Articles on metal–organic frameworks (MOFs) and porous coordination polymers usually start with the great promises these materials have for future applications,1 and the report on the “retrofitting” of MOF-520 by Kapustin et al. in this issue of ACS Central Science is no exception.2 I have no objection to that, but I want to take the opportunity to also highlight something that is perhaps taken for granted in the field, but seldom explicitly communicated: how MOFs and reticular chemistry with its origins in crystal engineering have completely transformed the way we think of the solid state, and specifically the solid state constructed from molecular building blocks.
The work by Kapustin et al. is an attractive example: it shows how one type of crystal, containing a particular framework (think of it as a symmetric three-dimensional network of balls-and-spokes extending in all three dimensions of space) can be reinforced by introducing extra spokes while in the solid state. By adding these components in a controlled fashion, the material can be strengthened to withstand much higher mechanical stress.1
The original MOF-520 built from Al3+ ions, formate ions, and 1,3,5-benzenetribenzoate withstands hydrostatic compression in a diamond-anvil cell up to 2.81 GPa, whereupon it turns amorphous. However, by replacing some formate ions with bridging carboxylates, the new material tolerates up to 5.5 GPa. At the same time, it loses a counterintuitive property of some of these materials: the tendency to inflate upon exposure to external pressure.
The replacement was not done fortuitously. Geometrically suitable formates were chosen, the distance between them calculated, and the 4,4′-biphenyldicarboxylate ligand was found to fit the bill as a retrofitter. Subsequently, single crystal diffraction confirmed the replacement and the topology change from fon to skl (see Figure 1).
Figure 1.
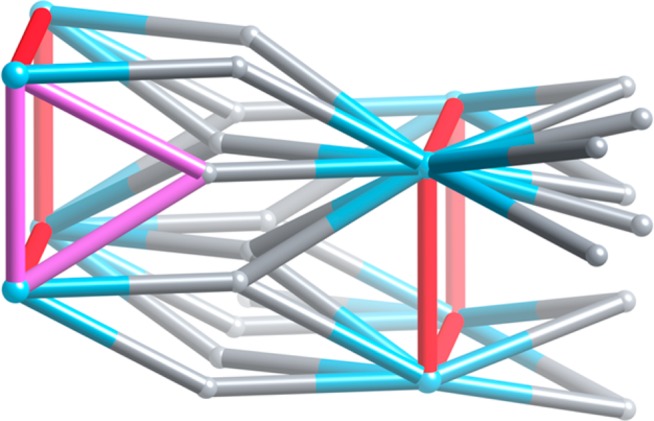
Schematic view of how the original 12- (octanuclear Al units) and 3-connected (1,3,5-benzenetribenzoate) fon topology of MOF-520 transforms into the MOF-520-BPDC with the 16- and 3-connected skl topology by addition of the red 4,4′-biphenyldicarboxylate links. Note how now trusses, rigid mechanical units, are formed, the pink triangle highlighting one of them. There are no such three-membered rings in the fon topology.
This is no mean feat if we look back a little. Crystal structures of organic and coordination compounds used to be a means to an end, to establish the molecular structure of the compound synthesized. True, there were early embryos to another, more integral, way of looking at these materials, i.e., by Wells,3 but essentially molecular structure determination was the crystallographer’s task.
In 1990, when Hoskins and Robson in a general way introduced the idea of what they called “scaffolding” like materials, they stated that they expected them “to show unusual and unexpected properties”.4 Today, this seems like something of an understatement. Not only have we remarkable properties, but both the understanding, and the ideas of what we can do with, the molecule-based solid-state has fundamentally changed.
So we now have materials that can be modeled by mechanical spokes and hinges based on the topology of the network, explaining among other things why some inflate under pressure and other do not, but at the same time having “soft” properties.5 We also have MOFs displaying negative Poisson’s ratio, known as auxeticity, becoming thicker when stretched along certain directions, or volume reduction with expansion along a specific direction under mechanical pressure, both unusual properties in other materials.6
We have also learned how to do chemistry inside MOFs by, for example, postsynthetic modification of the organic linkers, by replacing nonbridging components, or by “defect engineering”,7 that is, the removal of some linkers once the MOF has formed, resulting in chemically active pores. And, in the present case, by adding new linkers to an existing network, thus, in some sense the inversion of “defect engineering”.
Related to this is the notion that we need to move away from the idea that the average molecular positions in a crystal structure is all we need to know. In fact, it is just a starting point. In practice, many material properties will depend on imperfections, engineered or not, and we are also adding amorphous, or partly amorphous, materials as an additional MOF flavor.8 A recent example is the mechanochemical incorporation and protection of a molecular catalyst during amorphization and network transformation.9
All unthinkable 20 years ago, when even the very idea that permanent porosity could be created inside crystals of this type was met with a certain, sometimes vocal, skepticism.
Just as defect engineering may reduce the mechanical strength of the material,10 Kapustin et al. show how the “retrofitting” strengthens the material and changes its properties.1 However, we also should note that uniform hydrostatic pressure, in principle giving us the bulk modulus, as used in this work, is just one form of mechanical stress. For a more complete mechanical characterization, though outside the scope of the work presented, one would for example like to see measures of the elastic modulus and hardness of the material. It would be informative to be able to add the two materials to a two-dimensional property map as the one by Tan and Cheetham11 reproduced in Figure 2.
Figure 2.
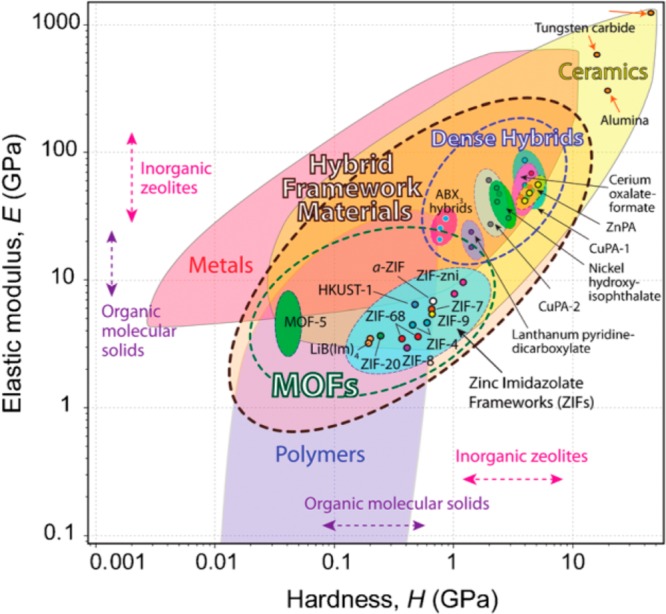
Elastic modulus versus hardness in a materials property map for various types of compounds. Reproduced from ref (11) with permission of The Royal Society of Chemistry. Copyright 2011.
References
- For a recent overview of commercial applications of MOFs and PCPs, seeNotman N.MOFs Find a Use. Chem. World, 8 March 2017. [Google Scholar]
- Kapustin E. A.; Lee S.; Alshammari A. S.; Yaghi O. M.. Molecular Retrofitting Adapts a Metal-Organic Framework to Extreme Pressure. ACS Cent. Sci. 2017, DOI: 10.1021/acscentsci.7b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. F. The Geometrical Basis of Crystal Chemistry 1. Acta Crystallogr. 1954, 7, 535–544. 10.1107/S0365110X5400182X. [DOI] [Google Scholar]
- Hoskins B. F.; Robson R. Design and Construction of A New Class of Scaffolding-Like Materials Comprising Infinite Polymeric Frameworks of 3-D-Linked Molecular Rods - A Reappraisal of The Zn(CN)2 and Cd(CN)2 Structures and The Synthesis and Structure of the Diamond-Related Frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4″,4‴-tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. 10.1021/ja00160a038. [DOI] [Google Scholar]
- a Sarkisov L.; Martin R. L.; Haranczyk M.; Smit B. On the Flexibility of Metal–Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 2228–2231. 10.1021/ja411673b. [DOI] [PubMed] [Google Scholar]; b Horike S.; Shimomura S.; Kitagawa S. Soft Porous Crystals. Nat. Chem. 2009, 1, 695–704. 10.1038/nchem.444. [DOI] [PubMed] [Google Scholar]
- Bennett T. D.; Cheetham A. K.; Fuchs A. H.; Coudert F.-X. Interplay between Defects, Disorder and Flexibility in Metal-Organic Frameworks. Nat. Chem. 2017, 9, 11–16. 10.1038/nchem.2691. [DOI] [PubMed] [Google Scholar]
- Fang Z.; Bueken B.; De Vos D. E.; Fischer R. A. Defect-Engineered Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2015, 54, 7234–7254. 10.1002/anie.201411540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. D.; Cheetham A. K. Amorphous Metal-Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. 10.1021/ar5000314. [DOI] [PubMed] [Google Scholar]
- Spekreijse J.; Öhrström L.; Sanders J. P. M.; Bitter J. H.; Scott E. L. Mechanochemical Immobilisation of Metathesis Catalysts in a Metal–Organic Framework. Chem. - Eur. J. 2016, 22, 15437–15443. 10.1002/chem.201602331. [DOI] [PubMed] [Google Scholar]
- Thornton A. W.; Babarao R.; Jain A.; Trousselet F.; Coudert F.-X. Defects in Metal–Organic Frameworks: A Compromise between Adsorption and Stability?. Dalton Trans. 2016, 45, 4352–4359. 10.1039/C5DT04330A. [DOI] [PubMed] [Google Scholar]
- Tan J. C.; Cheetham A. K. Mechanical Properties of Hybrid Inorganic–Organic Framework Materials: Establishing.. Chem. Soc. Rev. 2011, 40, 1059–1080. 10.1039/C5DT04330A. [DOI] [PubMed] [Google Scholar]


