Abstract
Background
Dermatophytosis is the common cutaneous infections in humans and animals, which is caused by the keratinophylic fungus called dermatophytes. In recent years, drugs resistance in pathogenic fungi, including dermatophyte strains to the current antifungals have been increased.
Objectives
The aim of this study was to evaluate the antifungal efficacy of AgNPs against Microsporum canis, Trichophyton mentagrophytes , and Microsporum gypseum.
Materials and Methods
The antifungal susceptibility of nanosilver particles compared with griseofulvin (GR). Its efficacy was investigated against three strains of dermatophytes by both agar dilution and broth microdilution test (BMD).
Results
The average minimum inhibitory concentration (MIC) AgNPs on M. canis, T. mentagrophytes and M. gypseum were 200, 180 and 170 μg.mL-1, respectively. Whereas these strains showed MIC of 25, 100 and 50 μg.mL-1 for GR.
Conclusions
Our finding indicated that the AgNPs was less active than GR but it had anti-dermatophytic effect.
Keywords: AgNPs, Antifungal efficacy, Microsporum canis, Microsporum gypseum, Trichophyton mentagrophytes
1. Background
Silver nanoparticles (AgNPs) are the particles with size of 2-100 nm, which contain 20-15,000 silver atoms (1). These particles are used in medicine, dental cements, treatment of wounds and burns, water purification, and textile engineering (2-4). Several studies have been carried out concerning the antimicrobial properties of AgNPs against various pathogens such as viruses, fungi, and some bacterial species. Most of which have confirmed the antimicrobial properties of AgNPs (5, 4, 6). The mechanisms of action of AgNPs referred to their accumulation on the membrane of microorganisms, formation of pores, change in permeability of cell wall, and inhibition of respiration process. In addition, it has been shown that AgNPs can greatly inhibit cellular respiration, DNA replication, and cell division, which result in the loss of cell viability, and lead to cell death (7, 8).
Dermatophytosis is the most common cutaneous fungal infections with worldwide distribution. Dermatophytes can grow in keratinized tissues such as hair, nails, and the outer skin layer (9, 10). This infection occurs in humans skin, pets, and farm animals. Dermatophyte species divided into three genera: Epidermophyton, Microsporum, and Trichophyton, and consist of 40 accepted species (11, 12). Clinical features of dermatophytosis are observed as tinea capitis, tinea corporis, tinea barbae, tinea faciei, tinea cruris, tinea pedis, tinea manuum, tinea unguium (onychomycosis), and allergy to dermatophyte antigens (13).
Depending on different types and severity of infection, various therapeutic agents such as griseofulvin and oral and/or topical formulations of azoles or allylamines, particularly itraconazole and terbinafine are used in the treatment of dermatophytosis (14, 15).
2. Objectives
According to increase in number of antifungal-resistance reports in some strains including M. gypseum and T. mentagrophytes (16-18), antifungal efficacy of AgNPs against M. canis, T. mentagrophytes, and M. gypseum was evaluated in this study.
3. Materials and Methods
3.1. Reagents and Fungal Strains
Nanosilver (Nanocid®) was purchased from Nano Nasb Pars Co, Tehran, Iran. The silver nanoparticles with average particle size of 4 nm were synthesized by a novel process that involved the photo-assisted reduction of Ag+to metallic nanoparticles and theirbio-stabilization based on undisclosed US-patent (United State Patent Application under No. US/2009/ 0013825) (17). Dermatophyte strains including M. canis PTCC 5069, M. gypseum PTCC5070, and T. mentagrophaytes PTCC 5054 were purchased from Iranian Research Organization for Science and Technology (IROST) in Tehran, Iran.
3.2. Susceptibility Testing
3.2.1. Broth Microdilution Method
Antifungal susceptibility testing was performed by microdilution assay and agar dilution method, according to guideline of Clinical and Laboratory Standards Institute (CLSI) in M38-A document for filamentous fungi (19). For broth microdilution test, dermatophyte strains were subcultured on Potato Dextrose Agar (PDA) (Merck Co., Darmstadt, Germany) and incubated at 30°C for 5-7 days. Conidia were moved to sterile saline and allowed to rest for 15 min. Conidia was counted by a hemocytometer, and the suspension was adjusted to 1×104 CFU.mL-1 in RPMI 1640 medium (with L-glutamine, without sodium bicarbonate; GIBCO-BRL, Grand Island, NY) buffered with MOPS (3-(N-morpholino) propanesulfonic acid; Serva, Feinbochemica GmbH, Germany). Serial dilutions of drugs (200-0 μg.mL-1 for AgNPs and griseofulvin) and inoculum were combined in 96-well microtiter plates and incubated at 32°C for 5 days (20). Inhibited growth by 90% of dermatophyte strains compared with the positive control determined as minimum inhibitory concentration (MIC). Griseofulvin was used as positive control for the evaluation of antifungal activity. A plate for each fungal strain with no AgNPs was used as negative control. The experiments were performed for each fungi sample in triplicate.
3.2.2. Agar Dilution Method
The inhibitory effects of various concentrations of AgNPs (0, 40, 80, 120, 160, 170 and 200 μg.mL-1) were assayed on three dermatophyte strains. An in vitro assay was carried out on a PDA (Merck Co., Darmstadt, Germany) treated with different concentrations of AgNPs as above and GR (0, 3.125, 6.25, 12.5, 25, 50, 100, 200 μg.mL-1). Various concentrations of AgNPs and GR were poured to PDA medium prior to plating in petri dish. Inoculum containing 1×104 CFU.mL-1 of dermatophyte strains was added to the hole in center of the plates. The plates were incubated for 14 days in 28°C. When the control plate was covered completely with fungal growth, the MIC was read. The MIC was determined as the lowest AgNPs and GR concentration that inhibited visible growth (21, 22). The experiments were replicated three times.
3.3. Data Analysis
Data were expressed as mean±SD of at least three independent experiments. One-way ANOVA was used to calculate statistical significance between positive control and culture medium treated with AgNPs at p-value < 0.05.
4. Results
The inhibitory effects of AgNPs at various concentrations were tested on the growth of M. canis PTCC 5069, M. gypseum PTCC 5070, and T. mentagrophytes PTCC 5054. Comparison between MICs of AgNPs and GR indicated that the antifungal efficacy of GR on dermatophayte strains was significantly higher than AgNPs (p<0.001). Susceptibility results of dermatophayte strains to AgNPs and GR are illustrated in (Figure 1). M. Canis had the highest resistance (200 μg.mL-1), following T. mentagrophytes (180 μg.mL-1) and M. gypseum (170 μg.mL-1). Mean MIC for GR were 25, 100 and 50 μg.mL-1, respectively. The colony diameter dermatophyte strains (mm) in various concentrations of griseofulvin and AgNPs are shown in (Tables 1 and 2) respectively.
Figure 1 .
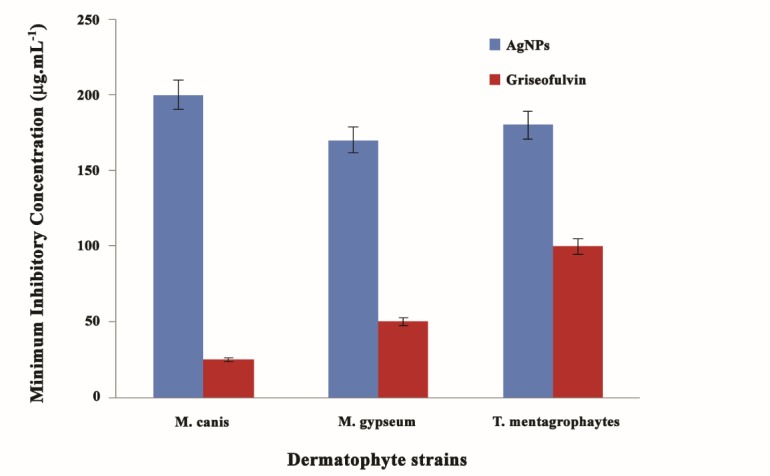
The minimum inhibitory concentration (MIC) of dermatophyte strains against AgNPs compared with griseofulvin (μg.mL-1)
Table 1. Colony diameter dermatophyte strains (mm) in various concentrations of griseofulvin .
| Griseofulvin concentrations (μg.mL-1) | ||||||||||
| Strains | 0 | 0.78 | 1.56 | 3.125 | 6.25 | 12.5 | 25 | 50 | 100 | 200 |
|
M. canis
M. gypseum T. mentagrophaytes |
54(±3.06) 63(±2.01) 68(±2.5) |
32(±3.34) 35(±1.53) 32(±3.7) |
22(±2.78) 28(±2.06) 27(±2.9) |
20.21(±1.98) 21(±1.71) 22.5(±2.07) |
16(±1.38) 15.1(±3.06) 22(±2.1) |
3(±0.9) 8(±1.82) 19(±1.39) |
- 2(±1.01) 12(±1.87) |
- - 5(±1.5) |
- - - |
- - - |
Table 2. Colony diameter dermatophyte strains (mm) in various concentrations of AgNPs .
| AgNPs concentrations (μg.mL-1) | ||||||||
| Strains | 0 | 40 | 80 | 120 | 160 | 170 | 180 | 200 |
|
M. canis
M. gypseum T. mentagrophytes |
48(±2.6) 58(±3.00) 55(±4) |
41(±2.7) 49(±1.53) 51(±1.8) |
36(±2.02) 34(±2.4) 47(±2.56) |
29(±3.04) 22(±1.33) 35(±2.00) |
25(±2.8) 12(±1.7) 21(±2.31) |
23(±2.33) - 15(±1.29) |
17(±1.00) - - |
- - - |
5. Discussion
Dermatophytosis is caused by the keratinophylic fungus called dermatophytes (23). Transmissibility from infected humans or animals to human is one important public health problem caused by dermatophyte species (24). In some cases, treatment of the disease with the current therapeutic agents can result in the damage of host tissues due to the similarity between eukaryotic cells in human and fungi structure, emergence of drugs resistance to fungal strains, and treatment failures (25, 26). Different research groups have investigated the efficacy of AgNPs on yeasts, molds, bacteria, and viruses (5, 27). But, information about anti-dermatophyte activities of nano-silver particles is few (28, 29).
This study was performed to investigate a new antifungal drug for the treatment of dermatophyte infection caused by M. Canis, T. Mentagrophytes, and M. gypseum. Our findings revealed that GR had higher anti-dermatophyte activity than AgNPs. Comparison of the three tested dermatophyte strains showed that M. canis was more resistant to AgNPs. Dermatophyte strains demonstrated an antifungal activity to AgNPs with the following order of resistance: M. canis> T. mentagrophytes> M. gypseum. Ability of AgNPs in destroying of fungi, pore in cell wall and plasma membrane are the potential mechanisms of its inhibitory effect on different organisms (7, 30). Here, the most GR-susceptible strains were M. canis followed by M. gypseum and T. mentagrophytes.
Previous data indicated that the AgNPs had good antifungal and antimicrobial effects (31-33). Atef et al. (33) reported the growth inhibition of the AgNPs on T. mentagrophytes and C. albicans. In their study, MIC100 AgNPs against C. albicans and T. mentagrophytes were 4±2.0 μg.mL-1 and 5±0.10 μg.mL-1, respectively. Kim et al. (29) showed that AgNPs had inhibitory effects on the growth of T. mentagrophytes, C. albicans, C. tropicalis, and C. glabrata. AgNPs (IC80, 1-7 μg.mL-1) exhibited greater efficacy than fluconazole (IC80: 10-30 μg.mL-1), but less active than Amphotericin B (IC80: 15 g.mL-1).
Petica et al. (32) indicated that the colloidal solutions containing up to 35 ppm AgNPs could inhibit the growth of Aspergillus, Penicillium, and Trichoderma species. Moreover, the inhibition effects of low concentrations of AgNPs on yeasts and E. coli were noted by Sondi et al. (8). Khaydarov et al. (34) reported that the AgNPs MIC for E. coli and S. aureus were 3 and 2 mg.L-1, respectively. Azimi et al. (35) demonstrated that the greater antifungal effect of AgNPs on S. mutans and S. sanguis than Nigella sativa oil. The inhibitory effects of AgNPs on the growth of Gram-negative bacteria E. coli and Gram-positive bacteria S. aureus and S. mutans were confirmed (36, 31). All of which appeared to be in agreement with our findings and other results reported about the antimicrobial activity of AgNPs.
Rathod et al. (37) demonstrated that synthesized AgNPs by Rhizopus stolonifer has a considerable antifungal activity on T. mentagrophytes and Candida species compared with Amphotericin B and fluconazole. Similarly, the antifungal effect of AgNPs alone and combined with griseofulvin against T. rubrum was studied. The results showed that AgNPs had superior efficiency than fluconazole (40 μg.mL-1), but less antifungal efficiency than griseofulvin (0.8 μg.mL-1). They confirmed that the antifungal activities of fluconazole and griseofulvin were increased in the presence of AgNPs (28). Gajbhiye et al. (38) showed that the increasing inhibitory effect of fluconazole was occurred in combination with AgNPs against C. albicans, Phoma, Glomerata and Trichoderma species. In conclusion, our data showed that (1) AgNPs had anti-dermatophytic effect and (2) the AgNPs was less active against dermatophyte strains.
Authors’ Contribution
SS performed the experiments, analyzed data and wrote the manuscript. SAAM designed, provided consultation, supervised the study and analyzed data. SH performed the experiments.
References
- 1.Chen X, Schluesener H. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Gao C, Xu Y, Xu C. In vitro Activity of nano-silver against Ocular Pathogenic Fungi. Life Sci J. 2012;9(4):750–753. [Google Scholar]
- 3.Mirshokraei P, Hassanpour H, Akhavan Taheri M, Riyahi M, Shams-Esfandabadi N. The in vitro effects of nanosilver colloid on kinematic parameters of ram spermatozoa. Iran J Vet Res. 2011;12(4):317–323. [Google Scholar]
- 4.Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16(10):8894–8918. doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrbod P, Motamed N, Tabatabaian M, Estyar RS, Amini E. et al. In vitro antiviral effect of ”Nanosilver” on influenza virus. DARU J Pharm Sci. 2009;17(2):88–93. [Google Scholar]
- 6.Inbaneson SJ, Ravikumar S, Manikandan N. Antibacterial potential of silver nanoparticles against isolated urinary tract infectious bacterial pathogens. Appl Nanosci. 2011;1(4):231–236. doi: 10.1007/s13204-011-0031-2. [DOI] [Google Scholar]
- 7.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E coli as a model for Gram-negative bacteria. J Colloid Interf Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Garg J, Tilak R, Garg A, Prakash P, Gulati AK, Nath G. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;2(1):1–6. doi: 10.1186/1756-0500-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rippon JW. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. Eastbourne, UK; WB Saunders Company; 1982.
- 11.Lakshmipathy DT, Kannabiran K. Review on dermatomycosis: pathogenesis and treatment. Nat Sci. 2010;2(7):726–731. doi: 10.4236/ns.2010.27090. [DOI] [Google Scholar]
- 12.Chah KF, Majiagbe KA, Kazeem HM, Ezeanyika O, Agbo IC. Dermatophytes from skin lesions of domestic animals in Nsukka, Enugu State, Nigeria. Vet Dermatol. 2012;23(6):522–528. doi: 10.1111/j.1365-3164.2012.01089.x.. [DOI] [PubMed] [Google Scholar]
- 13.Woodfolk JA. Allergy and dermatophytes. Clin Microbiol Rev. 2005;18(1):30–43. doi: 10.1128/CMR.18.1.30-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166(5-6):353–367. doi: 10.1007/s11046-008-9109-0. [DOI] [PubMed] [Google Scholar]
- 15.Khosravi AR, Shokri H, Nikaein D, Mansouri P, Erfanmanesh A, Chalangari R. Katalin M Yeasts as important agents of onychomycosis: in vitro activity of propolis against yeasts isolated from patients with nail infection. J Alter Complemen Med. 2013;19(1):57–62. doi: 10.1089/acm.2011.0722. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva Barros ME, Hamdan JS. Determination of susceptibility/resistance to antifungal drugs of Trichophyton mentagrophytes isolates by a macrodilution method. Can J Microbiol. 2005;51(11):983–987. doi: 10.1139/w05-100. [DOI] [PubMed] [Google Scholar]
- 17.Moaddab S, Ahari H, Shahbazzadeh D, Motallebi AA, Anvar AA, Rahman-Nya J, Shokrgozar MR. Toxicity study of nanosilver (Nanocid) on osteoblast cancer cell line. Int Nano Lett. 2011;1(1):11–6. [Google Scholar]
- 18.Lenhart K. Spontaneous mutants of Microsporon gypseum resistant to griseofulvin. Mycopathol Mycol Appl. 1968;36(2):150–60. doi: 10.1007/bf02049681. [DOI] [PubMed] [Google Scholar]
- 19. Wayne P. Clinical and Laboratory Standards Institute: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. CLSI document M27-A3 and Supplement S3. 2008.
- 20.Ghannoum M, Isham N, Sheehan D. Voriconazole susceptibilities of dermatophyte isolates obtained from a worldwide tinea capitis clinical trial. J Clin Microbiol. 2006;44(7):2579–2580. doi: 10.1128/JCM.00818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mota CRA, Miranda KC, Lemos JdA, Costa CR, Passos XS, Silva MdRR. Comparison of in vitro activity of five antifungal agents against dermatophytes, using the agar dilution and broth microdilution methods. Rev Soc Bras Med Trop. 2009;42(3):250–254. doi: 10.1590/S0037-86822009000300003. [DOI] [PubMed] [Google Scholar]
- 22.Niewerth M, Splanemann V, Korting HC, Ring J, Abeck D. Antimicrobial susceptibility testing of dermatophytes–comparison of the agar macrodilution and broth microdilution tests. Chemotherapy. 1998;44(1):31–35. doi: 10.1159/000007087. [DOI] [PubMed] [Google Scholar]
- 23.Coelho LM, Aquino-Ferreira R, Maffei CML, Martinez-Rossi NM. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J Antimicrob Chemother. 2008;62(4):758–61. doi: 10.1093/jac/dkn245. [DOI] [PubMed] [Google Scholar]
- 24.Khan MAAH, Khan SAH, Sarker MR, Hussain RF, Ahmed MU, Joarder Y, Hasan MB. Studies on microscopic technique and culture on Sabouraud’s dextrose agar medium for diagnosis of dermatophytes infection. KYAMC J. 2013;3(1):235–238. doi: 10.3329/kyamcj.v3i1.13658. [DOI] [Google Scholar]
- 25.Kauffman CA, Carver PL. Antifungal agents in the 1990s. Drugs. 1997;53(4):539–549. doi: 10.2165/00003495-199753040-00001. [DOI] [PubMed] [Google Scholar]
- 26.Pakshir K, Bahaedinie L, Rezaei Z, Sodaifi M, Zomorodian K. In vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur J Microbiol. 2009;2(4):158–163. doi: 10.1016/j.ijid.2008.05.762. [DOI] [Google Scholar]
- 27.Namasivayam S, Ganesh S, Avimanyu B. Evaluation of anti-bacterial activity of silver nanoparticles synthesized from Candida glabrata and Fusarium oxysporum. Int J Med Res. 2011;1:131–136. [Google Scholar]
- 28.Noorbakhsh F, Rezaie S, Shahverdi AR. Antifungal effects of silver nanoparticle alone and with combination of antifungal drug on dermatophyte pathogen Trichophyton rubrum. IJBBB. 2011;5:364–367. [Google Scholar]
- 29.Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol. 2008;18(8):1482–1484. [PubMed] [Google Scholar]
- 30.Vaidyanathan R, Kalishwaralal K, Gopalram S, Gurunathan S. Nanosilver-The burgeoning therapeutic molecule and its green synthesis. Biotechnol Adv. 2009;27(6):924–937. doi: 10.1016/j.biotechadv.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Wasif A, Laga S. Use of nano silver as an antimicrobial agent for cotton. AUTEX Res J. 2009;9(1):5–13. [Google Scholar]
- 32.Petica A, Gavriliu S, Lungu M, Buruntea N, Panzaru C. Colloidal silver solutions with antimicrobial properties. Mat Sci Eng B. 2008;152(1):22–27. doi: 10.1016/j.mseb.2008.06.021. [DOI] [Google Scholar]
- 33.Atef AH, Mogda KM, HH M. Biosynthesis of silver nanoparticles (AgNps) (a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans) New York Sci J. 2013;6(3):27–43. [Google Scholar]
- 34. Khaydarov RR, Khaydarov RA, Evgrafova S, Wagner S, Cho SY. Environmental and Human Health Issues of Silver Nanoparticles Applications. Environmental security and ecoterrorism. Springer 2011;p.117-127. DOI: http://dx.doi. 10.1007/978-94-007-1235-5.
- 35.Azimi Laysar H, Niakan M, Mohammad Taghi G, Jafarian Z, Mostafavizade M, Niakan S. Comparison of the antibacterial activity of various concentrations of Nigella Sativa and Nanosilver on the growth of S sanguis and S mutans. Res Dent Sci. 2013;9(4):179–186. [Google Scholar]
- 36.Magalhães APR, Santos LB, Lopes LG, Estrela CRdA, Estrela C, Torres ÉM. et al. Nanosilver application in dental cements. ISRN Nanotech. 2012;2012:1–6. doi: 10.5402/2012/365438. [DOI] [Google Scholar]
- 37.Rathod V, Banu A, Ranganath E. Biosynthesis of highly stabilized silver nanoparticles by Rhizopus stolonifer and their Anti-fungal efficacy. Int J Cur Biomed Phar Res. 2012;2(1):241–245. [Google Scholar]
- 38.Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine: Nanomed Nanotech Biol Med. 2009;5(4):382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]


