporium species. Comparative pharmacodynamic data for these molds, especially Fusarium and Scedosporium, could be informative in the development of susceptibility breakpoints as well as dosing regimen design for infections not evaluated in phase I and II clinical trials (19).
One factor that has hindered the study of antifungal pharmacodynamics for opportunistic molds both in vitro and in vivo is the lack of standardized, reliable, and sensitive techniques for quantifying viable mycelium. Methods developed for unicellular organisms (e.g., time-kill curves and quantitative organ cultures) that rely on CFU enumeration from agar medium are not well suited for multicellular filamentous fungi that may only grow as a single CFU when plated on agar (4). Moreover, in studies that have attempted to apply traditional time-kill methods for studies of antifungal activity against Aspergillus spp., CFU counts in control tubes decreased despite obvious increases in hyphal mass and the slope of antifungal “killing” in control tubes was often indistinguishable from that of drug-exposed isolates (11).
Recently, colorimetric assays and quantitative real-time PCR have been proposed as alternatives to CFU-based methods as in vitro and in vivo endpoints, respectively, for the study of drug activity against filamentous fungi (4, 13). In vitro, the reduction of the tetrazolium salt 2,3-bis-(2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide) (XTT) byviable hyphae can serve as an indicator of hyphal viability and has been shown to reliably and accurately quantify fungal burden following 24 h of incubation with as few as 102 conidia (13). Therefore, we used the XTT reduction assay combined with fluorescent microscopy with the cellular morbidity dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) as an early marker of hyphal damage to characterize the in vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium species with various susceptibilities to amphotericin B and itraconazole. With this combined approach, we were able to characterize the concentration-effect relationships of all three antifungals against a variety of pathogenic molds and confirm (qualitatively) differences in drug-induced hyphal damage between the three antifungals.
(This work was presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., abstr. M-1255, 2003.)
MATERIALS AND METHODS
Isolates and inoculum preparation.
Eleven isolates with various susceptibilities to amphotericin B and itraconazole were selected for testing from the laboratory collection at the Mycology Research Laboratories at the University of Texas M. D. Anderson Cancer Center, Houston. All isolates were subcultured twice on potato dextrose agar slants (Remel, Lenexa, Kans.) prior to testing. A standardized inoculum of conidia for each isolate was prepared by flooding 5- to 7-day-old cultures on potato dextrose agar slants with 5 ml of 0.85% sterile saline and 0.2% Tween 80 followed by 15 s of vortexing. The resultant suspension was then filtered twice through sterile syringes packed with glass wool to remove hyphal fragments. The conidial suspension was adjusted with a hemacytometer to a concentration of 1 × 106 to 5 × 106 in sterile water (16).
Culture medium.
RPMI 1640 culture medium (Sigma) buffered with 0.165 M MOPS (3-[N-morpholino]propanesulfonic acid) plus 2% glucose adjusted to a pH of 7.0 served as the growth medium for all experiments.
Drug stock solutions.
Drug stock solutions (1,280 mg/ml) were prepared for each antifungal in dimethyl sulfoxide (voriconazole and itraconazole) or water (amphotericin B deoxycholate) from pure powders (voriconazole from Pfizer, Inc, Sandwich, United Kingdom; itraconazole from Janssen Inc., Titusville, N.J.; amphotericin B deoxycholate from Sigma). Stock solutions were then prepared in RPMI culture medium and serially diluted (64 to 0.0625 μg/ml) for preparation of microtiter trays.
Antifungal susceptibility testing.
The MIC of each antifungal was determined in triplicate with standardized methods (NCCLS M38-A) (16). Briefly, conidial suspensions were diluted 1:50 in RPMI growth medium and dispensed (100 μl) into thawed microtiter trays containing serial twofold dilutions of antifungals. The trays were then incubated for 48 h at 37°C, and MICs were read at 24 and 48 h as the lowest drug concentrations that showed complete growth inhibition (100% inhibition). Candida parapsilosis ATCC 22019 served as a quality control isolate for each experimental run. The in vitro fungicidal activity (minimum fungicidal activity [MFC]) of each antifungal was measured for each isolate with previously reported methods (6). After 48 h of incubation, 20 μl was subcultured from each well that showed complete inhibition (100% or an optically clear well), from the last positive well (growth similar to that for the growth control well), and from the growth control (drug-free medium) onto potato dextrose agar plates (Remel, Lenexa, Kans.). The plates were incubated in a humid chamber at 35°C until growth was seen in the growth control subculture (usually before 48 h). The MFC was defined as the lowest drug concentration that showed either no growth or fewer than three colonies to achieve approximately 99 to 99.5% killing activity (6).
XTT reduction assay.
The XTT reduction assay was performed with methods developed by Meletiadis et al. (13). The assay relies on reduction of the tetrazolium salt XTT by intact cells with active biological redox capabilities to formazan derivatives, which can be measured colorimetrically (20). The relationship of formazan production versus fungal inoculum was determined by incubating standardized conidial suspensions of each isolate (102 to 105 conidia/ml) in RPMI growth medium for 24 h at 35°C. Additional wells containing medium alone were included in each plate to correct for background absorbance and to verify growth medium sterility. After 22 h of incubation, the trays were removed from the incubator and 50 ml of XTT solution (1 mg/ml) prepared in sterile water containing 125 mM menadione (Sigma) was added to each well. After 2 additional hours of incubation, formazan absorbance in each well was read at 492 nm with a microplate spectrophotometer (Powerwave X Select, Biotech Instruments, Winooski, Vt.). Absorbance readings were standardized in relation to unconverted XTT in the medium control wells and plate absorbance (692 nm) to determine the optical density at 492 nm. All experiments were performed in triplicate.
Pharmacodynamic studies were performed by inoculating 100 μl of a 1:5 dilution of the standardized conidial suspension (1 × 106 to 2 × 106 conidia/ml) into the wells of a flat-bottomed 96-well microtiter tray containing serial twofold dilutions of antifungals in RPMI growth medium (final concentration, 0.0625 to 32 μg/ml) plus a control (no drug). The trays were then incubated for 24 h at 35°C, and formazan absorbance was assayed as described previously. All experiments were performed in triplicate.
Fluorescent morbidity staining.
Fluorescent morbidity staining and microscopy were performed with a modification of the methods reported by Bowman et al. (5). Briefly, isolates were grown in RPMI medium for 18 h prior to the addition of amphotericin B, itraconazole, or voriconazole to the culture medium to a final concentration of 2 μg/ml. After incubation of cells for an additional 6 h, tubes were centrifuged at 10,000 × g for 5 min, culture medium was carefully removed with a sterile pipette, and fungi were washed twice in morpholinepropanesulfonic acid (MOPS, pH 7) buffer solution. DiBAC (Molecular Probes, Eugene, Oreg.) from a 1-mg/ml stock in 100% ethanol was then added to a final concentration of 2 mg/ml, and samples were incubated in the dark with shaking at room temperature for 1 h, washed twice again with MOPS, pH 7, and resuspended for photomicrography.
Photomicrographs of hyphae were taken with an Olympus BX-51 microscope equipped with Nomarski optics and epifluorescence (fluorescein isothiocyanate) filter. All pictures were taken at 400× magnification with a computerized digital camera (Spot Diagnostics, Sterling Heights, Mich.). The exposure times and light balance of micrographs were controlled through computer software (Image Pro Plus 4.1, Media Cybernetics, Silver Spring, Md.).
Data analysis.
Median MIC and MFC values were calculated from experiments performed in triplicate. Data from the XTT reduction assay were fitted to a four-parameter logistic model (Hill Equation) with computer curve-fitting software (Prism 4, Graphpad Software, Inc., San Diego, Calif.) to derive the 50% effective concentration (EC50) and the steepness of the inhibitory dose-response curve (Hill slope) (15). The goodness of fit for each isolate and drug combination was assessed by R2 and the standard error of the EC50 value. If the standard error of the EC50 was ≥0.5 log, data were refitted with a fixed value for the top plateau of the regression curve based on the formazan absorbance of the control wells. The EC50 and Hill slope data were not determined if minimal or no reduction in formazan absorbance was observed with increasing drug concentrations.
RESULTS
Antifungal susceptibility testing.
Median MIC and MFC results are reported in Table 1. Amphotericin B MICs were relatively lower for A. fumgiatus (MIC 0.25 μg/ml) than for Aspergillus terreus, Fusarium solani 001, Fusarium oxysporum 001 and 002, and Scedosporium apiospermun, all of which had MICs of ≥1 μg/ml. The MFCs for amphotericin B were 2 μg/ml or greater for all isolates with the exception of A. fumigatus isolates 99-2075 and 583 (0.5 μg/ml). Similarly, higher MICs of itraconazole (≥2 μg/ml) were observed with A. fumigatus 583 and all Fusarium and Scedosporium test isolates. Itraconazole MFCs ranged between 8 and 32 μg/ml for all test isolates. Median voriconazole MICs were 0.25 μg/ml, and MFCs ranged from 1 to 4 μg/ml for all Aspergillus species tested. Voriconazole MICs and MFCs were several dilutions higher with Fusarium species (2 to 16 μg/ml and ≥32 μg/ml, respectively) compared to Aspergillus. Voriconazole was considerably more active against S. apiospermum (MIC and MFC, 0.25 and 16 μg/ml, respectively) versus S. prolificans (16 and ≥32 μg/ml, respectively).
TABLE 1.
MICs and MFCsa
| Isolate | Amphotericin B
|
Itraconazole
|
Voriconazole
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC/MFC (μg/ml) | EC50 (μg/ml) ± 95% CI | Hill slope ± 95% CI | MIC/MFC (μg/ml) | EC50 (μg/ml) ± 95% CI | Hill slope ± 95% CI | MIC/MFC (μg/ml) | EC50 (μg/ml) ± 95% CI | Hill slope ± 95% CI | |
| A. fumigatus 293 | 0.25/2 | 0.10 ± 0.06 | −3.84 ± 2.10 | 0.25/2 | 0.30 ± 0.10 | −2.40 ± 1.00 | 0.25/0.50 | 0.18 ± 0.06 | −3.07 ± 1.10 |
| A. fumigatus 99-2075 | 0.25/0.5 | 0.12 ± 0.08 | −3.25 ± 1.20 | 0.5/16 | 0.85 ± 0.46 | −0.56 ± 0.45 | 0.25/1 | 0.10 ± 0.02 | −2.66 ± 0.60 |
| A. fumigatus 583 | 0.25/0.5 | 0.10 ± 0.01 | −4.27 ± 2.10 | 32/≥32 | 12.3 ± 2.00 | −0.56 ± 0.31 | 0.25/2 | 0.37 ± 0.07 | −1.20 ± 0.23 |
| A. terreus 0925 | 1/2 | 0.53 ± 0.10 | −5.33 ± 3.20 | 1/8 | 0.83 ± 0.12 | −3.37 ± 1.82 | 0.25/4 | 0.68 ± 0.19 | −2.15 ± 0.62 |
| A. terreus 0932 | 1/4 | 0.36 ± 0.06 | −2.65 ± 2.10 | 0.5/8 | 0.24 ± 0.05 | −3.13 ± 2.00 | 0.25/4 | 0.10 ± 0.04 | −1.43 ± 0.60 |
| F. solani 001 | 16/≥32 | NCb | NC | ≥32/≥32 | NC | NC | 16/≥32 | NC | NC |
| F. solani 002 | 1/2 | 0.27 ± 0.12 | −1.62 ± 0.63 | 8/≥32 | NC | NC | 4/32 | 3.30 ± 1.30 | −0.87 ± 0.50 |
| F. oxysporum 001 | 4/8 | 0.55 ± 0.35 | −0.86 ± 0.66 | ≥32/≥32 | NC | NC | 4/≥32 | 3.12 ± 1.70 | −0.81 ± 0.67 |
| F. oxysporum 002 | 2/4 | 0.41 ± 0.26 | −0.80 ± 0.30 | 8/≥32 | NC | NC | 2/≥32 | 0.53 ± 0.27 | −0.54 ± 0.32 |
| S. apiospermum | 4/>32 | 0.97 ± 0.35 | −0.57 ± 0.52 | 2/≥32 | 0.64 ± 0.35 | −1.17 ± 0.61 | 0.25/16 | 0.27 ± 0.025 | −1.21 ± 0.80 |
| S. prolificans | 1/4 | 0.65 ± 0.32 | −0.77 ± 0.22 | ≥32/≥32 | NC | NC | 16/≥32 | NC | NC |
MICs and MFCs were determined in triplicate with standardized methods and are reported as median values (16). Pharmacodynamics data were generated from 24-h XTT colorimetric assays performed in triplicate. The EC50 was calculated by fitting inhibition data to a four-parameter logistic model (Hill equation). All experiments were performed in triplicate. CI, confidence interval.
NC, not calculable due to minimal antifungal activity.
XTT reduction assay.
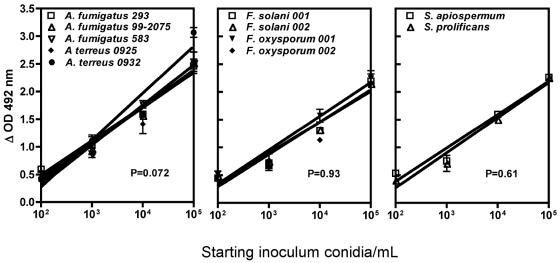
A linear relationship between XTT reduction to formazan, the colorimetrically assayed product, and starting inoculum was observed for isolates tested between 102 and 105 conidia/ml (regression plots are presented in Fig. 1). Slopes of regression plots for all 11 isolates ranged from 0.45 to 0.63, with coefficients of determination (R2) ranging from 0.78 to 0.94. Addition of amphotericin B, itraconazole, or voriconazole to culture medium containing susceptible molds reduced formazan absorbance from approximately 3 to ≤0.5, which was roughly equivalent to a 2.5 log reduction in hyphal viability based on data generated from inoculum-XTT reduction studies (Fig. 1).
FIG. 1.
Regression plots of the relationship of inoculum versus formazan production for test isolates at 24 h. The slopes of the regression lines were not significantly different by analysis of variance.
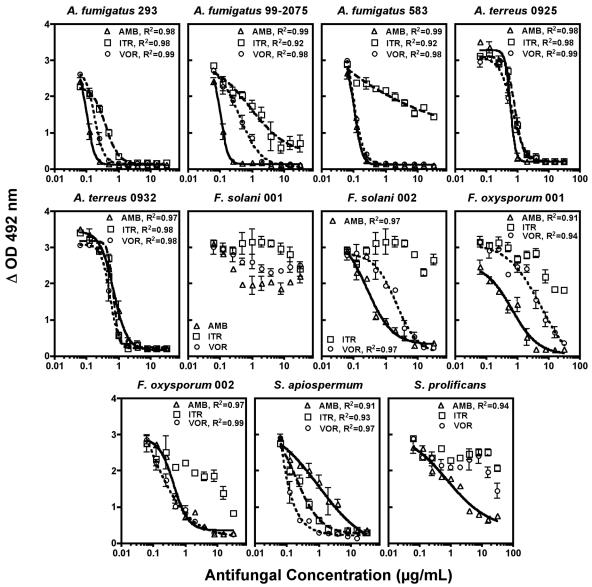
Pharmacodynamic results for all antifungal-isolate combinations are presented in Table 1, with concentration-effect plots presented in Fig. 2. Amphotericin B exhibited potent antifungal activity (EC50 range, 0.10 to 0.12 μg/ml) in a steep concentration inhibitory effect curve (Hill slope range, −3.25 to −4.27) against A. fumigatus isolates, including the itraconazole-resistant isolate A. fumigatus 583 (Fig. 2). Amphotericin B was somewhat less active against A. terreus 0925 (EC50 0.53 μg/ml) and A. terreus 0932 (EC50 0.36 μg/ml), although substantial reductions in hyphal viability were observed as drug concentrations approached 1 μg/ml. Similarly, amphotericin B inhibited the growth F. solani 002 at low concentrations (EC50 0.27 μg/ml), but showed minimal effects on the viability of F. solani 001 (Fig. 2), while moderate inhibitory activity against F. oxysporum and Scedosporium species with relatively higher EC50s (0.41 to 0.97 μg/ml) and much more shallow concentration-effect curves (Hill slopes −0.57 to −0.88) was observed (Fig. 2).
FIG. 2.
Sigmoidal concentration-inhibitory effect curves following exposure to amphotericin B (AMB), itraconazole (ITR), and voriconazole (VOR). Symbols represent the mean plus standard deviation of experiments performed in triplicate for each drug. Lines were generated by fitting data to a four-parameter logistic model (Hill equation).
Itraconazole was consistently less active against Aspergillus, Fusarium, and Scedosporium spp. than amphotericin B or voriconazole. Although effective against A. fumigatus 293 (EC50 0.30 μg/ml, Hill slope −2.40) and A. terreus isolates, itraconazole activity was attenuated for Aspergillus fumigatus isolates with MICs of ≥0.5 μg/ml (EC50 range, 0.85 to 12.3, and Hill slope −0.56). Itraconazole demonstrated minimal activity against Fusarium species and Scedosporium prolificans, but was moderately active against Scedosporium apiospermum, with an EC50 of 0.64 μg/ml and Hill slope of −1.17.
Voriconazole effectively inhibited the growth of A. fumigatus isolates (EC50 0.10 to 0.37 μg/ml, Hill slope −1.20 to −3.07), including the itraconazole-resistant isolate A. fumigatus 583 (Fig. 2). Voriconazole also inhibited the growth of A. terreus 0925 and 0932 at relatively low concentrations (EC50 0.68 and 0.10 μg/ml, respectively), although a more shallow concentration-response curves was observed. Voriconazole was active against Fusarium species (EC50 0.53 to 3.3 μg/ml, Hill slope −0.54 to −0.87) with the exception of the multidrug-resistant isolate Fusarium solani 001 (Fig. 2). Of the three agents tested, voriconazole was the most potent against S. apiospermum (EC50 0.27 μg/ml, Hill slope −1.2). Similar to itraconazole, minimal activity was noted for voriconazole against S. prolificans.
Fluorescent morbidity staining.
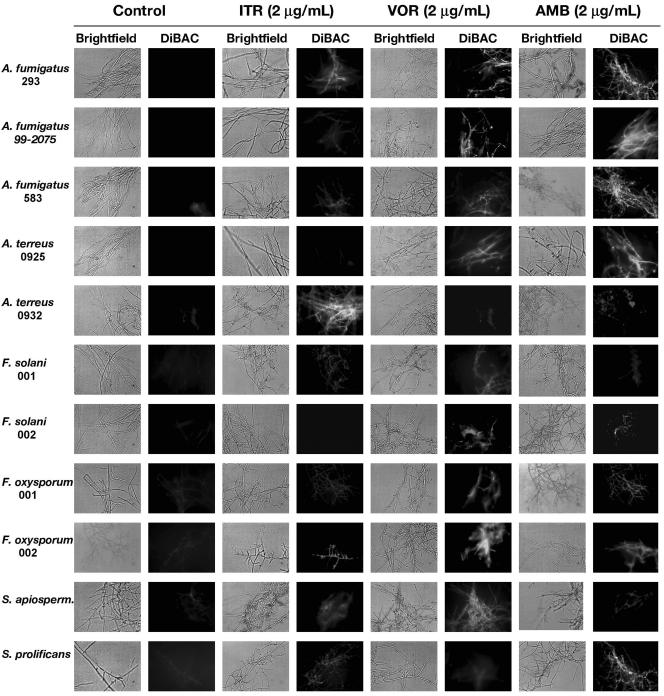
Control cells (non-drug exposed) demonstrated minimal diffuse or no fluorescence following DiBAC staining (Fig. 3). Hyphal damage following exposure to itraconazole (2 μg/ml) was clearly evident for isolates A. fumigatus 293, A. terreus 0925 and 0932, and S. apiospermum but less so in other isolates. For voriconazole, pronounced hyphal damage was seen for all Aspergillus and Fusarium isolates with the exception of the multidrug-resistant isolate F. solani 001. At a concentration of 2 μg/ml, voriconazole clearly induced hyphal damage against S. apiospermum but caused minimal injury to S. prolificans. For amphotericin B, hyphal damage was observed in all Aspergillus species, including A. terreus. Although some evidence of hyphal damage could be appreciated in Fusarium species, fluorescence was not as pronounced as seen with Aspergillus species. Consistent with the MIC/MFC and XTT viability data, considerable hyphal damage was evident with S. prolificans but not S. apiospermum following exposure of hyphae to 2 μg of amphotericin B per ml (Fig. 3).
FIG. 3.
Detection of hyphal damage by fluorescent microscopy with the cellular morbidity dye DiBAC following exposure to antifungals. Hyphae of each species were prepared after 18 h in growth medium, washed, and resuspended in culture medium containing 2 μg of the following antifungals per ml: itraconazole (ITR), voriconazole, (VOR), and amphotericin B (AMB). After 6 h of incubation, cells were washed and stained with the fluorescent morbidity dye DiBAC. Hyphae were then examined by bright-field (light boxes) and epifluoresence (dark boxes) microscopy at 400× with Nomarski optics and a fluorescein isothiocyanate (FITC) filter. Fluorescence in the dark boxes is indicative of early hyphal damage by the antifungal agent.
DISCUSSION
Although host immunosuppression and early laboratory diagnosis are widely recognized as the key factors in the outcome of opportunistic mold infections, antifungal therapy remains the most amendable day-to-day variable for improving survival in patients with invasive mycoses. As such, empirical strategies designed to hedge pharmacokinetic and pharmacodynamic uncertainties in patients (e.g., dosage escalation and combination therapy) have quickly become a standard approach for an increasingly ill and immunocompromised patient population. Unfortunately, few preclinical studies and even fewer organized clinical trials have attempted to address issues concerning antifungal pharmacodynamics or resistance breakpoints for invasive molds (1, 19). This is not surprising given the multitude of technical issues that complicate the in vitro measurement of antifungal activity against filamentous fungi as well as the numerous factors that confound interpretation of drug activity in patients. Nevertheless, several methods have been developed in the last 3 years that offer workable approaches for studying antifungal concentration-effect relationships in filamentous fungi.
Colorimetric assays that rely on reduction of tetrazolium salts (XTT and MTT) to colored formazan products by viable cells have long been used in the study of mammalian cell cytotoxicity (20). Meletiadis and colleagues recently standardized an assay method that can effectively quantify mold hyphal viability formed by inocula as low as 102 up to concentrations of 105 conidia/ml following 24 h of incubation (13). With this method, the authors were able to clarify diffuse visual MIC endpoints from microdilution broth susceptibility testing and decrease the variability of antifungal concentration-effect curves for amphotericin B and itraconazole against Aspergillus species (13, 14). Similarly, we found that with their XTT assay, formazan production by Aspergillus, Fusarium, and Scedosporium spp. correlated linearly with fungal biomass after 24 h of incubation, and we were able to correlate species-specific differences in antifungal pharmacodynamics for amphotericin B, itraconazole, and voriconazole.
Several different patterns were evident from our pharmacodynamic analysis. First, itraconazole was consistently less active than either amphotericin B or voriconazole against the test isolates. The major shift towards higher EC50s and shallower Hill slopes appears to occur when the MIC for the isolates approached or exceeded 1 μg/ml. As a result, itraconazole was not active alone against Fusarium (MIC, 8 to 32 μg/ml) or S. prolificans. Although itraconazole was active against S. apiospermum, the higher EC50 and shallower dose-response curve suggested a need for either higher itraconazole dosages or use in combination with a second agent to improve antifungal activity. Indeed, high-dose therapy and combination therapy have been recommended in the treatment of Scedosporium (Pseudallescheria) infections based on anecdotal reports of improved outcome with this approach (21).
Second, amphotericin B appeared by pharmacodynamic analysis to be remarkably active against Aspergillus, Fusarium, and Scedosporium species. The most notable exception was F. solani 001, which appeared to be cross-resistant to all of the antifungals tested. This potent activity is somewhat at odds with the sometimes high microbiological failure rates reported for amphotericin B in the treatment of these infections (21). The unusual biopharmaceutical properties and pharmacokinetics of this drug, however, may partially explain this discrepancy. Amphotericin B exhibits nonlinear, concentration-dependent binding to proteins in serum and tissue that increases with increasing drug concentrations (3). This peculiar pattern of protein binding is probably governed by the poor solubility of the amphotericin B molecule at a neutral pH (i.e., its amphoteric properties). With ultrafiltration and equilibrium dialysis, Bekersky et al. estimated that the maximum free-drug solubility of unbound amphotericin B in human plasma was 0.744 μg/ml (3). If this represents the maximal microbiologically active fraction of amphotericin B that can be achieved in human serum or tissue, then the activity of amphotericin B for the even more susceptible A. terreus, Fusarium, and Scedosporium spp. would appear to be marginal at best (see Table 1 and Fig. 2) (22).
Third, pharmacodynamic analysis of voriconazole revealed activity similar to that of amphotericin B for A. fumigatus (including itraconazole-resistant strains) and A. terreus. Voriconazole was active against most Fusarium isolates, but the EC50s were 5- to 10-fold higher and the inhibition slopes were much shallower than seen with Aspergillus species, suggesting a possible need for combination therapy or higher voriconazole doses in refractory infections. Unlike amphotericin B, voriconazole is only moderately protein bound (≈50%) and does not exhibit unlimited concentration-dependent protein binding (8). Therefore, the EC50s reported for Aspergillus, Fusarium, and S. apiospermum are well within the range of expected free plasma voriconazole levels of 2 to 5 μg/ml (8).
One major limitation of the XTT assay is that it primarily measures the inhibitory effects of antifungals. Similar to our findings, Meletiadis et al. reported that EC50 concentrations measured by the XTT assay are, on average, two- to fourfold lower than the MICs determined by the microdilution method (13). In some but not all cases, the EC90 may also correlate with MFC determinations made by colony enumeration techniques (6). As such, the XTT assay appears to correlate better with MIC results but may not identify fungicidal activity.
Quantitative descriptions of fungistatic and fungicidal activity are somewhat dubious for filamentous multicellular molds, which often display both viable and dead cellular compartments on the same hyphae (5, 9). Therefore, we qualitatively assessed the degree of hyphal damage in Aspergillus, Fusarium, and Scedosporium cells following antifungal exposure with the fluorescent morbidity dye DiBAC (2). When fungal cells collapse with mortal injury or cell death, DiBAC is able to bind to phospholipids inside of the fungal cells and exhibit intense fluorescence under UV stimulation. Overall, DiBAC staining correlated well with the in vitro susceptibility testing results and our pharmacodynamics analyses, which found voriconazole and amphotericin B to be the more effective agents. At a fixed concentration of 2 μg/ml, hyphae exposed to amphotericin B or voriconazole demonstrated roughly the same degree of hyphal damage, although voriconazole appeared to be more active against Fusarium species and S. apiospermum (Fig. 3). As expected from the pharmacodynamic studies, pronounced hyphal damage following exposure to 2 μg of itraconazole per ml was seen only in A. fumigatus isolates with itraconazole MICs of ≤1 μg/ml and S. prolificans.
In conclusion, we characterized species-specific differences in antifungal pharmacodynamics in vitro for amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium species. Amphotericin B and voriconazole exhibited similar pharmacodynamic characteristics and were consistently more active than itraconazole against the test isolates. The XTT reduction method, when coupled with some indicator of fungicidal activity, can provide insight into the pharmacodynamics of antifungal agents for invasive molds and focus preclinical investigations for dosage escalation and combination antifungal therapy. Studies are under way to further explore the in vitro-in vivo correlation of these assays.
Acknowledgments
This work is supported by a grant from Pfizer, Inc., to R.E.L. and M.E.K.
REFERENCES
- 1.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashford, C. L., B. Chance, and R. C. Prince. 1979. Oxonol dyes as monitors of membrane potential. Their behavior in photosynthetic bacteria. Biochim. Biophys. Acta 545:46-57. [DOI] [PubMed] [Google Scholar]
- 3.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, J. C., P. S. Hicks, M. B. Kurtz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. North Am. 16:915-933, vi-vii. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 9.Lass-Florl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Wurzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, S., J. Schranz, and S. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 11.Manavathu, E. K., J. L. Cutright, and P. H. Chandrasekar. 1998. Organism-dependent fungicidal activities of azoles. Antimicrob. Agents Chemother. 42:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 13.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, and P. E. Verweij. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motulsky, H., and A. Christopoulos. 2003. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. GraphPad Software Inc., San Diego, Calif.
- 16.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; accepted standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidler, E. 1991. The tetrazolium-formazan system: design and histochemistry. Prog. Histochem. Cytochem. 24:1-86. [DOI] [PubMed] [Google Scholar]
- 21.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 22.Wingard, J. R. 2002. Lipid formulations of amphotericins: are you a lumper or a splitter? Clin. Infect. Dis. 35:891-895. [DOI] [PubMed] [Google Scholar]