Abstract
Purpose
Advanced stage at diagnosis is a common feature of breast cancer in Sub-Saharan Africa (SSA), contributing to poor survival rates. Understanding its determinants is key to preventing deaths from this cancer in SSA.
Methods
Within the Nigerian Integrative Epidemiology of Breast Cancer Study (NIBBLE) multicentre case-control study on breast cancer, we studied factors affecting stage at diagnosis of cases, i.e. women diagnosed with histologically confirmed invasive breast cancer between January 2014 and July 2016 at six secondary and tertiary hospitals in Nigeria. Stage was assessed using clinical and imaging methods. Ordinal logistic regression was used to examine associations of socio-demographic, breast cancer awareness, health care access and clinical factors with odds of later stage (I, II, III or IV) at diagnosis.
Results
A total of 316 women were included, with a mean age (SD) of 45.4 (11.4) years. Of these, 94.9% had stage information: 5 (1.7%), 92 (30.7%), 157 (52.4%), and 46 (15.3%) were diagnosed at stages I, II, III and IV, respectively. In multivariate analyses, lower educational level (odds ratio (OR) 2.35, 95% confidence interval: 1.04, 5.29), not believing in a cure for breast cancer (1.81: 1.09, 3.01), and living in a rural area (2.18: 1.05, 4.51) were strongly associated with later stage, whilst age at diagnosis, tumour grade and oestrogen receptor status were not. Being Muslim (vs. Christian) was associated with lower odds of later stage disease (0.46: 0.22, 0.94).
Conclusion
Our findings suggest that factors that are amenable to intervention concerning breast cancer awareness and health care access, rather than intrinsic tumour characteristics, are the strongest determinants of stage at diagnosis in Nigerian women.
Keywords: breast cancer, stage, stage at diagnosis, awareness, Nigeria
Introduction
Breast cancer (BC) is the most common cancer in women worldwide and in Nigeria. BC incidence in Nigeria (estimated age-standardised incidence rate (ASR)=50.5 per 100,000) in 2012 was only half that in the United States (US) (ASR=92.9 per 100,000), but estimates of mortality rates from this cancer were higher in this West African country than in the US (ASR=25.9 vs 14.9 per 100,000, respectively)1, reflecting poorer survival.2
One of the most important prognostic factors for BC is stage at diagnosis, which has been shown to be relevant in the African setting.3-5 However, in contrast with BC diagnosed in developed countries, stage at diagnosis of BC in Nigeria, as in the rest of sub-Saharan Africa (SSA), has been widely reported to be late.3,6,7 Women are typically symptomatic at presentation as there are no organised, and little opportunistic, pre-clinical early detection. Moreover, presentation in majority of women is not in the early symptomatic stages, rather at advanced stage when regional spread and metastases are not uncommon. Although BC survival data in Nigeria are limited, available data support poor survival from this disease in women who present late.3,8
Recognizing the importance of early detection and treatment in BC control, an increasing body of research is examining factors associated with late stage at diagnosis, particularly in settings where stage has persistently remained late over decades and tumour size at presentation (mean 5-8 cm) is far beyond that of a palpable tumour (2 cm).9 In SSA, later stage at diagnosis of BC has been linked to various factors such as low educational level10, rural region of residence,11 lack of medical aid/insurance11 and poor health care access,6 e.g. long distance to health provider12. These factors would translate into delays in the time to diagnosis. On the other hand, clinical factors13, e.g. young age, poorly differentiated tumour grade14, and negative hormone receptor status14, may contribute to advanced stage as a result of a more rapid tumour growth rate. A significant number of papers that have investigated factors associated with stage at diagnosis have been reported from studies in western countries15-17 and SSA,12 but has been less well studied in Nigeria. Of particular relevance to the African setting, the extent to which the younger age at diagnosis distribution and the small excess of more aggressive tumour subtypes18 contribute to later stage diagnosis remains unknown.
In our study, we examined the role of socio-demographic, BC awareness, access to health care and clinical factors on stage at diagnosis of BC among women seeking care at tertiary and secondary health institutions in Nigeria as a first step to identify which of these factors may be amenable to intervention in the Nigerian setting.
Methods
Study Design and Setting
This study was conducted within the Nigerian Integrative Epidemiology of Breast Cancer Study (NIBBLE). NIBBLE is an on-going multicentre incident case-control study which began recruitment in January 2014 at six government hospitals in Nigeria. Five of these hospitals are located in the capital city of Abuja (population 1.4 million) - comprising two tertiary hospitals (National Hospital and University of Abuja Teaching Hospital Gwagwalada) and three secondary hospitals (Asokoro District Hospital, Garki Hospital and Wuse General Hospital). The sixth hospital, the University of Nigeria Teaching Hospital, is located 400 km south in Enugu, a city in South-Eastern Nigeria with a population of 3.3 million. The three large tertiary hospitals serve as major referral centres for cancer patients across Nigeria and all have facilities for chemotherapy, with one - the National Hospital Abuja - being one of four government hospitals in the country that currently also offer radiotherapy. The present analysis was restricted to NIBBLE cases, i.e. women newly-diagnosed with primary invasive BC at the six participating hospitals between January 2014 and July 2016. Only women diagnosed with breast cancer were included in the present analyses, thus analyses were concerned with the proportion of these who were diagnosed with late stage breast cancer.
Ethical approval was obtained for NIBBLE from the National Health Research Ethics Committee of Nigeria, health research ethics committees in each participating hospital, and institutional ethics committees at the University of Maryland Baltimore (US) and the London School of Hygiene and Tropical Medicine (UK). The study was carried out in compliance with the Nigerian National Code for Health Research Ethics and the Declaration of Helsinki. Written informed consent was obtained from all participants in the study.
Subject recruitment and interviewing
Participants were recruited at the surgical outpatient departments (SOPD) and the oncology departments in two of the participating hospitals (i.e. the National Hospital Abuja and the University of Nigeria Teaching Hospital, Enugu) and only at the SOPDs at the remaining four. All newly-diagnosed patients with a primary invasive BC aged 18 years and over were eligible regardless of their ethnicity or language. Potentially eligible patients were identified at their first visit by the surgeon, oncologist, or research nurse, informed about the study, and invited to participate prior to histological confirmation. Overall, 94.3% consented for whom a confidential structured face-to-face interview was conducted by a trained research nurse in English (70.6%), a predominant Nigerian language (20.6%) or other local language (8.8%) as per the patient's preference. Information was collected on socio-demographic variables, lifestyle, comorbidities, awareness of BC causes and symptoms and health care access (Tables 1 and 2). The research nurse also performed measurements, using a standard protocol, of the patient's height, weight, and waist and hip circumferences, from which body mass index (BMI, weight (kg)/height2 (m2)) and waist-hip ratio (WHR) measures were calculated. For these anthropometric measurements, participants were asked to remove shoes, heavy outer garments, hair ornaments and head scarves.
Table 1. Socio-demographic characteristics of women with breast cancer, by stage at diagnosis.
| Characteristics | Early BC (Stages I & II) N (%) a | Late BC (Stages III & IV) N (%) a | |
|---|---|---|---|
| Socio-demographic | Total no. of women | 97 (32.3) | 203 (67.7) |
| Age at BC diagnosis (years) | Mean age (SD) | 42.6 (11.5) | 46.4 (11.7) |
| Marital status | Married | 71 (33.6) | 140 (66.4) |
| Educational level | None | 5 (12.2) | 36 (87.8) |
| Primary/Secondary | 33 (29.2) | 80 (70.8) | |
| Tertiary/Post graduate (PG) | 59 (41.3) | 84 (58.7) | |
| Not reported | 0 (0) | 3 (100) | |
| Religion | Christianity | 80 (30.7) | 181 (69.3) |
| Islam | 17 (47.2) | 19 (52.8) | |
| Not reported | 0 (0) | 3 (100) | |
| Do you have a personal income? | Yes | 23 (25.6) | 67 (74.4) |
| No | 74 (35.2) | 136 (64.8) | |
| Socioeconomic class | Low | 37 (27.2) | 99 (72.8) |
| (using household data) | Middle | 38 (36.5) | 66 (63.5) |
| High | 22 (36.7) | 38 (63.3) | |
| Lifestyle | No. of ever smokers (%) | 1 (50.0) | 1 (50.0) |
| No. drinkers (%) (1 measure/day) | 10 (21.7) | 36 (78.3) | |
| (2-5 measures/day) | 1 (6.7) | 14 (93.3) | |
| Breast Cancer Awareness | |||
| Ever heard of BC | No | 8 (16.3) | 41 (83.7) |
| Yes | 88 (36.1) | 156 (63.9) | |
| Don’t Know/Not reported | 1 (33.3) | 2 (66.7) | |
| Knowledge of BC causes b | Poor | 57 (28.5) | 143 (71.5) |
| Fair | 25 (40.9) | 36 (59.1) | |
| Good | 15 (38.5) | 24 (61.5) | |
| Knowledge of BC symptoms c | Poor | 48 (28.4) | 121 (71.6) |
| Fair | 30 (33.3) | 60 (66.7) | |
| Good | 19 (46.3) | 22 (53.7) | |
| Belief in cure for BC | No | 26 (22.2) | 91 (77.8) |
| Yes | 71 (39.9) | 107 (60.1) | |
| Don’t know | 0 (0) | 5 (100.0) | |
| Practice of BSE | No | 37 (25.0) | 111 (75.0) |
| Yes | 53 (41.1) | 76 (58.9) | |
| Never heard of / Unknown | 7 (30.4) | 16 (69.6) | |
| First BC symptom | Breast Lump | 86 (33.0) | 175 (67.0) |
| Other Symptom d | 11 (28.2) | 28 (71.8) | |
| Health Care Access | |||
| Region of residence | North-Central (Abuja) | 85 (37.6) | 141 (62.4) |
| South-Eastern (Enugu) | 12 (16.2) | 62 (83.8) | |
| Diagnostic hospital type e | Tertiary | 68 (28.1) | 174 (71.9) |
| Secondary | 29 (50.0) | 29 (50.0) | |
| Travel time taken to diagnostic hospital | < 1 hour | 66 (36.1) | 117 (63.9) |
| 1 - < 2 | 15 (33.3) | 30 (66.7) | |
| >=2 | 5 (22.7) | 17 (77.3) | |
| Not reported | 11 (22.0) | 39 (78.0) | |
BC: breast cancer; BSE: breast self-examination; HCP: health care provider including traditional and spiritual healers; SD: standard deviation;
Unless otherwise specified
A score was assigned to each one of 8 items on BC causes: 2 to the correct answer, 1 to not sure/certain and 0 to the wrong answer. The 8 items included (i) lifestyle, (ii) not breastfeeding, (iii) getting older, (iv) family history of BC, (v) if cancer is caused by a curse, (vi) an insect bite, (vii) injury to the breast or if (viii) it is contagious. The sum of the 8 item-specific scores was then grouped into 3 categories of poor (score 0-8), fair (9-11) and good knowledge (12-16).
Scores 0, 1 and 2 were assigned as above to 7 common BC symptoms: (i) breast lumps, (ii) breast pain, (iii) change in the size or shape of the breast, (iv) dimpling of the skin or a wound to the breast, (v) fluid coming from the nipple in a woman not breastfeeding, (vi) swelling in the armpit and (vii) change in the shape of the nipple. The sum of the 7 item-specific scores was then grouped into poor (0-7), fair (8-11) and good knowledge of symptoms (12-14)
Other symptoms included swelling underarm, nipple discharge and change in shape or size of breast.
Recruitment numbers for tertiary hospitals were: National Hospital Abuja- 70, University of Abuja Teaching Hospital Gwagwalada - 98, University of Nigeria Teaching Hospital, Enugu - 74; for secondary hospitals: Asokoro District Hospital - 44, Garki Hospital -11 and Wuse General Hospital - 3)
Table 2. Clinical characteristics of women with breast cancer by stage at diagnosis.
| Clinical characteristics | Categories | Early BC (Stages I/II) (row %) | Late BC (Stages III/ IV) (row %) |
|---|---|---|---|
| Total | 97 (32.3) | 203 (67.7) | |
| Co-morbidities a | |||
| Previous HTN or diabetes | Yes | 29 (36.7) | 50 (63.3) |
| Previous history of BBD | Yes | 18 (43.9) | 23 (56.1) |
| Family history of BC | Yes | 7 (26.9) | 19 (73.1) |
| Other Clinical characteristics | |||
| BMI (kg/m2) | < 25 (Normal weight) | 22 (24.4) | 68 (75.6) |
| 25-29 (Overweight) | 38 (38.4) | 61 (61.6) | |
| >30 (Obese) | 36 (35.6) | 65 (64.4) | |
| Unknown | 1 (10.0) | 9 (90.0) | |
| WHR | <0.80 (low) | 15 (48.4) | 16 (51.6) |
| 0.8-0.85 (moderate) | 15 (27.8) | 39 (72.2) | |
| >0.85 (high) | 67 (32.2) | 141(67.8) | |
| Unknown | 0 (0) | 7 (100) | |
| Tumour laterality | Left breast | 42 (29.2) | 102 (70.8) |
| Right breast | 52 (34.7) | 98 (65.3) | |
| Other (underarm) | 3 (50.0) | 3 (50.0) | |
| Morphology | NST/IDC | 85 (34.3) | 163 (65.7) |
| Medullary | 7 (26.9) | 19 (73.1) | |
| Others (lobular, mucinous) | 5 (21.7) | 18 (78.3) | |
| Unknown | 0 (0) | 3 (100) | |
| Stage at BC diagnosis | I | 5 (100) | - |
| II | 92 (100) | - | |
| III | - | 157 (100) | |
| IV | - | 46 (100) | |
| Tumour grade (n=250) b | Well differentiated | 20 (38.5) | 32 (61.5) |
| Moderately differentiated | 55 (34.4) | 105 (65.6) | |
| Poorly differentiated | 6 (23.1) | 20 (76.9) | |
| Oestrogen receptor status (n=220) c | Positive | 32 (34.0) | 62 (66.0) |
| Negative | 45 (39.5) | 69 (60.5) | |
HTN: hypertension; BBD: benign breast diseases; BC: breast cancer; BMI: body mass index; WHR: waist-hip ratio;
NST: Not Otherwise Specified; IDC: invasive ductal carcinoma.
Number missing or unreported 7 or less in smoking, alcohol, hypertension, BBD and family history of BC categories.
There were 250 women with information on tumour grade, 12 patients with missing information on stage have been excluded.
There were 220 women with information on oestrogen receptor status, 12 patients with missing information on stage were excluded.
We adapted the scoring method previously used by Mena et al.19 to generate scores for knowledge of BC causes (based on 8 items; Table 1) and symptoms (based on 7 items; Table 1). For both domains, a woman was given a score of 2 for a correct answer, 1 if not sure/did not know and 0 for the wrong answer to each of its items. The total score for each domain was calculated as the sum of its item-specific scores and then categorised as poor, fair and good knowledge as described in Table 1 (footnotes a and b).
Tumour staging and pathology
Physical breast examination was performed at the time of enrolment by surgeons before a biopsy was conducted. Participants were asked to undergo mammography, chest-x-rays, abdominal ultrasound, and a bone scan to check for metastases. These tests are routinely recommended and majority of patients undergo them. Lymph node involvement was assessed on clinical examination at the time of presentation; no information was recorded in the clinical notes on possible re-assessment during surgery. Thereafter, the study assigned clinical tumour stage according to the tumour, node, metastasis (cTNM) classification (American Joint Committee of Cancer 7th Edition TNM classification) into stages I, II, III and IV.
The large majority of patients underwent a core needle biopsy (only a few had an excisional biopsy) for histological confirmation, the results of which were retrieved from pathologists’ reports within two weeks of a biopsy. Data were extracted on tumour characteristics (e.g. laterality, size, morphology, grade, receptor status). Tumours were graded as 1, 2 and 3 using the Scarf-Bloom-Richardson system.20 Immunohistochemistry (IHC) staining was used to assess oestrogen receptor (ER) status. A <1% positivity was the cut-off point for negative staining characteristics.21 IHC was done specifically for this study using a more rigorous and standardised approach.
Statistical analyses
Ordinal logistic regression models were used to identify associations with more advanced stage at diagnosis, i.e. assuming a common odds ratio (OR) for the binary outcomes: stage IV v I/II/III, IV/III vs I/II and IV/III/II vs I. Age at diagnosis was regarded as a priori confounder and thus examined alone and included in all models. Age-adjusted models were initially fit separately to each group of variables: socio-demographic, BC awareness, health care access and clinical. Subsequent models assessed the extent to which: (i) associations between socio-demographic variables and later stage at BC diagnosis were mediated by BC awareness or health care access factors; and (ii) associations between BC awareness and health care access variables with later stage at BC diagnosis were confounded by socio-demographic variables. Finally, a fully-adjusted model was fitted to identify independent correlates of later stage at BC diagnosis. This model included age at diagnosis, and the two variables found to be most strongly associated with later stage at BC diagnosis within each group in the age-adjusted models. Variables found to be associated with others already included in the model were excluded (e.g. hospital type was excluded because it was defined by region of residence in Enugu). Data analyses were performed using Stata14.1 (Stata Corporation, College Station, Texas, USA).
Principal Component Analyses (PCA) was applied, as previously described by Filmer and Pritchett22, to generate a single summary index of a woman's socio-economic status on the basis of her household assets. The variables included in the PCA were all binary variables (Y/N), e.g. for owning your home, living in an apartment, house or duplex; drinking water from outside, well, borehole, piped or bottled; use of various types of cooking fuel and having a separate room for cooking; type of toilet used; and ownership of certain household goods including a car, refrigerator, bicycle, electric fan, television, and motorcycle. The first component in the PCA was used, as it explained most of the variation, to generate wealth scores and categories of low (lowest 40% of the score distribution), middle (middle 40%) and high (highest 20%) socio-economic class.
Participants with WHR >1.2 or <0.6 and those with a BMI<10 kg/m2 or >50 kg/m2 were regarded as outliers and therefore excluded from analyses involving these variables.
Results
Participants’ characteristics
In all, 316 eligible participants were recruited into the study, but sufficient information to derive stage at diagnosis was available for only 300 (94.9%) women. Of these, five (1.7%) were diagnosed at stage I, 92 (30.7%) in stage II, 157 (52.4%) in stage III and 46 (15.3%) in stage IV. The characteristics of the study participants are summarized in Tables 1 and 2. The mean age at BC diagnosis was 45.4 years (SD=11.4 years; range:24-86 years). The majority (81%) were recruited in a tertiary hospital, 67.2% of these in Abuja. The commonest first BC symptom noticed by the women was a breast lump (Table 1). Median (interquartile range) self-reported time from symptom to hospital diagnosis at the participating hospital was 8.25 (4.24-18.5) months. In all, 46 (14.6%) BC patients were found to have metastases at the time of diagnosis, including to the lung (n=17, 36.9%), liver (n=9, 19.6%), bone (n=5, 10.9%), brain (n=2, 4.3%) and patients for which metastases could not be assessed at the time of presentation (Mx) (n=13, 28.3%).
Socio-demographic characteristics and later stage at diagnosis
There was no association between age and the odds of later stage at diagnosis (p for linear trend (pt)=0.16; Table 3). After adjusting for age, there was positive trend in the odds of later stage with lower educational level (pt=0.002), with women with no formal education having 2.75 (95% CI 1.37, 5.52; p=0.004) times the odds of being diagnosed at a later stage relative to those with tertiary or higher education (Table 3; Figure 1a). Higher educational level was associated with having ever heard about BC (p<0.001) and with believing in a cure for this disease (p<0.001), but the trend in the odds of later stage with educational level persisted, albeit attenuated, upon further adjustment for these two BC awareness variables (p=0.02; Figure 1b). Similarly, the association between educational level and the odds of later stage at diagnosis persisted after further adjustment for health care access variables (i.e. region of residence, type of hospital and travelling time to diagnostic hospital) (Figure 2b).
Table 3. Age-adjusted associations between socio-demographic, BC awareness, health care access and clinical variables with odds of later stage BC estimated using ordinal logistic regression.
| Socio-demographic characteristics | Age adjusted OR (95% CI) | p value | |
|---|---|---|---|
| Age at BC diagnosis (years) | <40 | 1 | |
| 40-49 | 1.44 (0.85, 2.42) | 0.18 | |
| 50-59 | 1.76 (0.94, 3.28) | 0.08 | |
| >60 | 1.44 (0.69, 3.01) | 0.33 (pt = 0.16) | |
| Marital Status | Married | 1 | |
| Unmarried | 1.31 (0.77, 2.23) | 0.32 | |
| Educational level | Tertiary/PG | 1 | |
| Primary/Secondary | 1.63 (1.01, 2.64) | 0.045 | |
| None | 2.75 (1.37, 5.52) | 0.004 (pt =0.002) | |
| Religion | Christian | 1 | |
| Muslim | 0.46 (0.24, 0.90) | 0.02 | |
| Do you have a personal income? | Yes | 1 | |
| No | 1.21 (0.74, 1.99) | 0.45 | |
| Socio-economic class | High | 1 | |
| Middle | 0.99 (0.53, 1.84) | 0.97 | |
| Low | 1.43 (0.79, 2.60) | 0.24 (pt =0.15) | |
| Breast Cancer Awareness | |||
| Ever heard of BC | Yes | 1 | |
| No | 2.24 (1.25, 4.03) | 0.01 | |
| Knowledge of BC causes | Good | 1 | |
| Fair | 1.09 (0.50, 2.38) | 0.82 | |
| Poor | 1.47 (0.74, 2.79) | 0.29 (pt = 0.18) | |
| Knowledge of BC symptoms | Good | 1 | |
| Fair | 1.67 (0.81, 3.47) | 0.17 | |
| Poor | 2.02 (1.03,4.00) | 0.04 (pt = 0.08) | |
| Practise of BSE | Yes | 1 | |
| No | 1.89 (1.20, 2.99) | 0.01 | |
| Belief in a cure for BC | Yes | 1 | |
| No | 2.23 (1.40, 3.56) | 0.001 | |
| Health Care Access | |||
| Region of residence | North-Central (Abuja) | 1 | |
| South-Eastern (Enugu) | 2.21 (1.33, 3.68) | 0.002 | |
| Diagnostic hospital type | Tertiary | 1 | |
| Secondary | 0.40 (0.22, 0.70) | 0.001 | |
| Travel time taken to diagnostic hospital (hrs) | <1 | 1 | |
| 1 - <2 | 1.42 (0.75, 2.71) | 0.28 | |
| ≥ 2 | 2.14 (0.89, 5.13) | 0.09 (pt =0.04) | |
| Clinical Characteristics | |||
| Previous HTN or diabetes | Yes | 1 | |
| No | 1.40 (0.81, 2.42) | 0.22 | |
| Previous history of BBD | Yes | 1 | |
| No | 1.46 (0.77, 2.77) | 0.25 | |
| Family History of BC | Yes | 1 | |
| No | 1.07 (0.49, 2.34) | 0.86 | |
| BMI (kg/m2) | < 25 (Normal weight) | 1 | |
| 25-29 (Overweight) | 0.55 (0.32, 0.95) | 0.03 | |
| >30 (Obese) | 0.66 (0.38, 1.14) | 0.14 | |
| Unknown | 1.45 (0.40, 5.18) | 0.57 (pt =0.41) | |
| WHR | <0.80 (low) | 1 | |
| 0.8-0.85 (moderate) | 2.80 (1.18, 6.68) | 0.02 | |
| >0.85 (high) | 2.20 (1.06, 4.60) | 0.04 (pt =0.098) | |
| Tumour grade | Well differentiated | 1 | |
| Moderately differentiated | 0.93 (0.50, 1.72) | 0.82 | |
| Poorly differentiated | 1.42 (0.58, 3.49) | 0.44 | |
| Unknown | 1.56 (0.76, 3.23) | 0.23 (pt =0.09) | |
| Oestrogen receptor status | Positive | 1 | |
| Negative | 1.18 (0.70, 2.01) | 0.531 | |
BC: breast cancer; BSE: breast self-examination; HCP: health care provider including traditional and spiritual healers; HTN: hypertension; BBD: benign breast disease; BMI: body mass index; WHR: waist-hip ratio; PG: post graduate; OR: odds ratio; CI: confidence interval; pt: p-value for linear trend
Figure 1.
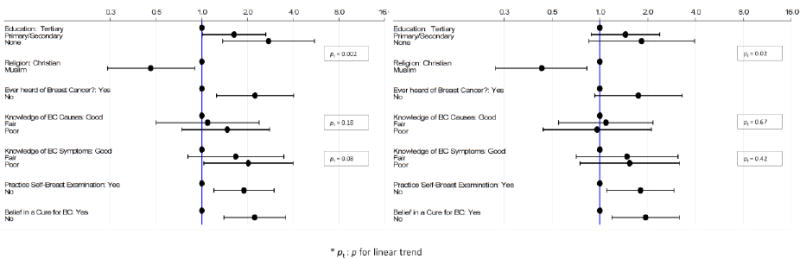
Odds of later stage at BC diagnosis by: (a) a woman's educational level, religion and BC awareness adjusting for age; and (b) a woman's educational level and religion adjusting for age and BC awareness, and by BC awareness variables adjusting for age, educational level and religion.
Figure 2.
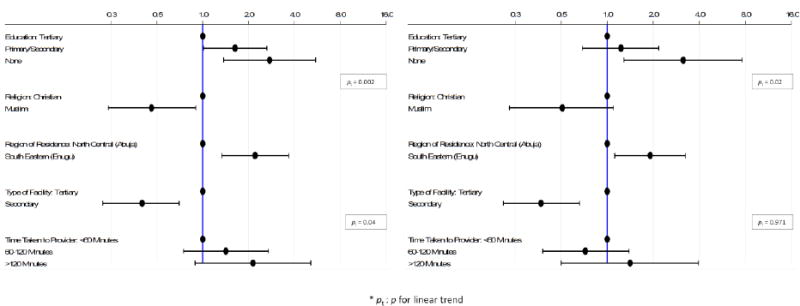
Odds of later stage at BC diagnosis by: (a) a woman's educational level, religion and health care access adjusting for age; and (b) a woman's educational level and religion adjusting for age and health care access, and by health care access variables adjusting for age, educational level and religion.
Muslim women were less likely to be diagnosed at later stages than Christian women (age-adjusted OR=0.46; 95% CI 0.24, 0.90; p=0.02), with this association strengthening slightly after further adjustment for educational level (OR=0.38; 95% CI 0.19, 0.75; p=0.005). Further adjustment for BC awareness or health care access variables did not change, however, the magnitude of the ORs (Figures 1 and 2).
The age-adjusted analyses showed no associations between later stage at BC diagnosis and a woman's marital status, self-reported personal income, or socioeconomic status (Table 3). There was also no association between later stage and self-reported alcohol consumption. Only 2 out of the 316 women in our study were ever smokers so the role of this lifestyle variable could not be assessed.
BC awareness and later stage at diagnosis
Overall, 80.4% women had ever heard of BC, but few displayed good knowledge of its causes (12.6%) or symptoms (13%) (Table 1). Only 59.5% of women believed in a cure or treatment for BC, and only 42.4% of women practised breast self-examination (BSE). After adjustment for age, never having heard of BC was significantly associated with an increased odds of later stage (OR=2.24; 95% CI 1.25, 4.03; p=0.01; Figure 1a). Women who did not believe in a BC cure (OR=2.23; 95% CI 1.40, 3.56; p=0.001) and those who did not practice BSE (OR=1.89; 95% CI 1.20, 2.99; p=0.01) were also more likely to be diagnosed at a later stage (Figure 1a). These associations were slightly attenuated upon further adjustment for a woman's educational level and religion.
In contrast, there were no clear trends in the odds of later stage with knowledge of BC causes or symptoms either in age-adjusted analyses or in those further adjusted for educational level and religion (Figure 1).
Access to health care and later stage at diagnosis
The proportion of participants with later stage was higher at the three tertiary hospitals (National Hospital Abuja: 62.3% (43/69); University of Abuja Teaching Hospital Gwagwalada: 69.4% (68/98); and University of Nigeria Teaching Hospital, Enugu: 83.8% (62/74)) than at the three secondary care hospitals in Abuja (50.8% (30/59)). These associations persisted after further adjustment for educational level and religion (Table 3; Figure 2a). In age-adjusted analysis, the odds of later stage were positively associated with the amount of travel time taken by the woman to reach the first healthcare provider she visited (pt=0.04; Table 3 and Figure 2a), but this trend was no longer significant upon further adjustment for educational level and religion (pt=0.97; Figure 2b).
Clinical factors and later stage BC diagnosis
Invasive ductal carcinoma (non-otherwise specified (NST)) was the commonest morphological type (83.2%). Information on tumour grade was available for 79.1% women, with 10.8% of these being poorly differentiated (Table 2). ER status was known for 69.6% women, with 45.2% of these being ER-positive overall. 66% of ER positive and 60.5% of ER negative tumours were diagnosed at stages III/IV (Table 2), but age-adjusted analyses revealed no associations between later stage at BC diagnosis and tumour grade, morphology, or ER (Table 3). There were also no clear trends in the odds of later stage with BMI or WHR at diagnosis, and no evidence of associations of later stage with a positive family history of BC or with having ever been diagnosed with diabetes, hypertension, or benign breast disorders (Tables 3 and 4).
Table 4. Fully-adjusted model showing associations between predictor variables and late stage BC.
| Variable | Categories | Age adjusted OR (95% CI) | p value | Fully-adjusted a OR (95% CI) | P value |
|---|---|---|---|---|---|
| Age at BC Diagnosis (years) | <40 | 1 | 1 | ||
| 40-49 | 1.44 (0.85, 2.43) | 0.18 | 1.53 (0.87, 2.68) | 0.139 | |
| 50-59 | 1.76 (0.94, 3.29) | 0.08 | 1.63 (0.84, 3.18) | 0.149 | |
| >60 | 1.44 (0.69, 3.02) | 0.33 | 0.89 (0.38, 2.08) | 0.789 | |
| pt | 0.16 | 0.86 | |||
| Educational level | Tertiary/PG | 1 | 1 | ||
| Primary/Secondary | 1.63 (1.01, 2.64) | 0.045 | 1.48 (0.89, 2.47) | 0.133 | |
| None | 2.75 (1.37, 5.52) | 0.004 | 2.35 (1.04, 5.29) | 0.039 | |
| Pt | 0.002 | 0.033 | |||
| Religion | Christian | 1 | 1 | ||
| Muslim | 0.46 (0.24, 0.90) | 0.02 | 0.46 (0.22, 0.94) | 0.033 | |
| Region of residence | North-Central (Abuja) | 1 | 1 | ||
| South-Eastern (Enugu) | 2.21 (1.33, 3.68) | 0.002 | 2.18 (1.05, 4.51) | 0.037 | |
| Travel time taken to diagnostic hospital | <1 | 1 | 1 | ||
| 1 - <2 | 1.42 (0.75, 2.71) | 0.28 | 1.45 (0.72, 2.93) | 0.300 | |
| ≥ 2 | 2.14 (0.89, 5.13) | 0.09 | 1.50 (0.59, 3.83) | 0.396 | |
| Pt | 0.04 | 0.786 | |||
| Ever heard of BC | Yes | 1 | 1 | ||
| No | 2.24 (1.25, 4.03) | 0.01 | 1.57 (0.80, 3.09) | 0.189 | |
| Belief in cure for BC | Yes | 1 | 1 | ||
| No | 2.23 (1.40, 3.56) | 0.001 | 1.81 (1.09, 3.01) | 0.022 | |
| WHR | <0.80 (low) | 1 | 1 | ||
| 0.8-0.85 (moderate) | 2.80 (1.18, 6.68) | 0.02 | 2.22 (0.88, 5.60) | 0.093 | |
| >0.85 (high) | 2.20 (1.06, 4.60) | 0.04 | 1.75 (0.84, 4.16) | 0.166 | |
| pt | 0.10 | 0.154 | |||
| Tumour grade | Well Differentiated | 1 | 1 | ||
| Moderately Differentiated | 0.93 (0.50, 1.72) | 0.82 | 0.86 (0.45, 1.65) | 0.647 | |
| Poorly Differentiated | 1.42 (0.58, 3.49) | 0.44 | 1.27 (0.50, 3.27) | 0.616 | |
| Unknown | 1.56 (0.76, 3.23) | 0.23 | 1.09 (0.49, 2.42) | 0.828 | |
| pt | 0.09 | 0.85 |
BC: breast cancer; HCP: health care provider including traditional and spiritual healers; WHR: waist hip ratio; pt: p-value for linear trend; PG: post-graduate; pt: p-value for linear trend; OR: odds ratio; CI: confidence interval;
Mutually-adjusted for all the other variables in the table.
Fully-adjusted model
A woman's educational level, religion, region of residence and belief in a cure for BC were identified as being independent correlates of later stage at BC diagnosis in the fully-adjusted model (Table 4). Notably, the association of lower educational level with the odds of later stage persisted in the fully-adjusted model, albeit with a slightly weakened trend (pt=0.033). The association of later stage with religion, though slightly attenuated when region of residence was included in the model, reflecting the fact that a higher percentage of Muslims resided in the North-Central than in the South-East region, also remained significant in the fully adjusted model (OR=0.46;95% CI 0.22, 0.94; p=0.033). This association also held when restricted to women diagnosed in Abuja (OR=0.42; 95% CI 0.20, 0.88; p=0.02). The association of belief in a BC cure with later stage, which was slightly attenuated upon adjustment for educational level and religion (Figure 1), was little affected with further adjustment for the other variables included in the fully-adjusted model (OR 1.81; 95% CI 1.09, 3.01; p=0.022). In contrast, the association between having ever heard of BC and later stage, which was weakened upon adjustment for educational level and religion (Figure 1), was further attenuated in the fully-adjusted analysis and no longer significant (Table 4).
Discussion
In this study, we examined the relationship between socio-demographic, BC awareness, access to health care and clinical factors with late stage diagnosis of BC at six tertiary and secondary level hospitals in two distinct regions of Nigeria. The findings showed that 67.7% women were diagnosed at late (III/IV) stages. The study identified lower educational level, being Christian, poor BC awareness and poor health care access as being independent correlates of later stage at BC diagnosis in Nigeria. In contrast, clinical and tumour features were not found to be related to stage at BC diagnosis.
This study is unique being the first multi-centre study to investigate socio-demographic, BC awareness, health care access and clinical determinants of late stage diagnosis of BC in two different regions in Nigeria. As the study was hospital-based, BC cases that do not reach a secondary or tertiary health facility to be diagnosed could not be included. However, given our high response rate of ∼95%, it is highly likely that our findings are a true representation of the all BC cases that are histologically diagnosed in the cities included. In addition, information from the Abuja and Enugu population based cancer registries showed that only 13.2% and 6.1% of all cancers cases recorded were from the private sector.23 The mean age at BC diagnosis was similar to that reported by other studies conducted in Nigeria6,24 and other SSA countries.25,26 Although some authors have associated younger age at diagnosis with later stage at presentation,27 others have found the reverse.14 We did not find an association between age at diagnosis and stage.
Breast cancer awareness is low in most African countries.28 Most women in our study had heard about BC, but only a few had good knowledge of its causes or symptoms, in line with the findings reported by other Nigerian studies.29,30 Women in our study who did not practice BSE had higher odds of presenting later than women who did. Other authors have reported an association between education and practice of BSE.31 While BSE has not been shown to be effective in early detection of BC, the awareness it generates may prove useful in low-resource settings.32
Access to health care is an important determinant of stage at diagnosis. Previous authors have reported variations in late stage BC by region of residence.33 Residing in areas with poorer access to health care, or taking longer time to travel for care, may increase the likelihood of a late stage diagnosis.33 Women diagnosed in Enugu had greater odds of later stage than those diagnosed in Abuja, perhaps reflecting differences in access to health care between the two cities. Abuja is a more affluent city with many secondary and tertiary health care facilities within easy reach whereas in Enugu participants had to travel long distances, often from rural areas, to get to the participating hospital which is located on the outskirts of the city. In addition, with the high prevalence of private practice and the frequent strike action in government hospitals, it is possible that only those who could not afford private care sought care at the participating hospital. High percentages of late stage at diagnosis have also been reported from other studies in South-Eastern and Western regions of Nigeria which cater to a predominantly more rural population than Abuja.6,24,34 In other similar settings, less developed areas reportedly have more advanced stage at diagnosis than the cities.27 We also found stage at diagnosis to be significantly better in secondary level facilities than at tertiary centres, perhaps because women may first present at secondary facilities, which may delay their presentation at tertiary hospitals, but more research is needed to confirm this.
Level of education was a strong determinant of stage at diagnosis in our study. Women with no education had significantly higher odds of later stage disease than women with tertiary education. Several studies in Nigeria10, other SSA countries35 and other developing countries (e.g. Brazil27) as well as multi-ethnic studies in the US36 have associated low educational level with later stage diagnosis of BC. Educational level was also positively correlated with knowledge of BC symptoms in our study similar to findings by Marcu et al. in the United Kingdom.37 Interestingly, the association of educational level did not appear to be fully mediated by differences in breast cancer awareness or health care access, but the variables examined here may be too crude to fully capture these domains. Nevertheless, this finding has important implications for the development of educational interventions that can potentially improve the stage at diagnosis of breast in developing countries.
We found no evidence of associations between tumour characteristics and later stage at BC diagnosis. This is in contrast with a study in South Africa where McCormack et al. reported that late stage tumours were more likely to be estrogen and progesterone receptor negative, but noted no association with HER2 status.38 In New Zealand, researchers have reported poorly differentiated tumour grade (II and III) in women with late stage disease,16 similar to findings in the United States, by Lipscomb et al. who found advanced stage breast cancer to be positively related with poorly differentiated tumour grade and HER2 type tumours.14 Our sample size for this analysis was, however, very limited, and thus, the influence of tumour characteristics on stage at diagnosis still needs to be fully explored in the SSA setting. The majority of biopsies in our study were core needle biopsies whose results tend to differ from excisional biopsies perhaps due to under-sampling of a heterogeneous tumour and insufficient amount of tumour in the cores taken.39,40 However, the lack of a strong association between tumour characteristics and tumour grade in our study suggests that other factors such as breast cancer awareness and delays to diagnosis may be more important drivers of late stage diagnosis in our setting. Obesity has been identified as a strong risk factor for late stage diagnosis in BC.41 However, in our study there was no association between measured BMI, or WHR, at diagnosis and later stage. As factors associated with later BC stage were not related to the tumour biology, but to woman's characteristics, they must act through increasing the time from onset of symptomatic disease to diagnosis at a health care facility. There are reports from other SSA settings of long patient-level and system delays which could result in considerable change in stage.35 We observed a long delay from time of a woman noticing the first BC symptoms to diagnosis at the recruiting hospitals, in line with previous reports of 12.1 months in Nigeria42 and 10 months in Ghana.43 Racial disparities have also been documented in the US with African-Americans reporting longer delays from first contact to diagnosis and from diagnosis to BC treatment than Whites and significantly more advanced stage at diagnosis.44
In this study, Muslim women were diagnosed at earlier stages than Christian women. This finding contrasts with previous studies that have reported less BC early detection practices among Muslim women. We speculate that the Muslim women in this study may belong to a higher socio-economic class than the Christian women, therefore may have the financial ability to seek care more readily. Although we adjusted for socioeconomic indicators, residual confounding may still be present. Secondly, Muslim women may be less likely to work outside the home and therefore would have the time to seek help once a breast symptom is felt without having to obtain permission from employers to get medical care. However, more research is needed to confirm this.
In SSA, ensuring early diagnosis and treatment of symptomatic women with breast cancer is crucial for stage-migration of the disease and, hence, achieve better outcomes. In our study, BC awareness and health care access related factors were identified as independent determinants of later stage at diagnosis in Nigeria. However, a limitation of this study is that it did not investigate a wider range of health service related factors such as misdiagnosis by health professionals, and delays in getting appointments and in obtaining test results. Stage migration interventions have proven to be successful and cost effective in several low- and middle-income countries such as India45, Malaysia46, South Africa38, Tanzania47 and Sudan48 following educational interventions coupled with appropriate diagnosis and treatment. In Sudan, the implementation of a cancer awareness and breast examination intervention program using trained local volunteers improved the early detection of breast abnormalities in women in rural communities.48 In Tanzania, late stage diagnosis (stages III/IV) was reduced by 51% over three years after trained health personnel delivered an educational intervention that focused on the signs and symptoms of cancer, and subsequently screened women by clinical examinations and taking pictures of suspicious lesions.47 More research is needed to characterize delays and factors associated with diagnostic delays in Nigeria, as well as in other SSA populations43, as such knowledge is crucial to the development of context appropriate BC control programs.
Acknowledgments
The project described was supported by the Training Program in Nigeria for Non-communicable Diseases Research (TRAPING NCD) grant number FIC/NIH D43TW009106 from the Fogarty International Centre. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Centre or the National Institutes of Health.
Footnotes
Author Contributions: EJA had the idea for the study, contributed to the study design, implementation and data analysis, drafted the manuscript and made subsequent revisions to the manuscript; VM had the idea for the study, supervised data analyses and provided critical revisions to the manuscript; OO, WB, MY, TY, EE, MA, IS, EM, IA, and BA contributed to data collection, data quality and approved the final draft; SNA contributed to the study design, data quality and manuscript revision; IDS supervised data analyses and provided critical revisions to the manuscript; CA had the idea for the study design, obtained funding, supervised data analyses and provided critical revisions to the manuscript. All authors read and approved the final draft.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest disclosed.
References
- 1.Gathani T, Ali R, Balkwill A, et al. Ethnic differences in breast cancer incidence in England are due to differences in known risk factors for the disease: prospective study. British journal of cancer. 2014;110(1):224–9. doi: 10.1038/bjc.2013.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Kene TS, Odigie VI, Yusufu LM, Yusuf BO, Shehu SM, Kase JT. Pattern of presentation and survival of breast cancer in a teaching hospital in north Western Nigeria. Oman medical journal. 2010;25(2):104–7. doi: 10.5001/omj.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller H, Henson K, Luchtenborg M, et al. Short-term breast cancer survival in relation to ethnicity, stage, grade and receptor status: national cohort study in England. British journal of cancer. 2016 doi: 10.1038/bjc.2016.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantelhardt EJ, Zerche P, Mathewos A, et al. Breast cancer survival in Ethiopia: a cohort study of 1,070 women. Int J Cancer. 2014;135(3):702–9. doi: 10.1002/ijc.28691. [DOI] [PubMed] [Google Scholar]
- 6.Ezeome ER. Delays in presentation and treatment of breast cancer in Enugu, Nigeria. Nigerian journal of clinical practice. 2010;13(3):311–6. [PubMed] [Google Scholar]
- 7.Jedy-Agba E, McCormack V, Adebamowo C, dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet glob health. 2016;4(12):e923–35. doi: 10.1016/S2214-109X(16)30259-5. http://www.thelancet.com/pdfs/journals/langlo/PIIS2214-109X(16)30259-5.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anyanwu SN. Survival following treatment of primary breast cancer in eastern Nigeria. East African medical journal. 2000;77(10):539–43. [PubMed] [Google Scholar]
- 9.Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2016;4(12):e923–e35. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruitt L, Mumuni T, Raikhel E, et al. Social barriers to diagnosis and treatment of breast cancer in patients presenting at a teaching hospital in Ibadan, Nigeria. Global public health. 2015;10(3):331–44. doi: 10.1080/17441692.2014.974649. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman M, de Pinho H, Cooper D, et al. Breast cancer incidence and determinants of cancer stage in the Western Cape. S Afr Med J. 2000;90(12):1212–6. [PubMed] [Google Scholar]
- 12.Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. International journal of cancer. 2014;135(9):2173–82. doi: 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Annals of surgical oncology. 2008;15(7):1983–8. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 14.Lipscomb J, Fleming ST, Trentham-Dietz A, et al. What Predicts an Advanced-Stage Diagnosis of Breast Cancer? Sorting Out the Influence of Method of Detection, Access to Care, and Biologic Factors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(4):613–23. doi: 10.1158/1055-9965.EPI-15-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayanju OM, Jeffe DB, Elmore L, Ksiazek DN, Margenthaler JA. Patient and process factors associated with late-stage breast cancer diagnosis in Safety-Net patients: a pilot prospective study. Annals of surgical oncology. 2013;20(3):723–32. doi: 10.1245/s10434-012-2558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seneviratne S, Lawrenson R, Harvey V, et al. Stage of breast cancer at diagnosis in New Zealand: impacts of socio-demographic factors, breast cancer screening and biology. BMC Cancer. 2016;16:129. doi: 10.1186/s12885-016-2177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores YN, Davidson PL, Nakazono TT, Carreon DC, Mojica CM, Bastani R. Neighborhood socio-economic disadvantage and race/ethnicity as predictors of breast cancer stage at diagnosis. BMC public health. 2013;13:1061. doi: 10.1186/1471-2458-13-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS medicine. 2014;11(9):e1001720. doi: 10.1371/journal.pmed.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mena M, Wiafe-Addai B, Sauvaget C, et al. Evaluation of the impact of a breast cancer awareness program in rural Ghana: a cross-sectional survey. International journal of cancer. 2014;134(4):913–24. doi: 10.1002/ijc.28412. [DOI] [PubMed] [Google Scholar]
- 20.Howell LP, Gandour-Edwards R, O'Sullivan D. Application of the Scarff-Bloom-Richardson tumor grading system to fine-needle aspirates of the breast. Am J Clin Pathol. 1994;101(3):262–5. doi: 10.1093/ajcp/101.3.262. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(16):2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 23.Nigerian National System of Cancer Registries. [accessed 3rd April, 2017]; https://nigeriancancerregistries.net/ [PubMed]
- 24.Anyanwu SN, Egwuonwu OA, Ihekwoaba EC. Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria. Breast. 2011;20(Suppl 2):S51–3. doi: 10.1016/j.breast.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Mabula JB, McHembe MD, Chalya PL, et al. Stage at diagnosis, clinicopathological and treatment patterns of breast cancer at Bugando Medical Centre in north-western Tanzania. Tanzania journal of health research. 2012;14(4):269–79. [PubMed] [Google Scholar]
- 26.Galukande M, Wabinga H, Mirembe F, Karamagi C, Asea A. Difference in Risk Factors for Breast Cancer by ER Status in an Indigenous African Population. ISRN oncology. 2013;2013:463594. doi: 10.1155/2013/463594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahao Kde S, Bergmann A, Aguiar SS, Thuler LC. Determinants of advanced stage presentation of breast cancer in 87,969 Brazilian women. Maturitas. 2015;82(4):365–70. doi: 10.1016/j.maturitas.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Brinton LA, Figueroa JD, Awuah B, et al. Breast cancer in Sub-Saharan Africa: opportunities for prevention. Breast cancer research and treatment. 2014;144(3):467–78. doi: 10.1007/s10549-014-2868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azubuike S, Okwuokei S. Knowledge, attitude and practices of women towards breast cancer in benin city, Nigeria. Annals of medical and health sciences research. 2013;3(2):155–60. doi: 10.4103/2141-9248.113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okobia MN, Bunker CH, Okonofua FE, Osime U. Knowledge, attitude and practice of Nigerian women towards breast cancer: a cross-sectional study. World journal of surgical oncology. 2006;4:11. doi: 10.1186/1477-7819-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amoran OE, Toyobo OO. Predictors of breast self-examination as cancer prevention practice among women of reproductive age-group in a rural town in Nigeria. Nigerian medical journal : journal of the Nigeria Medical Association. 2015;56(3):185–9. doi: 10.4103/0300-1652.160362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter N, Canadian Task Force on Preventive Health, C Preventive health care, 2001 update: should women be routinely taught breast self-examination to screen for breast cancer? CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2001;164(13):1837–46. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, McLafferty S, Escamilla V, Luo L. Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois. Prof Geogr. 2008;60(1):54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntekim A, Nufu FT, Campbell OB. Breast cancer in young women in Ibadan, Nigeria. African health sciences. 2009;9(4):242–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Pace LE, Mpunga T, Hategekimana V, et al. Delays in Breast Cancer Presentation and Diagnosis at Two Rural Cancer Referral Centers in Rwanda. The oncologist. 2015;20(7):780–8. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farooqi B, Smith B, Chowdhary M, Pavoni S, Modi A, Schnell F. Racial disparities in breast cancer diagnosis in Central Georgia in the United States. The Journal of community and supportive oncology. 2015;13(12):436–41. doi: 10.12788/jcso.0179. [DOI] [PubMed] [Google Scholar]
- 37.Marcu A, Lyratzopoulos G, Black G, Vedsted P, Whitaker KL. Educational differences in likelihood of attributing breast symptoms to cancer: A vignette-based study. Psycho-oncology. 2016 doi: 10.1002/pon.4177. [DOI] [PubMed] [Google Scholar]
- 38.McCormack VA, Joffe M, van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast cancer research : BCR. 2013;15(5):R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris GC, Denley HE, Pinder SE, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol. 2003;27(1):11–5. doi: 10.1097/00000478-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol. 2007;60(12):1300–6. doi: 10.1136/jcp.2006.045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung J, Martin J, McLaughlin D. Rural-urban disparities in stage of breast cancer at diagnosis in Australian women. The Australian journal of rural health. 2016 doi: 10.1111/ajr.12271. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim NA, Oludara MA. Socio-demographic factors and reasons associated with delay in breast cancer presentation: a study in Nigerian women. Breast. 2012;21(3):416–8. doi: 10.1016/j.breast.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Clegg-Lamptey J, Hodasi W. A study of breast cancer in korle bu teaching hospital: assessing the impact of health education. Ghana medical journal. 2007;41(2):72–7. doi: 10.4314/gmj.v41i2.55305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(7):1227–38. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadgil A, Sauvaget C, Roy N, et al. Cancer early detection program based on awareness and clinical breast examination: Interim results from an urban community in Mumbai, India. Breast. 2016;31:85–9. doi: 10.1016/j.breast.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18(7):1172–6. doi: 10.1093/annonc/mdm105. [DOI] [PubMed] [Google Scholar]
- 47.Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. International journal of cancer. 2015;136(12):2875–9. doi: 10.1002/ijc.29348. [DOI] [PubMed] [Google Scholar]
- 48.Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. The Lancet Oncology. 2013;14(4):363–70. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]


