Abstract
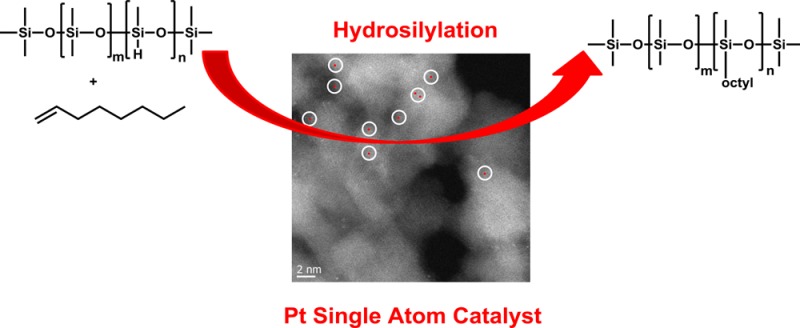
Catalytic hydrosilylation represents a straightforward and atom-efficient methodology for the creation of C–Si bonds. In general, the application of homogeneous platinum complexes prevails in industry and academia. Herein, we describe the first heterogeneous single atom catalysts (SACs), which are conveniently prepared by decorating alumina nanorods with platinum atoms. The resulting stable material efficiently catalyzes hydrosilylation of industrially relevant olefins with high TON (≈105). A variety of substrates is selectively hydrosilylated including compounds with sensitive reducible and other functional groups (N, B, F, Cl). The single atom based catalyst shows significantly higher activity compared to related Pt nanoparticles.
Short abstract
We present the first heterogeneous Pt single atom catalysts (SACs) supported on alumina nanorods, which show high catalytic activity for the hydrosilylation of industrially relevant olefins (TON ≈ 105) and stability compared to related nanoparticles.
Olefin hydrosilylation is an important process to form alkylsilanes by catalytic addition of Si–H bonds to olefins.1,2 The resulting products find widespread application in the manufacture of silicone-based aerogels, surfactants, release coatings, lubricants, and adhesives.3,4 Furthermore, organosilicon compounds are of interest for the design of novel catalysts, defense materials and life science products. In general, catalytic hydrosilylations are performed using molecularly defined (pre)catalysts containing mainly Pt,5,6 but also Ru,7,8 Pd,9 and Rh.10,11 In fact, some of these reactions constitute the largest-scale examples of homogeneous catalysis with noble metals. Nowadays, platinum-based complexes such as Speier’s6 and Karstedt’s catalysts formed by reaction of 1,3-divinyl-1,1,3,3-tetramethyldisiloxane with chloroplatinic acid and PtCl2(cyclooctadiene) are the most extensively used industrial catalysts, despite the cost of the precious metal. A disadvantage of these platinum catalysts in some applications is that they are trapped in the product and cannot be easily recovered due to the viscous morphology of them. Notably, catalyst costs might account for up to 30% of the cost of silicones.12
Hence, the development of less expensive catalysts is an important and ongoing goal for industrial and academic researchers. Apart from new developments based on common hydrosilylation metals,13,14 in recent years the use of non-noble metals attracted significant interest. In this respect, Chirik and co-workers15 showed that well-defined iron complexes are highly active for the selective anti-Markovnikov addition of silanes to alkenes under mild conditions. Since the original report in 2012, a series of catalysts based on Fe,15−17 Co,12,17−20 Ni,21−23 and B24 were successfully explored in hydrosilylation reactions.
An alternative approach to overcome the drawbacks (vide supra) is the development of highly active supported catalysts. However, most of the known heterogeneous materials are rather inactive. For example, Béland and Pagliaro25 reported small Pt(0) nanoparticles (4–6 nm) encapsulated in a sol–gel derived porous matrix of methyltriethoxysilane for the hydrosilylation of different olefins with triethoxysilane in the presence of 1 mol % catalyst. Simple PtO2 was studied in the hydrosilylation of heptamethyltrisiloxane with n-octene, which revealed that initially Pt(IV) is reduced during the reaction to form the active sites.26 So far, only a silica-supported Karstedt Pt-type catalyst and crystalline Pt nanoparticles embedded into the walls of a mesostructured silica framework showed high catalytic activity on this reaction.27,28 Thus, to the best of our knowledge general heterogeneous Pt-based catalysts with broad substrate scope, high regioselectivity, and high activity comparable to homogeneous systems do not exist.
Recently, heterogeneous catalysts with atomically dispersed active sites were developed as model systems to understand as well as to design such materials at the molecular level and build a bridge between the catalysis subdisciplines.1,21,27,29 In addition to recycling advantages for liquid products, such heterogeneous single atom catalysts (SACs) may display improved activity and selectivity compared to catalysts containing bulk metal or metal nanoparticles. Indeed, SACs based on Pt,22,30−36 Pd,37,38 Ir,39 Rh,40 Co,41−43 and Fe44 were presented in the past 5 years and displayed interesting activity, e.g., in hydrogenations and CO oxidation. Inspired by these works, we were curious if reducing the size of Pt nanoparticles to single atoms would create improved catalysts for alkene hydrosilylation chemistry. Herein, we present the first heterogeneous SAC for the selective hydrosilylation of all kinds of terminal olefins and show that supported Pt on the surface of specific aluminum oxide nanorods exhibits excellent activity.
To design SACs, it is crucial to select a support which allows creation of defined metal sites on the surface and inhibits the aggregation of the metal atoms toward nanoparticles. In this context, the use of an oxidic support is preferred due to the stronger interaction of unsaturated surface centers with the isolated metal species. Indeed, it has been demonstrated that ZnO, Fe2O3, Al2O3, and TiO2 stabilize single noble metal atoms.27,39,40,45 For our initial catalyst preparation, Al2O3 nanorods (NR-Al2O3)46 were selected as carrier because of their high surface area (SBET = 330 m2/g) and controlled pore size (≈12 nm). Deposition of Pt (270 ppm, measured by ICP-AES) onto this support was performed by two different methods, namely, reductive precipitation (RP) and impregnation (IP) (see the Supporting Information).
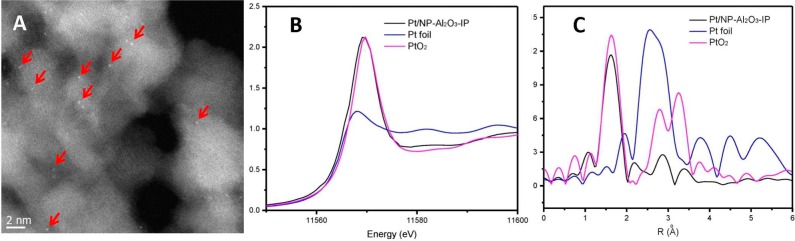
When impregnation (IP) was used to deposit Pt on NR-Al2O3, TEM images of the resulting material (Pt/NR-Al2O3-IP) showed the rodlike morphology of the NR-Al2O3 support (Figure S1) and single Pt atoms (white dots in Figure 1A), which are formed exclusively. For comparison, reductive precipitation using NaBH4 led to the formation of uniform Pt nanoparticles (NPs) of 2–5 nm on NR-Al2O3 (Figures S2 and S3). The valence state and environment of Pt were analyzed by X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy at the Pt LIII edge. The white line peak of fresh Pt/NR-Al2O3-IP in the XANES spectrum is similar to the PtO2 reference (Figure 1B), indicating the presence of oxidic platinum centers.
Figure 1.
HRSTEM images of Pt/NR-Al2O3-IP (A), Pt LIII edge XANES for Pt/NR-Al2O3-IP (B), and k3-weighted Fourier transform of Pt LIII edge EXAFS for Pt/NR-Al2O3-IP (C).
To further verify that Pt was dispersed atomically on the support, EXAFS spectra were recorded (Figure 1C and Table S2). The strong peak between 1 and 2 Å is due to a Pt–O shell. No peak in the region of 2–3 Å, which is characteristic for a Pt–Pt shell, is observed, thus confirming the presence of isolated Pt atoms only. The Pt–O coordination number in Pt/NR-Al2O3-IP was ≈5.0 considering the fitting error of EXAFS analysis (ca. 20%).
To evaluate the activity of the prepared single atom Pt catalyst and related materials, the hydrosilylation reaction of 1-octene and diethoxymethylsilane under solvent free conditions was chosen as a benchmark test. Obviously, Al2O3 nanorods were not active in this reaction (Table 1, entry 1). Platinum nanoparticles (Pt/NR-Al2O3-RP catalysts prepared by the reductive precipitation) showed low activity (entry 2). In contrast, the single atom platinum catalyst (SAC) prepared by the impregnation precipitation method showed under optimal conditions high activity achieving the desired product (3a) in 95% yield with 2.06 × 105 TON (TON = turnover number, moles of product per mole of catalyst; entries 3 and 4). The benchmark reaction occurred with high anti-Markovnikov selectivity and low alkene isomerization compared with previously reported heterogeneous27 and homogeneous6 platinum catalysts. Notably, a commercial Pt/Al2O3 catalyst showed no activity at all (entry 7). Meanwhile, the material prepared using commercial Al2O3 instead of Al2O3 nanorods showed significantly lower activity (entry 8). Platinum immobilized on SiO2, CeO2, carbon black (Vulcan XC72), MnO, and ZnO2 formed catalysts, which gave the desired product in no or only low yields (0–26%; entries 9–13). TEM images for Pt/CeO2-IP, Pt/SiO2-IP, and Pt/C showed the formation of Pt nanoparticles on the surface of these materials. These results clearly indicate that the NR-Al2O3 plays a crucial role for the formation of single platinum sites with high activity for the hydrosilylation process. Notably, in terms of activity our Pt/NR-Al2O3-IP is at least comparable to homogeneous state-of-the-art catalysts such as H2PtCl6 and Karstedt’s system (entries 14 and 15).
Table 1. Hydrosilylation of 1-Octene in the Presence of Different Pt Catalystsa.
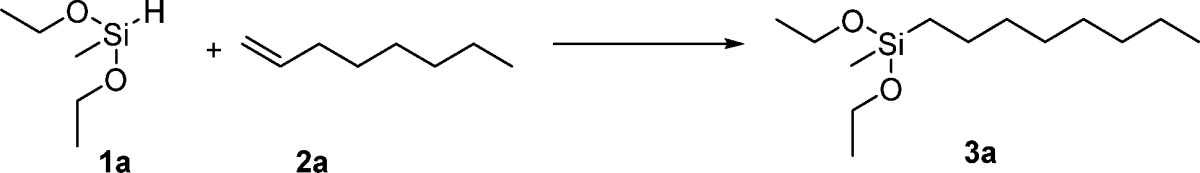
| entry | catalyst | time (h) | yield (%) | TON (104)b | TOF (104 h–1)b |
|---|---|---|---|---|---|
| 1 | NR-Al2O3 | 2 | 0 | 0 | 0 |
| 2 | Pt/NR-Al2O3-RPc | 2 | 33 | 7.1 | 3.6 |
| 3 | Pt/NR-Al2O3-IP | 2 | 95 | 20.6 | 10.3 |
| 4 | Pt/NR-Al2O3-IPd | 2 | 73 | 31.6 | 15.8 |
| 5 | Pt/NR-Al2O3-IPe | 2 | 40 | 8.7 | 4.3 |
| 6 | Pt/NR-Al2O3-IP | 1 | 41 | 8.9 | 4.5 |
| 7 | Pt/Al2O3f | 2 | 0 | 0 | 0 |
| 8 | Pt/Al2O3-IPg | 2 | 57 | 12.2 | 6.1 |
| 9 | Pt/CeO2-IP | 2 | 6 | 1.3 | 0.7 |
| 10 | Pt/SiO2-IP | 2 | 26 | 5.6 | 2.8 |
| 11 | Pt/C-IP | 2 | 6 | 2.6 | 1.3 |
| 12 | Pt/MnO-IP | 2 | 0 | 0 | 0 |
| 13 | Pt/ZnO2-IP | 2 | 0 | 0 | 0 |
| 14 | H2PtCl6 | 2 | 46 | 10 | 5.0 |
| 15 | Karstedt catalyst | 2 | 96 | 20.6 | 10.3 |
Reaction conditions: 3 mmol of olefin, 3 mmol of silane, 2 h, N2 (10 bar), 100 °C, isolated yields. IP = impregnation precipitation.
Calculated for the total Pt content.
RP = reductive precipitation.
30 mmol of olefin, 30 mmol of silane, 5.0 × 10–6 mmol of Pt.
100 °C.
Commercial 5 wt % Pt/Al2O3, 30 mmol of olefin, 30 mmol of silane, 1.02 × 10–5 mmol of Pt.
Commercial Al2O3. TON = mol product/mol Pt. TOF = TON/reaction time.
Next, we investigated the stability and recyclability of selected catalysts, which are important factors for potential applications in industrial processes. For this purpose, we compared the Pt-SAC and supported Pt-NPs, which both were recycled by centrifugation and reused six times without any reactivation. Gratifyingly, when Pt/NR-Al2O3-IP was used after the sixth recycling, still 92% yield (total TONs of 1.18 × 106) was achieved (Figure 3). In contrast, a significantly lower activity was observed in the presence of Pt/NR-Al2O3-RP, where almost no product was obtained after the third recycling. These results demonstrate the higher activity of Pt-SAC—which can be anticipated due to the better dispersion of active catalyst centers—compared to Pt nanoparticles. However, contrary to expectations, the former catalyst also showed an improved stability at least on a shorter time scale.
Figure 3.
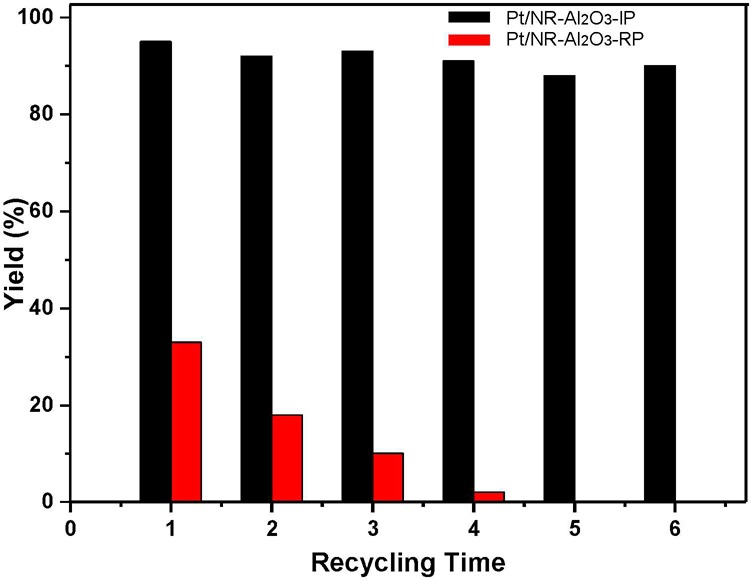
Recyclability of Pt/NR-Al2O3-IP and Pt/NR-Al2O3-RP in the hydrosilylation of 1a and 2a.
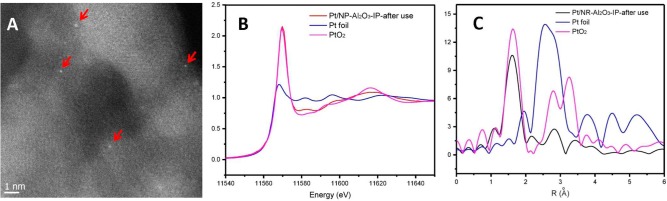
To prove the stability of the SAC, a sample of the recycled material (after 6 runs) was characterized by STEM, XANES, and EXAFS and compared to the fresh one. As shown in Figure 2, no significant differences were observed. For example, the Pt–O coordination number in fresh Pt/NR-Al2O3-IP was ≈5.0 and did not change much after catalysis (Table S2). Hence, we conclude that single Pt atoms on NR-Al2O3 are stable during the hydrosilylation reactions.
Figure 2.
HRSTEM images of Pt/NR-Al2O3-IP after use (A), Pt LIII edge XANES for Pt/NR-Al2O3-IP after use (B), and k3-weighted Fourier transform of Pt LIII edge EXAFS for Pt/NR-Al2O3-IP after use (C).
To demonstrate the general synthetic applicability of this single atom catalyst, we explored the hydrosilylation of 1-octene with a variety of tertiary silanes. In all cases we used the active platinum in neat olefin and obtained excellent to quantitative yields of the desired linear products. Hence, hydrosilylations with dimethylphenylsilane (1b) as well as with multisilicon containing compounds proceeded smoothly (Table 2). Reaction with 1c is of special interest because the hydrosilylated products have been widely applied in both inorganic chemistry and organic syntheses.2 In addition, hydrosilylations of the benchmark alkene with silanes containing two Si–H bonds were accomplished, and again, excellent yields were obtained using 1d–1g as starting materials (entries 4–7). In all cases, no side products were observed and the single atom Pt possessed catalytic performance with high turnover numbers.
Table 2. Single Atom Pt Catalyzed Selective Hydrosilylation of 1-Octene with Various Tertiary Silanesa.
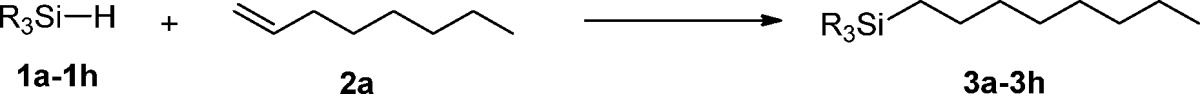
| entry | substrate | T (°C) | yield (%) | TON (105) |
|---|---|---|---|---|
| 1 | Me(EtO)2SiH (1a) | 100 | 95 | 2.06 |
| 2 | PhMe2SiH (1b) | 120 | 94 | 2.04 |
| 3 | HSi(Me)2OSi(Me)3 (1c) | 100 | 95 | 2.06 |
| 4 | HSi(Me)2O(Me)2SiH (1d) | 100 | 94b | 1.36 |
| 5 | HSi(Me)2OSi(Me)2O(Me)2SiH (1e) | 120 | 93b | 1.35 |
| 6 | HSi(Me)NH(Me)2SiH (1f) | 120 | 92b | 1.33 |
| 7 | TDMSS (1g) | 120 | 96c | 0.70 |
Reaction conditions: 3 mmol of 1-octene, 3 mmol of silane, 2 h, 10 mg of Pt/NR-Al2O3-IP, N2 (10 bar), isolated yields. TON = mol product/mol Pt.
4 mmol of 1-octene, 2 mmol of silane.
4 mmol of 1-octene, 1 mmol of silane, TDMSS = tetrakis(dimethylsilyloxy)silane. TON = mol product/mol Pt.
Furthermore, we tested the hydrosilylation of diverse alkenes using the Pt-SAC. Simple linear and branched aliphatic olefins gave very good yields of the corresponding silylated products (Table 3, entries 1 and 2). More challenging and sensitive oxygen-containing allylic compounds, such as cyclohexyl vinyl ether (2d), 1,2-epoxy-4-vinylcyclohexane (2e), and allyl glycidyl ether (2f), were also hydrosilylated with the single atom catalyst to form the corresponding products in high yields (entries 3–5). Notably, the catalyst system even tolerates epoxy rings in allyl glycidyl ether, which is susceptible to various ring-opening reactions. Furthermore, the reaction of 6-chloro-1-hexene (2g) with 1a yielded the desired product and no dehalogenated side product was observed (entry 6). Hydrosilylations of allylbenzene (2h), allyl phenyl ether (2i), and allyl benzyl ether (2j) with 1a also afforded the corresponding hydrosilylated products in quantitative isolated yields with high selectivity (entries 7–9).
Table 3. Single Atom Pt Catalyzed Selective Hydrosilylation of Various Olefins with 1aa.
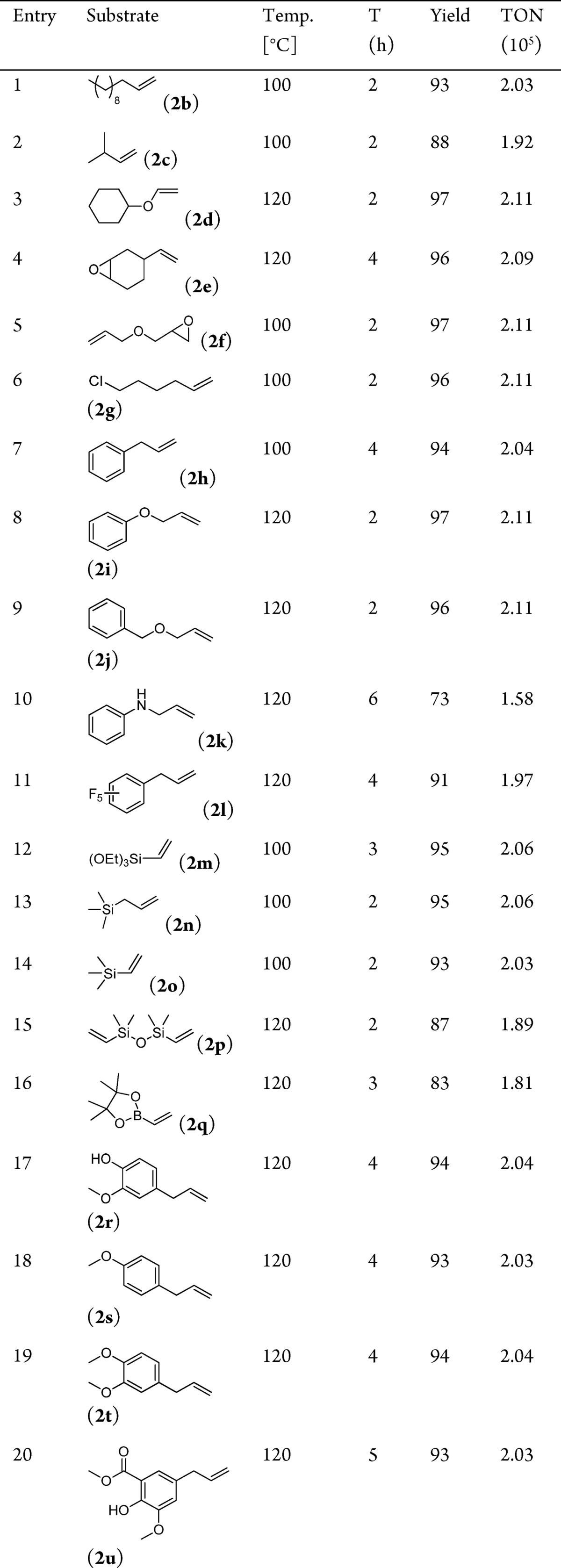
Reaction conditions: 3 mmol of olefin, 3 mmol of silane, 2 h, 10 mgPt/NR-Al2O3-IP, N2 (10 bar), isolated yields. TON = mol product/mol Pt.
N-Allylaniline (2k) and allylpentafluorobenzene (2l) were hydrosilylated smoothly to the desired products in 73% and 91% yields, respectively (entries 10 and 11). Moreover, we performed the hydrosilylation of 1a with vinylsiloxanes such as triethoxyvinylsilane (2m), allyltrimethylsilane (2n), vinyltrimethylsilane (2o), and 1,3-divinyltetramethyldisiloxane (2p) and formed the corresponding products in high yields selectively (entries 12–15). For the first time, the boron-containing alkene (2q) was transformed successfully to the hydrosilylated product in 83% yield, which might be interesting for applications in material sciences (entry 16). Moreover, the single atom catalyst showed high activity for the hydrosilylation of renewable substrates with 1a. In this respect, lignin-derived alkenes such as eugenol (2r), 4-allylanisole (2s), 4-allyl-1,2-dimethoxybenzene (2t), and methyl 5-allyl-3-methoxysalicylate (2u) reacted in excellent yields (entries 17–20).
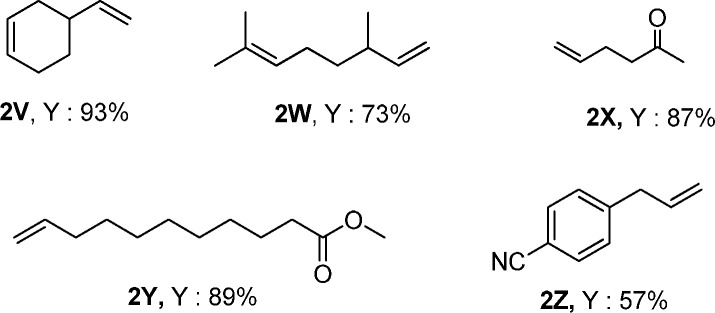
In general, hydrosilylation reactions allow controlling the hydrophilicity/hydrophobicity of a given material/surface. In this respect, such transformations offer interesting possibilities for innovations and find increasing interest for the development of consumer and life science products such as elastic skin.47,48 Hence, selective Si–C bond formation in the presence of different functional groups is of increasing concern. For this purpose, the chemoselectivity of the single atom catalyst was tested with substrates including other reducible groups. As shown in Scheme 1, the hydrosilylation of 4-vinyl-1-cyclohexene (2v) took place selectively at the terminal position without hydrosilylation of the internal cyclohexyl group or hydrogenation of the C=C bonds. Furthermore, (+)-β-citronellene (2w) underwent selective hydrosilylation retaining the internal C=C bond in the substrate. In the case of 5-hexen-2-one (2x), the desired product was obtained in 87% yield without touching the ketone, which easily occurred in the presence of homogeneous Pt and other metal catalysts. In addition, the single site Pt catalyst exhibited good activity in the hydrosilylation of an unsaturated ester affording an ester group tagged silane in 89% yield. Finally, the reaction of 4-allylbenzonitrile (2z) with 1a gave the corresponding nitrile in 57%.
Scheme 1. Selective Hydrosilylation of Alkenes with Other Functional Groups.
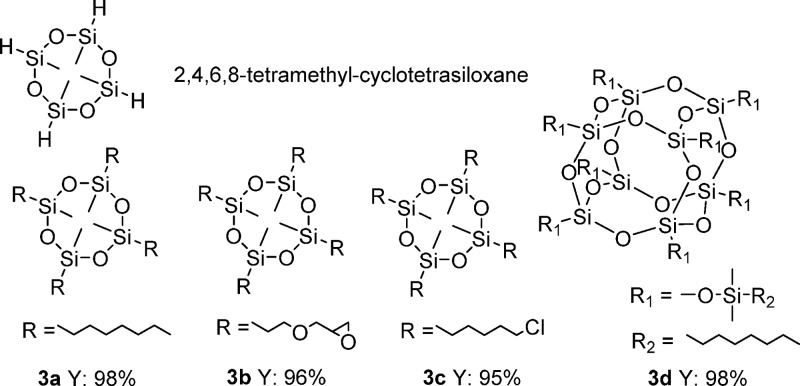
Among the industrially applied silanes, there is a substantial interest in the reaction of 2,4,6,8-tetramethylcyclotetrasiloxane: The derived products find widespread applications in cosmetic ingredients due to their excellent spreading, lubrication properties, and unique volatility characteristics. Thus, the hydrosilylation of different alkenes such as 1-octene, allyl glycidyl ether, and 6-chloro-1-hexene with 2,4,6,8-tetramethylcyclotetrasiloxane was done in the presence of the single atom Pt catalyst (Scheme 2).
Scheme 2. Selective Hydrosilylation of Cyclotetrasiloxane and POSS Derivatives.
To our delight, in all experiments the products were obtained in good yields as colorless fluids with high selectivity and low alkene isomerization. Furthermore, polyhedral oligomeric silsesquioxanes (POSS) have attracted attention as precursors to ceramic materials and nanocomposites. Traditionally, substituted POSS derivatives are prepared by hydrolysis of organotrichlorosilanes where large amounts of HCl are formed. Complementarily, the cross-coupling of 1-octene and POSS-SiH proceeded in the presence of Pt-SAC with excellent yield.
To demonstrate the utility of our catalyst, two industrially relevant reactions of polysilanes with olefins were studied. The resulting materials exhibit distinctive optical and electrical properties, and some of them constitute important precursors for novel materials such as nanostructured silicon carbide.49Hence, the hydrosilylation of 1-octene with PMHS and PL6020 proceeded smoothly in the presence of the single atom Pt catalyst (Scheme 3). Finally, the cross-coupling of PL6020 and vinyl-trimethylsilane gave 5c, which represents an example for cross-linked silicone polymers.
Scheme 3. Cross-Linked Silicone Fluids Prepared Using Single Atom Pt Catalyst.

270 mg of PMHS (Sigma-Aldrich, MFCD00084478), 3 mmol of 1-octene. 270 mg of SL6020 (Sigma-Aldrich, MFCD01325013), 2 mmol of olefin.
In summary, we present the first heterogeneous single atom Pt catalyst, which allows for efficient and selective Si–C bond formation. Contrary to all previously known heterogenized catalysts for hydrosilylation, the prepared Pt/NR-Al2O3-IP offers high activity as well as wide substrate scope and excellent chemoselectivity. In a more general sense, this example demonstrates the superiority of SAC compared to more common heterogenized nanoparticles.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00105.
Extended data about optimization of reaction conditions, preparation and characterization of catalyst, and synthesis and spectroscopic data of products (PDF)
Author Contributions
M.B. designed the method and coordinated the project. X.C. and K.J. conducted the experiments and developed the project. We thank S.W. for his kind support on the preparation of SAC. X.D., C.K., and M.-M.P. helped to characterize the SACs. We thank F.S. and A.B. for their kind suggestions and improvements on this project. M.B., X.C., and K.J. wrote the manuscript.
This work is supported by the state of Mecklenburg-Vorpommern.
The authors declare no competing financial interest.
Supplementary Material
References
- Marciniec B. Catalysis by transition metal complexes of alkene silylation - recent progress and mechanistic implications. Coord. Chem. Rev. 2005, 249, 2374–2390. 10.1016/j.ccr.2005.02.025. [DOI] [Google Scholar]
- Marciniec B.; Gulinski J.; Urbaniak W.; Kornetka Z. W.. Hydrosilylation; Marciniec B., Ed.; Pergamon: Oxford, U.K., 1992. [Google Scholar]
- Ojima I. In The Chemistry of Organic Silicon Compounds; Patai S., Rappoport Z., Eds.; Wiley: Chichester, U.K., 1989; Vol. 1. [Google Scholar]
- Hill R. M. In Silicone Surfactants, Surfactants Science Series; Hill R. M., Ed.; Marcel Dekker: New York, 1999; Vol. 86, p 1–48. [Google Scholar]
- Meister T. K.; Kuck J. W.; Riener K.; Pothig A.; Herrmann W. A.; Kuhn F. E. Decoding catalytic activity of platinum carbene hydrosilylation catalysts. J. Catal. 2016, 337, 157–166. 10.1016/j.jcat.2016.01.032. [DOI] [Google Scholar]
- Speier J. L. Homogeneous Catalysis of Hydrosilation by Transition Metals. Adv. Organomet. Chem. 1979, 17, 407–447. 10.1016/S0065-3055(08)60328-7. [DOI] [Google Scholar]
- Tuttle T.; Wang D. Q.; Thiel W.; Kohler J.; Hofmann M.; Weis J. Ruthenium based catalysts for olefin hydrosilylation: dichloro(p-cymene)ruthenium and related complexes. Dalton Trans. 2009, 5894–5901. 10.1039/b820115c. [DOI] [PubMed] [Google Scholar]
- Bokka A.; Hua Y. D.; Berlin A. S.; Jeon J. Mechanistic Insights into Grubbs-Type Ruthenium-Complex-Catalyzed Intramolecular Alkene Hydrosilylation: Direct sigma-Bond Metathesis in the Initial Stage of Hydrosilylation. ACS Catal. 2015, 5, 3189–3195. 10.1021/acscatal.5b00431. [DOI] [Google Scholar]
- Komine N.; Abe M.; Suda R.; Hirano M. Markovnikov-Selective Hydrosilylation of Electron-Deficient Alkenes with Arylsilanes Catalyzed by Mono(phosphine)palladium(0). Organometallics 2015, 34, 432–437. 10.1021/om500964g. [DOI] [Google Scholar]
- Bai Y.; Zhang F. X.; Li J. Y.; Xu Y. S.; Peng J. J.; Xiao W. J. Application of polyethyleneglycol (PEG) functionalized ionic liquids for the rhodium-catalyzed hydrosilylation reaction of alkenes. J. Organomet. Chem. 2015, 794, 65–69. 10.1016/j.jorganchem.2015.06.024. [DOI] [Google Scholar]
- Wu Y.; Karttunen V. A.; Parker S.; Genest A.; Rosch N. Olefin Hydrosilylation Catalyzed by a Bis-N-Heterocyclic Carbene Rhodium Complex. A Density Functional Theory Study. Organometallics 2013, 32, 2363–2372. 10.1021/om301236n. [DOI] [Google Scholar]
- Schuster C. H.; Diao T. N.; Pappas I.; Chirik P. J. Bench-Stable, Substrate-Activated Cobalt Carboxylate Pre-Catalysts for Alkene Hydrosilylation with Tertiary Silanes. ACS Catal. 2016, 6, 2632–2636. 10.1021/acscatal.6b00304. [DOI] [Google Scholar]
- Marko I. E.; Sterin S.; Buisine O.; Mignani G.; Branlard P.; Tinant B.; Declercq J. P. Selective and efficient platinum(0)-carbene complexes as hydrosilylation catalysts. Science 2002, 298, 204–206. 10.1126/science.1073338. [DOI] [PubMed] [Google Scholar]
- Du X. Y.; Huang Z. Advances in Base-Metal-Catalyzed Alkene Hydrosilylation. ACS Catal. 2017, 7, 1227–1243. 10.1021/acscatal.6b02990. [DOI] [Google Scholar]
- Tondreau A. M.; Atienza C. C. H.; Weller K. J.; Nye S. A.; Lewis K. M.; Delis J. G. P.; Chirik P. J. Iron Catalysts for Selective Anti-Markovnikov Alkene Hydrosilylation Using Tertiary Silanes. Science 2012, 335, 567–570. 10.1126/science.1214451. [DOI] [PubMed] [Google Scholar]
- Bart S. C.; Lobkovsky E.; Chirik P. J. Preparation and molecular and electronic structures of iron(0) dinitrogen and silane complexes and their application to catalytic hydrogenation and hydrosilation. J. Am. Chem. Soc. 2004, 126, 13794–13807. 10.1021/ja046753t. [DOI] [PubMed] [Google Scholar]
- Noda D.; Tahara A.; Sunada Y.; Nagashima H. Non-Precious-Metal Catalytic Systems Involving Iron or Cobalt Carboxylates and Alkyl Isocyanides for Hydrosilylation of Alkenes with Hydrosiloxanes. J. Am. Chem. Soc. 2016, 138, 2480–2483. 10.1021/jacs.5b11311. [DOI] [PubMed] [Google Scholar]
- Chen C.; Hecht M. B.; Kavara A.; Brennessel W. W.; Mercado B. Q.; Weix D. J.; Holland P. L. Rapid, Regioconvergent, Solvent-Free Alkene Hydrosilylation with a Cobalt Catalyst. J. Am. Chem. Soc. 2015, 137, 13244–13247. 10.1021/jacs.5b08611. [DOI] [PubMed] [Google Scholar]
- Mo Z.; Xiao J.; Gao Y.; Deng L. Regio- and Stereoselective Hydrosilylation of Alkynes Catalyzed by Three-Coordinate Cobalt(I) Alkyl and Silyl Complexes. J. Am. Chem. Soc. 2014, 136, 17414–17417. 10.1021/ja510924v. [DOI] [PubMed] [Google Scholar]
- Sun J.; Deng L. Cobalt Complex-Catalyzed Hydrosilylation of Alkenes and Alkynes. ACS Catal. 2016, 6, 290–300. 10.1021/acscatal.5b02308. [DOI] [Google Scholar]
- Yang X. F.; Wang A. Q.; Qiao B. T.; Li J.; Liu J. Y.; Zhang T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- Liu J. L.; Lucci F. R.; Yang M.; Lee S.; Marcinkowski M. D.; Therrien A. J.; Williams C. T.; Sykes E. C. H.; Flytzani-Stephanopoulos M. Tackling CO Poisoning with Single-Atom Alloy Catalysts. J. Am. Chem. Soc. 2016, 138, 6396–6399. 10.1021/jacs.6b03339. [DOI] [PubMed] [Google Scholar]
- Buslov I.; Song F.; Hu X. L. An Easily Accessed Nickel Nanoparticle Catalyst for Alkene Hydrosilylation with Tertiary Silanes. Angew. Chem., Int. Ed. 2016, 55, 12295–12299. 10.1002/anie.201606832. [DOI] [PubMed] [Google Scholar]
- Simonneau A.; Oestreich M. Formal SiH4 chemistry using stable and easy-to-handle surrogates. Nat. Chem. 2015, 7, 816–822. 10.1038/nchem.2329. [DOI] [PubMed] [Google Scholar]
- Ciriminna R.; Pandarus V.; Gingras G.; Beland F.; Pagliaro M. Closing the Organosilicon Synthetic Cycle: Efficient Heterogeneous Hydrosilylation of Alkenes over SiliaCat Pt(0). ACS Sustainable Chem. Eng. 2013, 1, 249–253. 10.1021/sc3001096. [DOI] [Google Scholar]
- Putzien S.; Louis E.; Nuyken O.; Kuhn F. E. PtO2 as a ″self-dosing″ hydrosilylation catalyst. Catal. Sci. Technol. 2012, 2, 725–729. 10.1039/C2CY00367H. [DOI] [Google Scholar]
- Miao Q. J.; Fang Z. P.; Cai G. P. Silica-supported Karstedt-type catalyst for hydrosilylation reactions. Catal. Commun. 2003, 4, 637–639. 10.1016/j.catcom.2003.10.006. [DOI] [Google Scholar]
- Galeandro-Diamant T.; Sayah R.; Zanota M.; Marrot S.; Veyre L.; Thieuleux C.; Meille V. Pt nanoparticles immobilized in mesostructured silica: a non-leaching catalyst for 1-octene hydrosilylation. Chem. Commun. 2017, 53, 2962–2965. 10.1039/C6CC10154B. [DOI] [PubMed] [Google Scholar]
- Liu J. Y. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. 10.1021/acscatal.6b01534. [DOI] [Google Scholar]
- Dvorak F.; Camellone M. F.; Tovt A.; Tran N. D.; Negreiros F. R.; Vorokhta M.; Skala T.; Matolinova I.; Myslivecek J.; Matolin V.; Fabris S. Creating single-atom Pt-ceria catalysts by surface step decoration. Nat. Commun. 2016, 7, 10801–10807. 10.1038/ncomms10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao B. T.; Wang A. Q.; Yang X. F.; Allard L. F.; Jiang Z.; Cui Y. T.; Liu J. Y.; Li J.; Zhang T. Single-atom catalysis of CO oxidation using Pt-1/FeOx. Nat. Chem. 2011, 3, 634–641. 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- Wei H. S.; Liu X. Y.; Wang A. Q.; Zhang L. L.; Qiao B. T.; Yang X. F.; Huang Y. Q.; Miao S.; Liu J. Y.; Zhang T. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634–5640. 10.1038/ncomms6634. [DOI] [PubMed] [Google Scholar]
- Yang S.; Kim J.; Tak Y. J.; Soon A.; Lee H. Single-Atom Catalyst of Platinum Supported on Titanium Nitride for Selective Electrochemical Reactions. Angew. Chem., Int. Ed. 2016, 55, 2058–2062. 10.1002/anie.201509241. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Asakura H.; Zhang J.; Zhang J. G.; De S.; Yan N. Stabilizing a Platinum(1) Single-Atom Catalyst on Supported Phosphomolybdic Acid without Compromising Hydrogenation Activity. Angew. Chem., Int. Ed. 2016, 55, 8319–8323. 10.1002/anie.201602801. [DOI] [PubMed] [Google Scholar]
- Moses-DeBusk M.; Yoon M.; Allard L. F.; Mullins D. R.; Wu Z. L.; Yang X. F.; Veith G.; Stocks G. M.; Narula C. K. CO Oxidation on Supported Single Pt Atoms: Experimental and ab Initio Density Functional Studies of CO Interaction with Pt Atom on theta-Al2O3(010) Surface. J. Am. Chem. Soc. 2013, 135, 12634–12645. 10.1021/ja401847c. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhao X. C.; Lei N.; Li L.; Zhang L. L.; Xu S. T.; Miao S.; Pan X. L.; Wang A. Q.; Zhang T. Hydrogenolysis of Glycerol to 1,3-propanediol under Low Hydrogen Pressure over WOx-Supported Single/Pseudo-Single Atom Pt Catalyst. ChemSusChem 2016, 9, 784–790. 10.1002/cssc.201501506. [DOI] [PubMed] [Google Scholar]
- Gao G. P.; Jiao Y.; Waclawik E. R.; Du A. J. Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 6292–6297. 10.1021/jacs.6b02692. [DOI] [PubMed] [Google Scholar]
- He P. L.; Xu B. A.; Xu X. B.; Song L.; Wang X. Surfactant encapsulated palladium-polyoxometalates: controlled assembly and their application as single-atom catalysts. Chem. Sci. 2016, 7, 1011–1015. 10.1039/C5SC03554F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Wang A. Q.; Qiao B. T.; Liu X. Y.; Yang X. F.; Wang X. D.; Liang J. X.; Li J. X.; Liu J. Y.; Zhang T. Remarkable Performance of Ir-1/FeOx Single-Atom Catalyst in Water Gas Shift Reaction. J. Am. Chem. Soc. 2013, 135, 15314–15317. 10.1021/ja408574m. [DOI] [PubMed] [Google Scholar]
- Lang R.; Li T. L.; Matsumura D.; Miao S.; Ren Y. J.; Cui Y. T.; Tan Y.; Qiao B. T.; Li L.; Wang A. Q.; Wang X. D.; Zhang T. Hydroformylation of Olefins by a Rhodium Single-Atom Catalyst with Activity Comparable to RhCl(PPh3)3. Angew. Chem., Int. Ed. 2016, 55, 16054–16058. 10.1002/anie.201607885. [DOI] [PubMed] [Google Scholar]
- Liu W. G.; Zhang L. L.; Yan W. S.; Liu X. Y.; Yang X. F.; Miao S.; Wang W. T.; Wang A. Q.; Zhang T. Single-atom dispersed Co-N-C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. 10.1039/C6SC02105K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P. Q.; Yao T.; Wu Y.; Zheng L. R.; Lin Y.; Liu W.; Ju H. X.; Zhu J. F.; Hong X.; Deng Z. X.; Zhou G.; Wei S. Q.; Li Y. D. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805. 10.1002/anie.201604802. [DOI] [PubMed] [Google Scholar]
- Zhang L. L.; Wang A. Q.; Wang W. T.; Huang Y. Q.; Liu X. Y.; Miao S.; Liu J. Y.; Zhang T. Co-N-C Catalyst for C-C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. 10.1021/acscatal.5b01223. [DOI] [Google Scholar]
- Guo X. G.; Fang G. Z.; Li G.; Ma H.; Fan H. J.; Yu L.; Ma C.; Wu X.; Deng D. H.; Wei M. M.; Tan D. L.; Si R.; Zhang S.; Li J. Q.; Sun L. T.; Tang Z. C.; Pan X. L.; Bao X. H. Direct, Nonoxidative Conversion of Methane to Ethylene, Aromatics, and Hydrogen. Science 2014, 344, 616–619. 10.1126/science.1253150. [DOI] [PubMed] [Google Scholar]
- Kwak J. H.; Hu J. Z.; Mei D.; Yi C. W.; Kim D. H.; Peden C. H. F.; Allard L. F.; Szanyi J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on gamma-Al2O3. Science 2009, 325, 1670–1673. 10.1126/science.1176745. [DOI] [PubMed] [Google Scholar]
- Park H. S.; Choi Y. S.; Jung Y. M.; Hong W. H. Intermolecular interaction-induced hierarchical transformation in 1D nanohybrids: Analysis of conformational changes by 2D correlation Spectroscopy. J. Am. Chem. Soc. 2008, 130, 845–852. 10.1021/ja073074k. [DOI] [PubMed] [Google Scholar]
- Yu B.; Kang S. Y.; Akthakul A.; Ramadurai N.; Pilkenton M.; Patel A.; Nashat A.; Anderson D. G.; Sakamoto F. H.; Gilchrest B. A.; Anderson R. R.; Langer R. An elastic second skin. Nat. Mater. 2016, 15, 911–918. 10.1038/nmat4635. [DOI] [PubMed] [Google Scholar]
- Rucker C.; Kummerer K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115, 466–524. 10.1021/cr500319v. [DOI] [PubMed] [Google Scholar]
- Madar R. Materials science - Silicon carbide in contention. Nature 2004, 430, 974–975. 10.1038/430974a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.