Abstract
A new pharmacokinetically enhanced formulation of amoxicillin-clavulanate (2,000 mg of amoxicillin/125 mg of clavulanate twice a day; ratio 16:1) has been designed, with sustained-release technology, to allow coverage of bacterial strains with amoxicillin-clavulanic acid MICs of at least 4/2 μg/ml. The bacteriological efficacy of amoxicillin-clavulanate, 2,000/125 mg twice a day, ratio 16:1, was compared in a rat model of respiratory tract infection versus four other amoxicillin-clavulanate formulations: 8:1 three times a day (1,000/125 mg), 7:1 three times a day (875/125 mg), 7:1 twice a day (875/125 mg), and 4:1 three times a day (500/125 mg); levofloxacin (500 mg once a day); and azithromycin (1,000 mg on day 1 followed thereafter by 500 mg once a day). Bacterial strains included Streptococcus pneumoniae, with amoxicillin-clavulanic acid MICs of 2/1 (one strain), 4/2, or 8/4 μg/ml (three strains each), and Haemophilus influenzae, one β-lactamase-positive strain and one β-lactamase-negative, ampicillin-resistant strain. Animals were infected by intrabronchial instillation. Antibacterial treatment commenced 24 h postinfection, with doses delivered by computer-controlled intravenous infusion to approximate the concentrations achieved in human plasma following oral administration. Plasma concentrations in the rat corresponded closely with target human concentrations for all antimicrobials tested. Amoxicillin-clavulanate, 2,000/125 mg twice a day, ratio 16:1, was effective against all S. pneumoniae strains tested, including those with amoxicillin-clavulanic acid MICs of up to 8/4 μg/ml and against β-lactamase-producing and β-lactamase-negative ampicillin-resistant H. influenzae. These results demonstrate the bacteriological efficacy of pharmacokinetically enhanced amoxicillin-clavulanate 2,000/125 mg twice a day (ratio 16:1) against S. pneumoniae with amoxicillin-clavulanic acid MICs of at least 4/2 μg/ml and support clavulanate 125 mg twice a day as sufficient to protect against β-lactamase in this rat model.
Antimicrobial agent-resistant strains of the key respiratory tract pathogens are widespread, and their prevalence is increasing in many areas (12, 15). For Haemophilus influenzae collected in the Alexander Project in 2001, the prevalence of β-lactamase production exceeded 24% in the United States and was more than 10% in 9 of the 16 countries included within the study (27). In the same study, the prevalence of penicillin-resistant Streptococcus pneumoniae in 2001 was approximately 20% in the United States and over 30% in Spain, Japan, France, and Hong Kong. The prevalence of macrolide-resistant S. pneumoniae was also high in the United States (28%) and was at least 10% in 12 of the 16 countries included within the study (27). Data from the 1998 to 2000 Alexander Project and other surveillance studies have reported similar findings (15-17, 26).
Amoxicillin-clavulanate is a combination of the β-lactam antibiotic amoxicillin and the β-lactamase inhibitor clavulanic acid in the potassium form. Clavulanic acid protects amoxicillin against hydrolysis by β-lactamase, extending the spectrum of amoxicillin to include β-lactamase-producing species such as H. influenzae (27). Penicillin-resistant Streptococcus pneumoniae strains with amoxicillin MICs of ≥4 μg/ml are emerging in some regions. These strains are difficult to treat, as they are often multiply resistant to most of the commonly used antimicrobials, including macrolides and, in some areas, particularly the Far East, fluoroquinolones (15, 27).
A greater understanding of the role of pharmacokinetics and pharmacodynamics in predicting antimicrobial efficacy allows the development of new antimicrobials or new formulations of existing agents based on specific pharmacokinetic and pharmacodynamic targets to eradicate resistant organisms (6, 14, 21). The pharmacokinetics and pharmacodynamics of amoxicillin-clavulanate are well understood, with maximum efficacy predicted when serum concentrations are above the MIC for approximately 35 to 40% of the dosing interval (6, 14, 21, 28, 30, 31). A new pharmacokinetically enhanced formulation of amoxicillin-clavulanate, 2,000 mg of amoxicillin/125 mg of clavulanate twice a day (ratio 16:1), has been developed to provide amoxicillin levels over the dosing duration sufficient to eradicate penicillin-resistant Streptococcus pneumoniae with amoxicillin MICs of ≤4 μg/ml. This formulation is given as two tablets, each of which consists of a bilayer comprising a sustained-release layer of sodium amoxicillin (437.5 mg) and an immediate-release layer of amoxicillin trihydrate (562.5 mg)/potassium clavulanate (62.5 mg). This bilayer design extends the duration of the dosing interval for which serum amoxicillin concentrations are above the MIC to 49% for strains with MICs of 4 μg/ml and 35% for strains with MICs of 8 μg/ml (18).
The aim of this study was to compare the in vivo bacteriological efficacy of the pharmacokinetically enhanced formulation of amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), with other conventional amoxicillin-clavulanate formulations against strains of S. pneumoniae with elevated amoxicillin MICs and against H. influenzae strains. Azithromycin and levofloxacin were included as examples of macrolide and quinolone antimicrobials widely used for the treatment of community-acquired respiratory tract infections.
MATERIALS AND METHODS
Compounds.
Sodium amoxicillin was obtained from GlaxoSmithKline (Piscataway, N.J.). Potassium clavulanate was obtained from GlaxoSmithKline (Worthing, United Kingdom). Azithromycin (Pfizer) and levofloxacin (Ortho McNeil, Raritan, N.J.) were used as commercial intravenous preparations. All antimicrobials were used as pure, free acid/base equivalents. All test antimicrobials were prepared as sterile solutions in phosphate-buffered saline except levofloxacin, which was prepared in 0.9% sodium chloride.
Bacterial strains.
Test strains were either laboratory strains or recent clinical isolates obtained from the Alexander Project (11), Alert (24), and the U.S. Surveillance Study (20) and were chosen for their proven ability to produce infection in animal models and for their antimicrobial susceptibility profiles. Susceptibility was defined according to current National Committee for Clinical Laboratory Standards guidelines. MICs were determined by broth microdilution in accordance with National Committee for Clinical Laboratory Standards methods (Table 1) (23). MIC determinations were repeated multiple times, and results were consistent across all replicates.
TABLE 1.
Antimicrobial susceptibilities (MICs) of isolates used in the experimental infection modela
| Strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Amoxicillin | Amoxicillin/clavulanic acid | Levofloxacin | Azithromycin | |
| S. pneumoniae | ||||
| 05010S | 2 | 2/1 | 1 | ≤0.06 |
| 16001S | 4 | 4/2 | 1 | 0.006 |
| 30005S | 4 | 4/2 | 0.5 | 4 |
| 20009S | 4 | 4/2 | 0.5 | >32 |
| 05003S | 8 | 8/4 | 0.5 | 0.016 |
| 404053 | 8 | 8/4 | 0.5 | >64 |
| 47003S | 8 | 8/4 | 0.5 | 4 |
| H. influenzae | ||||
| H128 (Bla+) | 32 | 1/0.5 | ≤0.016 | 0.5 |
| Chesterfieldb | 4 | 4/2 | ≤0.016 | 2 |
MICs were determined by broth microdilution (23).
β-Lactamase negative, ampicillin resistant.
Animals.
Specific-pathogen-free Sprague-Dawley CD rats (Charles River, Raleigh, N.C.) weighing approximately 200 g were used throughout the study. Animals were housed two to a cage, with individual animals separated by a clear Plexiglass partition. Each therapy group included three to six animals. The use of animals in this study was approved by the GlaxoSmithKline Animal Care and Usage Committee and met or exceeded the standards of the American Association for the Accreditation of Laboratory Animal Care, the U.S. Department of Health and Human Services, and all local and federal animal welfare laws.
Cannula implantation.
Animals were obtained with the carotid artery and/or jugular vein previously cannulated for blood sampling and antimicrobial administration, respectively, with polyethylene tubing (0.58 mm internal diameter by 0.96 mm outside diameter) (Portex Ltd., Kent, England). Four to five days prior to infection, animals were anesthetized with 2.5 liters/min O2 plus 2.5% isoflurane (Aerrane, Fort Dodge Animal Health, Iowa). The previously implanted cannulas were exteriorized dorsally and taken through a flexible metal sheath to the top of the cage. A polytetrafluoroethylene flange attached to one end of the metal sheath was implanted subcutaneously on the back of the rat, and the distal end was affixed to a brass ferrule and swivel joint. The device allowed full movement of the animal and prevented the sheath from being pulled into the cage. The incisions were closed, and a local anesthetic was applied (4% lidocaine; Roxane Labs, Inc., Columbus, Ohio). The animals were allowed to recover for at least 4 days prior to infection. During this time, cannulas were kept patent by flushing with heparinized dextrose solution (500 U/ml of solution in 50% dextrose).
Respiratory tract infection model.
S. pneumoniae strains were grown overnight on nutrient agar plates containing 7% sterile defibrinated horse blood. Inocula were prepared by harvesting the growth from three plates and suspending in 3 ml of phosphate-buffered saline (approximately 8.5 log10 CFU/ml). The culture was diluted 1:10 in cooled, molten nutrient agar to give final bacterial inocula, in 100 μl of molten agar, of approximately 6.5 log10 CFU/rat. For H. influenzae strains, colonies were grown overnight at 37°C in Mueller-Hinton broth (BBL, Becton Dickinson & Co., Cockeysville, Md.) supplemented with 5% Fildes extract (Oxoid, Ltd., Basingstoke, United Kingdom) to give approximately 8.0 log10 CFU/ml. The culture was diluted 1:5 in cooled, molten nutrient agar to give final bacterial inocula, in 100 μl of molten agar, of approximately 6 log10 CFU/ml. For both S. pneumoniae and H. influenzae strains, molten agar was maintained at a temperature of approximately 40°C, and the inocula were prepared immediately prior to infection.
Rats were anesthetized by combined intramuscular injection of 100 μl of ketamine HCl (40 mg/kg Ketaset; Fort Dodge Animal Health) and xylazine (5 mg/kg Rompun; Bayer Corp., Shawnee Mission, Kans.). Anesthetized rats were then infected by intrabronchial instillation of a 100-μl bacterial inoculum in cooled, molten agar by means of nonsurgical intubation.
Antimicrobial administration.
Dosing commenced 24 h postinfection and continued for a further 2 days, for a total of 3 days of dosing. In all studies, a group of six to eight animals received an infusion of saline at a rate similar to that of the antimicrobial-treated groups and acted as infected, saline-treated controls.
Antimicrobials were administered to the rats to simulate human oral dosing of the following regimens: amoxicillin-clavulanate, 2,000/125 mg twice a day (1,125 mg of immediate-release amoxicillin trihydrate plus 875 mg of sustained-release amoxicillin and 125 mg of conventional clavulanate, ratio 16:1); 1,000/125 mg three times a day, ratio 8:1; 875/125 mg three times a day, ratio 7:1; 875/125 mg twice a day, ratio 7:1; and 500/125 mg three times a day, ratio 4:1; levofloxacin, 500 mg once a day; and azithromycin, 1,000 mg on day 1, followed thereafter by 500 mg once a day.
The desired concentrations in plasma for conventional amoxicillin-clavulanate, azithromycin, and levofloxacin were determined by application of a linear, one-compartment absorption model, C(t) = A(e−kelt − e−kat), where C is the concentration in plasma at time t, A is the zero-time intercept assuming instantaneous absorption, e is the base of the natural log, kel is the elimination constant, and ka is the absorption rate constant (Table 2). Human target profiles were based on those published in the literature (4, 9, 13, 18).
TABLE 2.
Pharmacokinetic (PK) parameters in adult humans and rats used to determine infusion rates for simulation studies with ratsa
| Species | PK modelb | Parameter (unit) | Amoxicillin (500 mg) | Amoxicillin (875 mg) | Amoxicillin (1,000 mg) | Clavulanate (125 mg) | Azithromycin (500/1,000 mg) | Levofloxacin (500 mg) |
|---|---|---|---|---|---|---|---|---|
| Human | C(t) = A(e−kelt − e−kat) | A (μg/ml) | 40 | 70 | 80 | 9.0 | 1.0 | 10 |
| kel (h−1) | 1.0 | 1.0 | 1.0 | 1.3 | 0.5 | 1.0 | ||
| ka (h−1) | 0.65 | 0.65 | 0.65 | 0.78 | 0.04 | 0.1 | ||
| Rat | C = Ae−λt | A (μg/ml) | 240 | 240 | 240 | 26.7 | 2.5 | 50 |
| λ (h−1) | 2.1 | 2.1 | 2.1 | 2.4 | 0.2 | 0.56 | ||
| Dose (mg/kg) | 44.0 | 44.0 | 44.0 | 4.4 | 5.5 | 22 |
The 2,000/125 mg twice a day (ratio 16:1) regimen of amoxicillin-clavulanate has been designed with a sustained-release amoxicillin component to give a specific pharmacokinetic profile in humans following oral dosing (18). Concentrations for this novel formulation were determined from the addition of two individual one-compartment absorption models. The first described the immediate-release amoxicillin portion and the second described the sustained-release amoxicillin portion: C(t) = A(e−kelt − e−kat) + B(e−kel2(t − 2) − e−ka2(t − 2)), where B is the zero-time intercept assuming instantaneous absorption for the second portion, and with a separation of 2 h between the two simulation segments (Table 3). Standard oral dosing in animals would not result in the appropriate sustained profile; therefore, in these studies, continuous intravenous infusion into the jugular vein was used to mimic the pharmacokinetic profile achieved following oral dosing in humans.
TABLE 3.
Pharmacokinetic (PK) parameters predicted for adult humans for determination of infusion rates for simulation of amoxicillin-clavulanate 16:1 twice a day in rats
| Species | PK modela | Parameter | Amoxicillin (2,000 mg) | Clavulanate (125 mg) |
|---|---|---|---|---|
| Human | C(t) = A(e−kelt − e−kat) + B(e−kel2(t − 2) − e−ka2(t − 2))b | A (μg/ml) | 95.0 | 9.0 |
| kel (h−1) | 1.0 | 1.3 | ||
| ka (h−1) | 0.65 | 0.78 | ||
| B (μg/ml) | 60.0 | |||
| kel2 (h−1) | 1.3 | |||
| ka2 (h−1) | 0.8 | |||
| Rat | C = Ae−λt | A (μg/ml) | 240 | 26.7 |
| λ (h−1) | 2.1 | 2.4 | ||
| Dose (mg/kg) | 44.0 | 4.4 |
C is the concentration in plasma at time t, A is the zero-time intercept assuming instantaneous absorption, e is the base of the natural log, kel is the elimination constant and ka is the absorption rate constant.
Sustained-release portion starting 2 h (t − 2) after immediate release. B is the zero-time intercept assuming instantaneous absorption for the second portion.
The data for the pharmacokinetics of amoxicillin, azithromycin, levofloxacin, and potassium clavulanate in rats (Tables 2 and 3) used to calculate dosing parameters were determined from concentrations measured in uninfected animals after intravenous administration of a bolus of 100 mg of amoxicillin, azithromycin, or levofloxacin per kg of body weight and 20 mg of potassium clavulanate per kg of body weight.
Flow rates were controlled with infusion pumps (Pump 22; Harvard Instruments, Edenbridge, United Kingdom) linked serially to a microcomputer. Input rates were calculated, and the infusion flow rates for each pump were modified individually every 60 s. The system was programmed to administer the calculated rates either every 8 h (for three times a day), 12 h (for twice a day), or 24 h (for once a day).
Sampling and evaluation. (i) Pharmacokinetics studies.
Blood samples were taken from the carotid artery cannula at intervals up to 8 h after the start of the infusion. Blood samples were centrifuged to isolate plasma, and the samples were assayed within 1 h of collection. Concentrations of amoxicillin, clavulanate, azithromycin, and levofloxacin were determined by a large-plate agar diffusion assay. Amoxicillin and azithromycin were assayed with Micrococcus luteus NCTC 8340 in a blood agar base (Difco, Detroit, Mich.), which was adjusted to pH 7.8 with sodium hydroxide for azithromycin. Levofloxacin was assayed with a commercially available Bacillus subtilis NCTC 6633 spore suspension (Difco) in nutrient agar (BBL). Potassium clavulanate was assayed by an enzyme inhibition assay with Klebsiella pneumoniae NCTC 11228 in nutrient agar supplemented with 60 μg of benzylpenicillin per ml (18).
Samples were assayed in duplicate against standards over the concentration range of 0.015 to 2 μg/ml for amoxicillin and azithromycin, 0.4 to 50 μg/ml for levofloxacin, and 0.078 to 5 μg/ml for clavulanate. The lowest concentration was taken as the limit of detection for the assay. The correlation coefficients for the regression lines of the standard solutions were not less than 0.996. The within-day coefficients of variation were less than 5% for amoxicillin, between 7.1 and 10.1% for azithromycin, between 3.1 and 7.8% for levofloxacin, and between 6.1 and 9.4% for clavulanate. Between-day coefficients of variation were 3.7 to 6.2% for amoxicillin, 6.8 to 10.5% for azithromycin, 6.0 to 11.6% for levofloxacin, and 6.3 to 9.8% for clavulanate.
(ii) Efficacy.
Approximately 14 h after the cessation of therapy, animals were euthanized by inhalation of rising concentrations of CO2. Lungs were excised aseptically and homogenized in 1 ml of phosphate-buffered saline (0.1 M, pH 7.4). Tenfold serial dilutions were prepared in phosphate-buffered saline for the enumeration of viable bacteria, and samples were inoculated (20 μl) in triplicate onto blood (S. pneumoniae) or chocolate (H. influenzae) agar plates by a modified Miles-Misra technique. After overnight incubation at 37°C, colonies were counted. The lower limit of detection was ≤1.7 log10 CFU/lung.
Data analysis.
Bacteriological efficacy was determined based on the number of bacteria in the lungs at the conclusion of the study. Results are presented as group means ± standard deviations. Statistical analysis was performed with analysis of variance. A P value of ≤0.05 was considered significant.
Data were fitted with an iterative least-squares modeling program (MK-Model), and appropriate models were chosen on the basis of visual inspection and the Schwartz criterion.
The areas under the concentration-time curves (AUCs) from time zero to infinity for plasma were calculated by noncompartmental analysis with the trapezoidal rule for data up to the last concentration time point, with the remaining area to infinity calculated by dividing this point by the elimination rate constant.
RESULTS
Concentrations achieved in plasma.
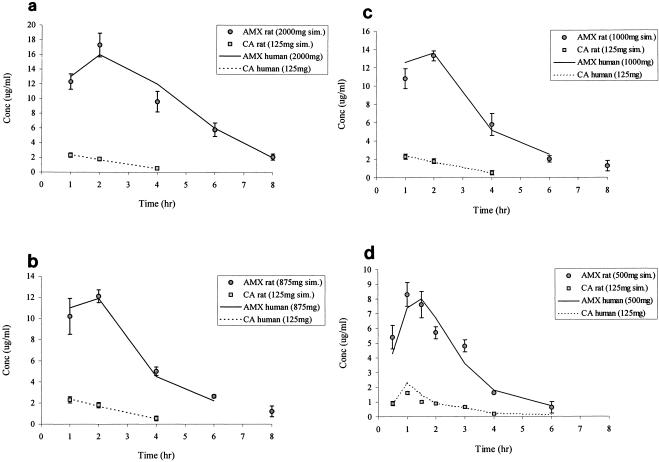
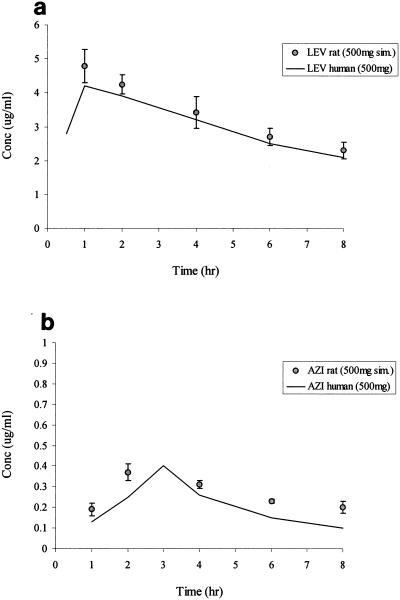
The pharmacokinetics of the formulation amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), which includes an 875-mg sustained-release amoxicillin component, in rat plasma closely followed the human target profile (Fig. 1a) (18). Simulated concentrations for the other conventional amoxicillin-clavulanate formulations were also similar to the target human profiles (Fig. 1b to d) (13) (data on file, GlaxoSmithKline). Table 4 compares the time above the MIC for amoxicillin-clavulanate formulations obtained in the rat versus human targets (13, 18) (data on file, GlaxoSmithKline). There were good similarities for all agents tested between the human target pharmacokinetics and pharmacodynamics profiles versus those obtained in the rats. Simulated human pharmacokinetics for levofloxacin and azithromycin in rat plasma were also close to target values (Fig. 2) (4, 9), the 0 to 8 h AUCs for levofloxacin and azithromycin being 34 and 9 μg · h/ml, respectively. Spot samples taken over time during the remainder of the infusion period also show good agreement with the pharmacokinetics profiles in humans.
FIG. 1.
Concentrations of amoxicillin-clavulanate in the plasma of rats and comparison with target levels in humans. Data for rats are presented as means ± standard deviations. (a) Amoxicillin-clavulanate 16:1 twice a day to simulate amoxicillin, 2,000 mg (1,125 mg of immediate release and 875 mg of sustained release), and clavulanate, 125 mg (n = 5 to 10). (b) Amoxicillin-clavulanate 7:1 three times a day to simulate amoxicillin 875 mg and clavulanate 125 mg (n = 5 to 10). (c) Amoxicillin-clavulanate 8:1 three times a day to simulate amoxicillin 1,000 mg and clavulanate 125 mg (n = 5 to 10). (d) Amoxicillin-clavulanate 4:1 three times a day to simulate amoxicillin 500 mg and clavulanate 125 mg (n = 5 to 10). AMX, amoxicillin; CA, clavulanate; sim., simulated.
TABLE 4.
Mean time above the MIC for amoxicillin-clavulanate formulations against MICs for the bacterial strains tested obtained in rat plasma simulating human serum pharmacokinetics and comparison with targets for human serum
| Amoxicillin-clavulanate formulationa | Time above the MIC (% of dosing interval) for MICs of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 μg/ml
|
2 μg/ml
|
4 μg/ml
|
8 μg/ml
|
|||||
| Rat | Human | Rat | Human | Rat | Human | Rat | Human | |
| 2,000/125 mg b.i.d. (ratio 16:1) | >70 | >70 | 64 | 60 | 50 | 49 | 38 | 35 |
| 1,000/125 mg t.i.d. (ratio 8:1) | 100 | 87.5 | 68 | 80.2 | 48 | 54 | 31 | 35.4 |
| 875/125 mg t.i.d. (ratio 7:1) | 100 | 87.5 | 60 | 71 | 45 | 47.5 | 27 | 25 |
| 875/125 mg b.i.d. (ratio 7:1) | 66 | 58 | 40 | 47.5 | 30 | 32 | 18 | 17 |
| 500/125 mg t.i.d. (ratio 4:1) | 62.5 | 62 | 40 | 52 | 30 | 33.3 | NTb | 12.5 |
b.i.d., twice a day; t.i.d., three times a day; o.d., once a day.
NT, not tested.
FIG. 2.
Concentrations of levofloxacin and azithromycin in the plasma of rats and comparison with target levels in humans. Data for rats are presented as means ± standard deviations. (a) Levofloxacin to simulate 500 mg (n = 5 to 10). (b) Azithromycin to simulate 500 mg on day 2 following a loading dose of 1,000 mg administered on day 1 (n = 5 to 10).
Efficacy against S. pneumoniae.
Table 5 reports the bacterial count in the lungs for the test antimicrobials and controls at the end of the experiment against S. pneumoniae.
TABLE 5.
Efficacy of test antimicrobials against seven S. pneumoniae strains with amoxicillin-clavulanic acid (AMX/CA) MICs of ≥2/1 μg/ml
| Treatment groupa | Mean log10S. pneumoniae (CFU/lung)b ± SD for strain (AMX/CA MIC):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 05010S (2 μg/ml) | 16001Sc (4 μg/ml) | 16001S (4 μg/ml) | 30005S (4 μg/ml) | 20009S (4 μg/ml) | 05003S (8 μg/ml) | 404053 (8 μg/ml) | 47003S (8 μg/ml) | |
| Control | 7.3 ± 0.4 | 7.0 ± 0.8 | 5.7 ± 1.2 | 7.1 ± 0.7 | 7.1 ± 0.4 | 6.4 ± 0.6 | 6.8 ± 0.4 | 6.0 ± 0.3 |
| AMX/CA | ||||||||
| 2,000/125 mg b.i.d. (ratio 16:1) | ≤1.7** | 2.8 ± 1.2** | 2.2 ± 0.8** | 2.3 ± 0.9** | 2.5 ± 0.9** | 2.0 ± 0.9** | 3.8 ± 1.4** | 1.8 ± 0.2** |
| 1,000/125 mg t.i.d. (ratio 8:1) | NT | NT | 2.8 ± 0.5** | 2.7 ± 1.3** | 3.3 ± 0.9** | 4.9 ± 0.5** | 6.6 ± 1.3 | 4.7 ± 0.7** |
| 875/125 mg t.i.d. (ratio 7:1) | NT | NT | 3.0 ± 1.3* | 2.1 ± 0.7** | 3.2 ± 0.4** | 5.2 ± 0.9* | 6.4 ± 0.6 | 4.9 ± 0.4** |
| 875/125 mg b.i.d. (ratio 7:1) | NT | NT | 4.5 ± 1.2* | 5.6 ± 1.3* | 5.2 ± 0.7** | 6.3 ± 0.7 | 6.4 ± 0.7 | 5.5 ± 0.4 |
| 500/125 mg t.i.d. (ratio 4:1) | 3.1 ± 1.3** | 4.5 ± 0.9** | NT | NT | NT | NT | NT | NT |
| Azithromycin, 1,000/500 mg o.d. | NT | NT | NT | 5.8 ± 2.0 | 7.1 ± 0.9 | 3.1 ± 1.0** | 6.6 ± 0.4 | 6.0 ± 0.6 |
| Levofloxacin, 500 mg o.d. | NT | NT | 4.2 ± 0.8* | 5.7 ± 0.8* | 4.9 ± 0.8** | 3.0 ± 1.3** | 3.5 ± 0.2** | 3.9 ± 0.8** |
See Table 4, footnote a.
The limit of detection was <1.7. *, significantly different from the control (P ≤ 0.05). **, significantly different from the control (P ≤ 0.01). NT, not tested.
Strain 16001S was tested in two separate experiments.
Against all S. pneumoniae strains tested, amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), produced a significant reduction in bacterial numbers versus the controls (P ≤ 0.01). Against S. pneumoniae strains 05010S and 16001S (amoxicillin-clavulanic acid MICs of 2/1 and 4/2 μg/ml, respectively), amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than the dose of 4:1 three times a day (P ≤ 0.05). Against the three strains with amoxicillin-clavulanic acid MICs of 4/2 μg/ml, amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than the 7:1 twice a day dose (P ≤ 0.05 against strain 16001S and P ≤ 0.01 versus the other two strains). Against strains with amoxicillin-clavulanic acid MICs of 8/4 μg/ml, amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than all the other amoxicillin-clavulanate doses tested (P ≤ 0.01).
Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective (P ≤ 0.01) than azithromycin against the two strains with an amoxicillin-clavulanic acid MIC of 4/2 μg/ml and against two of the three strains with an amoxicillin-clavulanic acid MIC of 8/4 μg/ml (P ≤ 0.01); these two strains were also macrolide resistant (azithromycin MICs of 4 to >64 μg/ml) (Table 1). There was a significant difference between azithromycin and placebo only against strain 05003S.
Levofloxacin was significantly more effective than placebo for all S. pneumoniae strains tested, although numerical reductions in bacterial counts were not as large as those recorded with amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1) (Table 5). Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than levofloxacin against all three strains with amoxicillin-clavulanic acid MICs of 4/2 μg/ml (P ≤ 0.01) and one of the three strains with MICs of 8/4 μg/ml (P ≤ 0.01). All of these strains were susceptible to levofloxacin (Table 1).
Efficacy against H. influenzae.
A summary of viable bacterial numbers recovered from the lungs of the antimicrobial-treated and control animals infected with H. influenzae can be found in Table 6.
TABLE 6.
Efficacy of test antimicrobials against two H. influenzae strains
| Treatment groupa | Mean log10H. influenzae (CFU/lung) ± SD for strain:
|
|
|---|---|---|
| H128 (β-lactamase positive) | Chesterfieldb | |
| Control | 6.4 ± 0.6 | 6.7 ± 0.6 |
| AMX/CA | ||
| 2,000/125 mg b.i.d. (ratio 16:1) | 2.0 ± 0.7** | 3.1 ± 0.9** |
| 1,000/125 mg t.i.d. (ratio 8:1) | 2.1 ± 1.0** | 3.6 ± 1.1** |
| 875/125 mg t.i.d. (ratio 7:1) | 2.8 ± 1.3** | 3.8 ± 1.3** |
| 875/125 mg b.i.d. (ratio 7:1) | 2.0 ± 0.8** | 5.1 ± 1.1** |
| Azithromycin 1,000/500 mg o.d. | 3.2 ± 1.7* | 5.8 ± 1.1 |
| Levofloxacin 500 mg o.d. | ≤1.7** | ≤1.7** |
Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), reduced bacterial numbers in the lungs significantly versus controls for both H. influenzae strains tested (P ≤ 0.01). These strains were both susceptible to amoxicillin-clavulanate, having amoxicillin-clavulanic acid MICs of ≤ 4/2 μg/ml (Table 1). The amoxicillin-clavulanate formulation of 2,000/125 mg twice a day (ratio 16:1) provided a time above the MIC of ≥49% for both H. influenzae isolates (Table 4). There was no significant difference between the amoxicillin-clavulanate formulations against H. influenzae H128, although this strain was resistant to amoxicillin (amoxicillin MIC, 32 μg/ml) (Table 1). However, the 7:1 three times a day dose was significantly less effective than levofloxacin (P ≤ 0.05). Levofloxacin was also significantly more effective than azithromycin against this strain (P ≤ 0.05). Levofloxacin was the most effective agent tested against H. influenzae Chesterfield (P ≤ 0.01). Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than azithromycin and amoxicillin-clavulanate 7:1 twice a day (P ≤ 0.01), and it demonstrated similar efficacy to amoxicillin-clavulanate 7:1 three times a day and 8:1 three times a day against this strain of H. influenzae (P > 0.05).
DISCUSSION
Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was effective against all S. pneumoniae strains tested, including those with amoxicillin-clavulanic acid MICs of up to 8/4 μg/ml, and against β-lactamase-producing and β-lactamase-negative, ampicillin-resistant H. influenzae.
There were good similarities between target human pharmacokinetic profiles and time above the MIC for the amoxicillin-clavulanate formulations tested with those achieved in the rat. This is consistent with previous studies with this model (2, 3, 29, 30). The fact that the plasma concentrations measured in the infected animals in this study closely matched uninfected pharmacokinetics and pharmacodynamics parameters suggests that infection had little effect on the kinetics of amoxicillin-clavulanate. A 3-day treatment model was chosen as other comparative studies have indicated that this is optimal for showing treatment differences. The pharmacokinetics simulated were based on mean human data, and in the clinical situation there would be some interpatient variation in these parameters. However, there was also variability between rats— and, indeed, mean values were used to calculate pharmacokinetics and pharmacodynamics parameters, correcting for some of the clinical variation.
Based on pharmacokinetics and pharmacodynamics predictions from this animal model, amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), is expected to have significant efficacy against all S. pneumoniae strains tested, with a time above the MIC based on total compound of at least the 35% required for maximal efficacy (21, 30). Protein binding of amoxicillin in the rat is similar to that in humans, and the extent of binding does not appear to be influenced by the infection. These studies demonstrate that using sustained-release technology to increase the time over which amoxicillin exposure is above the MIC is a viable strategy in optimizing pharmacokinetics and pharmacodynamics (time above the MIC) and extending antibacterial coverage to include strains with amoxicillin-clavulanic acid MICs of at least 4/2 μg/ml.
Clinical studies support these findings. In a combined analysis of five studies in community-acquired pneumonia and two studies in acute bacterial sinusitis, amoxicillin-clavulanate, 2,000/125 mg, was effective in 46 of 48 (95.8%) patients with penicillin-resistant Streptococcus pneumoniae isolated at screening and against six of seven strains with amoxicillin-clavulanic acid MICs of 4/2 μg/ml and six of seven strains with amoxicillin-clavulanic acid MICs of 8/4 μg/ml (10).
Amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), was significantly more effective than levofloxacin against four S. pneumoniae strains that were susceptible to levofloxacin and nonsusceptible to amoxicillin, including one strain with an amoxicillin MIC of 8 μg/ml. The levofloxacin MICs of the test S. pneumoniae strains are similar to the MICs reported for clinical isolates (0.5 to 1 μg/ml) and are below the susceptible breakpoint for levofloxacin (≤2 μg/ml) (15, 24). As fewer blood samples were obtained at 23 h, calculation of a confident 0 to 24 h AUC for levofloxacin was not possible. However, the pharmacokinetics profile in the rat from 0 to 8 h was very similar to the target human profile, suggesting that the dose administered was comparable to human oral dosing.
The results for levofloxacin were consistent with a previous study in this model in which levofloxacin was similar in efficacy to ciprofloxacin, trovafloxacin, and grepafloxacin but was significantly less effective than gemifloxacin against four quinolone-susceptible S. pneumoniae strains (3). Gemifloxacin reduced bacterial counts to close to or below the limit of detection for all test strains, indicating that the poor efficacy of levofloxacin in this model is probably not an artifact caused by a general underperformance of quinolones (3). Given the current breakpoints for levofloxacin, it is therefore unclear why levofloxacin has performed so poorly in these two studies. A possible explanation is that levofloxacin does not concentrate in rat epithelial lining fluid to the same extent as in humans, and thus the serum levels are less predictive, depending on the species studied. As tissue concentrations were not measured in these studies, this postulation is impossible to either confirm or deny. In clinical studies, sporadic failures with levofloxacin have been documented against quinolone-resistant pneumococci, and failures against initially susceptible strains that developed quinolone resistance while on therapy have also been reported (7, 8, 19), although no changes in levofloxacin MICs were seen in this study.
As would be expected, azithromycin had very poor activity against macrolide-resistant S. pneumoniae strains but was effective against the one macrolide-susceptible strain tested (strain 05003S). This is consistent with previous findings in this model (2, 3). Activity has been demonstrated with azithromycin in a rat model of infection against an S. pneumoniae isolate with MIC of 8 μg/ml (MefE resistance phenotype); however, the doses administered were not designed to simulate human pharmacokinetics and may have resulted in plasma or lung concentrations unlikely to be achieved with the clinical azithromycin dose (22). In the clinical setting, macrolide failures have been documented in community-acquired pneumonia against pneumococcal strains with macrolide resistance due to either efflux (mef-mediated), with MICs of 4 to 64 μg/ml, or ribosomal modification (erm-mediated), with MICs of ≥64 μg/ml (20).
The amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), formulation contains the standard 125 mg of clavulanate. Clavulanate is an irreversible inhibitor of β-lactamase, and the 125-mg dose is sufficient to protect amoxicillin from degradation (5). The findings of the current study support this, with excellent efficacy demonstrated against a β-lactamase-producing H. influenzae strain, equivalent to that of levofloxacin.
β-Lactamase-negative, ampicillin-resistant H. influenzae strains are uncommon; in the 1998 to 2000 Alexander Project, 21 of 8,523 (0.2%) H. influenzae strains isolated were β-lactamase-negative, ampicillin-resistant H. influenzae; 12 of these were from Japan (15). Although amoxicillin-clavulanate 16:1 twice a day produced a 3.6 log decrease in bacterial counts against the β-lactamase-negative, ampicillin-resistant H. influenzae strain and was more effective than the conventional 7:1 twice a day formulation, it was less effective than levofloxacin. This would be expected, given the high susceptibility of H. influenzae to quinolones.
Azithromycin was the least effective agent tested against both H. influenzae strains. According to current National Committee for Clinical Laboratory Standards breakpoints, both H. influenzae strains are susceptible to azithromycin (susceptible breakpoint, ≤4 μg/ml) (24). Previous studies in this and similar models have also reported poor efficacy for azithromycin against H. influenzae following 3 days of therapy (1-3). It should be noted that azithromycin may penetrate into human epithelial lining fluid more readily than that of the rat, which may contribute to the lack of potency in rat models of infection. A macrolide efflux mechanism has recently been described for H. influenzae, which may also explain the discrepancy between the standard breakpoints and findings in these models and clinical studies (25).
It is interesting that while amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), provided numerically greater reductions in bacterial counts than the other formulations for most of the strains tested, this did not reach significance in all strains. This suggests that there is a maximal effect and highlights the need to consider the time above the MIC.
In conclusion, the pharmacokinetically enhanced 2,000/125 mg twice a day (ratio 16:1) formulation of amoxicillin-clavulanate shows advantages over existing formulations and other antimicrobial agents in this animal model. The results of these studies suggest that the pharmacokinetically enhanced formulation of amoxicillin-clavulanate, 2,000/125 mg twice a day (ratio 16:1), may be useful for the treatment of infections caused by penicillin-resistant Streptococcus pneumoniae with amoxicillin MICs of at least 4 μg/ml. It also demonstrated robust utility against both β-lactamase-positive and -negative strains of H. influenzae.
Acknowledgments
We acknowledge the help of Heather Karr in the preparation of the manuscript.
REFERENCES
- 1.Alder, J. D., P. J. Ewing, A. M. Nilius, M. Mitten, A. Tovcimak, A. Oleksijew, K. Jarvis, L. Paige, and S. K. T. Tanaka. 1998. Dynamics of clarithromycin and azithromycin efficacies against experimental Haemophilus influenzae pulmonary infection. Antimicrob. Agents Chemother. 42:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, V., C. E. Thorburn, S. J. Knott, and G. Woodnutt. 1998. Bacteriological efficacies of three macrolides compared with those of amoxicillin-clavulanate against Streptococcus pneumoniae and Haemophilus influenzae. Antimicrob. Agents Chemother. 42:3193-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, V., R. Page, J. Satterfield, C. Singley, R. Straub, and G. Woodnutt. 2000. Comparative in vivo activity of gemifloxacin in a rat model of respiratory tract infection. J. Antimicrob. Chemother. 45(Suppl. 1):79-85. [DOI] [PubMed] [Google Scholar]
- 4.Chien, S.-C., M. C. Rogge, L. G. Gisclon, C. Curtin, F. Wong, J. Natarajan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. T. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral and intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, C. E., B. Slocombe, and A. R. White. 1990. Effect of low concentrations of clavulanic acid on the in-vitro activity of amoxycillin against β-lactamase-producing Branhamella catarrhalis and Haemophilus influenzae. J. Antimicrob. Chemother. 26:371-380. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 8.Empey, P. E., H. R. Jennings, A. C. Thornton, R. P. Rapp, and M. E. Evans. 2001. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann. Pharmacother. 35:687-690. [DOI] [PubMed] [Google Scholar]
- 9.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25:73-82. [DOI] [PubMed] [Google Scholar]
- 10.Garau, J., M. R. Jacobs, B. Wynne, E. Berkowitz, and M. Twynholm. 2003. Pharmacokinetically enhanced amoxicillin/clavulanate (AMX/CA) 2,000/125 mg in the treatment of community-acquired pneumonia and acute bacterial sinusitis (ABS) caused by Streptococcus pneumoniae, abstr. L-1382, p. 422. In Program and abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.
- 11.Hoberman, A., R. Dagan, A. Rosenblut, E. Leibovitz, A. Huff, and B. Wynne. 2003. Extra-strength amoxicillin-clavulanate (A/C-ES) versus azithromycin (AZI) for acute otitis media (AOM) in children, abstr. G-459, p. 279. In Program and abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.
- 12.Huff, J., A. White, E. Power, J. Weil, and G. R. Mera. 2002. 10-year trends in penicillin- and erythromycin-resistant Streptococcus pneumoniae for 5 European countries and the USA: the Alexander Project, abstr. C2-1624, p. 108. In Program and abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.
- 13.Jackson, D., D. L. Cooper, R. Horton, P. F. Langley, D. H. Staniforth, and J. A. Sutton. 1983. Absorption pharmacokinetic and metabolic studies with augmentin, p. 88-101. In E. A. P. Croydon and M. F. Michel (ed.), Augmentin: clavulanate-potentiated amoxicillin. Proceedings of the European Symposium, June 1982. Excerpta Medica, Scheveningen, The Netherlands.
- 14.Jacobs, M. R. 2003. How can we predict bacterial eradication? Int. J. Infect. Dis. 7(Suppl. 1):S13-S20. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, M. R., D. Felmingham, P. C. Appelbaum, and R. N. Grüneberg. 2003. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J. Antimicrob. Chemother. 52:229-246. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, D. M., H. S. Sader, T. R. Fritsche, D. J. Biedenbach, and R. N. Jones. 2003. Susceptibility trends of Haemophilus influenzae and Moraxella catarrhalis against orally administered antimicrobial agents: five-year report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 47:373-376. [DOI] [PubMed] [Google Scholar]
- 17.Jones, M., R. Blosser-Middleton, I. Critchley, J. Karlowsky, C. Thornsberry, and D. Sahm. 2003. In vitro susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: a European multicenter study during 2000-2001. Clin. Microbiol. Infect. 9:590-599. [DOI] [PubMed] [Google Scholar]
- 18.Kaye, C. M., A. Allen, S. Perry, M. McDonagh, M. Davy, K. Storm, N. Bird, and O. Dewit. 2001. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin. Ther. 23:578-584. [DOI] [PubMed] [Google Scholar]
- 19.Kays, M. B., D. W. Smith, M. E. Wack, and G. A. Denys. 2002. Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy 22:395-399. [DOI] [PubMed] [Google Scholar]
- 20.Lonks, J. R., J. Garau, L. Gomez, M. Xercavins, A. Ochoa de Echaguen, I. F. Gareen, P. T. Reiss, and A. A. Medeiros. 2002. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 35:556-564. [DOI] [PubMed] [Google Scholar]
- 21.MacGowan, A. P. 2004.. Elements of design: the knowledge on which we build. Clin. Microbiol. Infect. 10(Suppl. 2):6-11. [DOI] [PubMed]
- 22.Mitten, M. J., J. Meulbroek, M. Nukkala, L. Paige, K. Jarvis, A. Oleksijew, A. Tovcimak, L. Hernandez, J. D. Alder, P. Ewing, Y. S. Or, Z. Ma, A. M. Nilius, K. Mollison, and R. K. Flamm. 2001. Efficacies of ABT-773, a new ketolide, against experimental bacterial infections. Antimicrob. Agents Chemother. 45:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing, document M100-S10, 10th informational supplement. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing, doc. M100-S13, 13th informational supplement. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waites, K., and S. Brown. 2003. Antimicrobial resistance among isolates of respiratory tract infection pathogens from the southern United States: data from the PROTEKT US surveillance program 2000/2001. South. Med. J. 96:974-985. [DOI] [PubMed] [Google Scholar]
- 27.White, A. R., C. Kaye, J. A. Poupard, R. Pypstra, G. Woodnutt, and B. Wynne. 2004. Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent. J. Antimicrob. Chemother. 53(Suppl. 1):I3-I20. [DOI] [PubMed] [Google Scholar]
- 28.Woodnutt, G. 2000. Pharmacodynamics to combat resistance. J. Antimicrob. Chemother. 46(Suppl. T1):25-31. [DOI] [PubMed] [Google Scholar]
- 29.Woodnutt, G., and V. Berry. 1999. Efficacy of high-dose amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodnutt, G., and V. Berry. 1999. Two pharmacodynamic models for assessing the efficacy of amoxicillin-clavulanate against experimental respiratory infections caused by strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodnutt, G., V. Berry, and J. Bryant. 1996. Evaluation of reduced dosing schedule of amoxicillin (AMX) using two pharmacodynamic models, abstr. A40, p. 8. In 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C.