Abstract
Gatifloxacin is an 8-methoxy fluoroquinolone effective against a broad spectrum of pathogens common in pediatric infections. The safety and pharmacokinetics of a single dose of gatifloxacin were studied in pediatric patients from 6 months to 16 years of age. Seventy-six pediatric patients (average age, 6.7 ± 5.0 years) were administered a single oral dose of gatifloxacin suspension (5, 10, or 15 mg/kg of body weight; 600-mg maximum) in a dose-escalating manner. Subjects were stratified by age into 4 groups. An additional 12 children, greater than 6 years of age, received gatifloxacin as the tablet formulation at a dose of approximately 10 mg/kg. Gatifloxacin's apparent clearance and half-life were 5.5 ± 2.1 ml/min/kg and 5.1 ± 1.4 h. The maximum concentration of drug in plasma and area under the concentration-time curve (AUC) increased in a manner approximately proportional to the dose. At the 10-mg/kg dose, the bioavailability was similar between the suspension and tablet formulation. The apparent oral clearance of gatifloxacin, normalized for body weight, exhibited a small but statistically significant decrease with increasing age. In all subjects receiving gatifloxacin at 10 mg/kg, the AUC exceeded 20 μg · h/ml (estimated free AUC/MIC ratio of ≥34 for MIC of ≤0.5 μg/ml). These data suggest that gatifloxacin at a dose of 10 mg/kg every 24 h will achieve therapeutic concentrations in plasma in infants and children.
The need for new, effective antimicrobial agents for treatment of infections caused by antibiotic-resistant bacteria has been well documented (5, 23). In infants and children, this need is exemplified by treatment failures caused by antibiotic-resistant strains of Streptococcus pneumoniae. This organism is the most common cause of upper and lower respiratory tract bacterial infections, bacteremia, and meningitis in the pediatric age group. Clinical isolates with documented resistance to β-lactams, macrolides, chloramphenicol, and sulfonamides have been noted with increasing frequency from both children and adults (12, 21).
Gatifloxacin is an 8-methoxy fluoroquinolone effective against a wide range of bacterial pathogens including antibiotic-resistant S. pneumoniae. This is consistent with its overall increased potency against gram-positive organisms compared to earlier fluoroquinolones, ciprofloxacin, ofloxacin, and levofloxacin. Gatifloxacin demonstrates increased activity against Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, group B streptococcus, and viridans group streptococcus (13, 14, 22). It also has excellent activity against a number of gram-negative enteric and respiratory pathogens including Haemophilus influenzae, Enterobacter spp., Klebsiella spp., Serratia marcescens, Legionella pneumophila, and Proteus spp. (6, 10). As a result, it is approved by the Food and Drug Administration for the treatment of community-acquired pneumonia, acute bacterial exacerbations of chronic bronchitis, sinusitis, and urinary tract infections in adults.
The pharmacokinetics of gatifloxacin in adults is characterized by rapid and complete absorption with an average bioavailability of 96% (16). It distributes extensively into body tissues and is eliminated almost exclusively in the urine with renal clearance exceeding glomerular filtration. Its relatively long half-life in adults (7 h) affords once-daily dosing.
These pharmacokinetic characteristics and antimicrobial activity make gatifloxacin a promising new compound for the treatment of pediatric gram-positive and gram-negative bacterial infections in children. It is currently in trials for the treatment of recurrent otitis media and acute otitis media treatment failures in children (K. Hamed, A. Arguedas, X. Saez-Llorens, A. Rodriguez, J. Yang, P. Pierce, and R. Echols, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. G1848, 2003). In designing these trials, detailed pharmacokinetic and safety data were needed in pediatric patients to determine the dosage most likely to result in clinical benefit. Our study evaluated the safety, tolerability, and pharmacokinetics of single-dose gatifloxacin in infants and children in both tablet and prototype suspension formulations. By studying several doses and pediatric age groups, we were also able to assess any potential age-related differences in gatifloxacin pharmacokinetics and dose proportionality.
MATERIALS AND METHODS
This was a nonrandomized, open-label, parallel-group, single-dose, dose escalation multicenter trial in pediatric patients aged 6 months to 16 years. All children were either receiving antibiotic treatment or were being considered for antibiotic therapy for the treatment of documented or suspected bacterial infection when enrolled in the study. Subjects were stratified by age into 4 groups: age 6 months to ≤2 years, >2 to ≤6 years, >6 to ≤12 years, and >12 to ≤16 years. The following laboratory tests were performed for all children prior to administration of gatifloxacin: complete blood count with differential, blood chemistries, blood urea nitrogen, serum creatinine, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transferase, lactate dehydrogenase, alkaline phosphatase, total and direct bilirubin, and serum albumin. These tests were repeated within 72 h after study drug administration.
Children were excluded from the study if they had significant underlying renal, hepatic, or hematopoietic dysfunction. Also excluded were infants and children with known hypersensitivity to quinolones, recent seizure disorders, cystic fibrosis, or juvenile rheumatoid arthritis. Written informed consent was obtained from the parents or legal guardians prior to enrollment in the study, and when appropriate, patient assent was also obtained. The study was approved by the local institutional review board at each participating center.
Drug administration and sample collection.
The gatifloxacin suspension prototype formulation (100 mg/5 ml) was dosed at 5, 10, or 15 mg/kg of body weight up to a maximum dose of 600 mg. Gatifloxacin tablets were dosed at 10 mg/kg (dose rounded to closest 50-mg increment) to the two older age groups only. At least six subjects were enrolled in each age group. All noninfants fasted for 2 h prior and 2 h following gatifloxacin administration. Serial venous blood samples were collected for pharmacokinetic analysis prior to and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after dosing. Plasma was stored at −20°C until analyzed. Urine was collected predose and in the intervals from 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdose in children with indwelling urine catheters or who were toilet trained.
Calculation of pharmacokinetic parameters.
Pharmacokinetic parameters were determined by noncompartmental methods. The following pharmacokinetic parameters for gatifloxacin were determined for each subject: maximum concentration of drug in serum (Cmax) (in micrograms per milliliter), time to Cmax (Tmax) (in hours), area under the concentration-time curve from 0 h to infinity (AUC0-∞) (in microgram-hours per milliliter), half-life (t1/2) (in hours), apparent clearance (CLT/F) (in milliliters per minute per kilogram), renal clearance (CLR) (in milliliters per minute per kilogram), and percent urinary excretion. The peak concentration in plasma (Cmax) and the time to reach peak concentration (Tmax) were recorded directly from experimental observations. The slope of the terminal phase of the plasma drug concentration profile (λ) was determined by log-linear regression of at least three data points. The absolute value of λ was used to estimate the apparent terminal t1/2 of 0.693/λ. The area under the plasma concentration-time curve to the last quantifiable concentration (AUC0-T) was calculated by trapezoidal and log trapezoidal summations. AUC0-∞ was calculated by summing AUC0-T and Clast/λ. Apparent clearance (CLT/F) was calculated by dividing the dose by AUC0-∞. The renal clearance (CLR) during each collection interval was determined as Ae0-T/AUC0-T, where Ae is the amount of drug excreted in the urine. The Ae0-24 was expressed as a percentage of the total dose. Unbound AUC was estimated as the total AUC multiplied by the previously published unbound fraction (8). Pharmacokinetic calculations were performed by using WinNonLin (version 3.0; Pharsight, Mountain View, Calif.) and SAS (version 6.12; SAS Institute, Cary, N.C.).
Assay.
Plasma and urine were analyzed for gatifloxacin concentrations by using a validated high-performance liquid chromatography method with fluorescence detection (16). The quantitative assay ranges were 0.01 to 5 μg/ml for plasma and 1 to 1,000 μg/ml for urine. The assay accuracies were greater than 94 and 91% for the plasma and urine assays, respectively, and within-day and between-day precisions were typically less than 5% for each assay run and always less than 10.7% for plasma and urine assays.
Statistical analysis.
Potential age-, dose-, and formulation-related differences in pharmacokinetic parameters were examined by using SAS (version 6.12). The log-transformed AUC0-∞ and Cmax and raw Tmax, t1/2, and CLT/F were analyzed by using an analysis of variance model, confidence intervals, and by Pearson correlation analysis. Statistical significance was defined in each analysis as a P value of less than 0.05.
RESULTS
A total of 118 pediatric patients were enrolled, and 111 patients were dosed with gatifloxacin. Ninety-one subjects completed the study, with most noncompletions due to vomiting of the study dose shortly after administration. Three additional subjects were discovered to have taken disallowed medications that interfere with gatifloxacin absorption and were excluded from the pharmacokinetic analysis. In all, evaluable pharmacokinetic data were obtained from 76 subjects receiving the oral gatifloxacin suspension and 12 subjects receiving the tablet formulation. The study population was predominately Caucasian (53%) and male (64%). A large number of the subjects in this study were hospitalized with acute medical conditions, and approximately 40% of subjects were receiving various concomitant systemic medications at the time of gatifloxacin administration.
Safety.
Gatifloxacin was safe at all dose levels studied, with no reports of serious adverse events related to the study drug. The most common adverse events were vomiting, headache, and rash in 17, 3, and 4% of the subjects, respectively. Only subjects receiving the suspension formulation experienced vomiting, which occurred in 0, 10, and 25% of subjects in the 5-, 10-, and 15-mg/kg dose groups, respectively.
Three subjects experienced increases in liver function tests: two with minor increases (<2 times the upper limit of normal) and one with a moderate increase (4 to 7 times the upper limit of normal). These elevations were transient and returned to normal without intervention.
Pharmacokinetics.
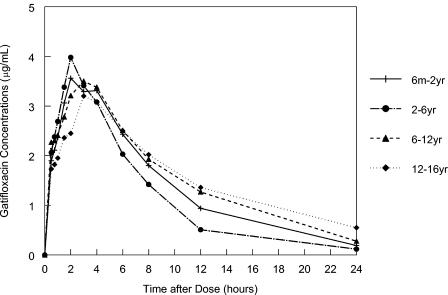
The mean concentration in plasma-versus-time profiles for the 10-mg/kg dose level in the selected age groups are presented in Fig. 1. As depicted, all four age groups achieved similar concentrations in plasma. The absorption of gatifloxacin was rapid, with median Tmax values ranging from 1.25 to 4 h across various age and dose groups.
FIG. 1.
Gatifloxacin concentrations in plasma (geometric mean) following administration of a 10-mg/kg suspension by age cohort.
Pharmacokinetic parameter values following administration of gatifloxacin suspension are shown in Table 1. Cmax and AUC0-∞ increased in a dose-related manner corresponding to the 1:2:3 gatifloxacin dose ratios. Furthermore, as shown in Table 1, the CLT/F and CLR values are similar among the 5-, 10-, and 15-mg/kg dose levels within each age group. A comparison of the gatifloxacin pharmacokinetic parameter estimations for the tablet and suspension formulations is shown in Table 2. The tablet formation resulted in a shorter Tmax (1.3 versus 3.0 h) and a higher Cmax (5.3 versus 3.8 μg/ml), although the AUC was similar (43.7 versus 38.6 μg · h/ml) to that of the suspension formulation, suggesting more rapid absorption of the tablet formulation.
TABLE 1.
Summary of gatifloxacin pharmacokinetic parameters for infants and childrena
| Parameter (units) | Dose (mg/kg of body wt) | Result [n] for age group
|
||||
|---|---|---|---|---|---|---|
| 6 mo-2 yr | 2-6 yr | 6-12 yr | 12-16 yr | Overall | ||
| Cmax (μg/ml) | 5 | 1.68 (35) [6] | 2.81 (24) [4] | 2.21 (21) [6] | 1.70 (31) [6] | 1.79 (39) [22] |
| 10 | 4.24 (25) [8] | 4.20 (28) [7] | 3.93 (24) [9] | 3.55 (16) [7] | 3.97 (24) [31] | |
| 15 | 5.25 (35) [6] | 5.32 (13) [6] | 5.66 (27) [5] | 4.58 (25) [6] | 5.17 (26) [23] | |
| AUC (μg · h/ml) | 5 | 11.16 (39) [6] | 15.95 (26) [4] | 12.91 (45) [6] | 15.86 (17) [6] | 12.11 (39) [22] |
| 10 | 33.09 (26) [8] | 27.50 (17) [7] | 35.37 (44) [9] | 43.23 (18) [7] | 34.37 (33) [31] | |
| 15 | 41.99 (49) [6] | 42.18 (24) [6] | 59.19 (11) [5] | 45.47 (32) [6] | 46.25 (32) [23] | |
| Tmax (h) | 5 | 2 (0.75-3) [6] | 1.75 (1-3) [4] | 1.25 (0.5-3) [6] | 3 (0.75-6) [6] | 2 (0.5-6) [22] |
| 10 | 3 (0.75-4) [8] | 2 (2-4) [7] | 3 (0.5-6) [9] | 3 (1.5-4) [7] | 3 (0.5-6) [31] | |
| 15 | 3 (2-4) [6] | 2.5 (1.5-6) [6] | 4 (3-8) [5] | 3 (0.75-4) [6] | 3 (0.75-8) [23] | |
| t1/2 (h) | 5 | 4.45 (0.50) [6] | 4.27 (0.50) [4] | 4.71 (1.43) [6] | 5.73 (0.62) [6] | 4.80 (1.01) [22] |
| 10 | 4.76 (1.35) [8] | 4.35 (0.57) [7] | 5.21 (1.31) [9] | 7.53 (2.07) [7] | 5.42 (1.80) [31] | |
| 15 | 4.21 (0.76) [6] | 4.46 (1.07) [6] | 5.09 (0.50) [5] | 5.97 (1.38) [6] | 4.93 (1.17) [23] | |
| CLT/F (ml/min/kg) | 5 | 8.09 (3.91) [6] | 5.36 (1.43) [4] | 6.89 (2.53) [6] | 5.34 (1.11) [6] | 6.52 (2.67) [22] |
| 10 | 5.16 (1.13) [8] | 6.17 (1.07) [7] | 5.02 (1.70) [9] | 3.88 (0.70) [7] | 5.06 (1.42) [31] | |
| 15 | 6.56 (3.32) [6] | 6.09 (1.58) [6] | 4.05 (0.57) [5] | 4.13 (1.03) [6] | 5.25 (2.17) [23] | |
| CLR (ml/min/kg) | 5 | 5.09 (0.11) [2] | 4.77 (1.62) [6] | 4.14 (1.07) [6] | 6.46 (3.37) [14] | |
| 10 | 4.53 (0.68) [4] | 3.46 (1.16) [8] | 3.02 (0.60) [7] | 4.87 (2.91) [19] | ||
| 15 | 3.44 (1.17) [5] | 2.18 (0.50) [5] | 2.47 (0.95) [6] | 3.27 (1.65) [16] | ||
| % UR | 5 | 82 [1] | 70 (15) [6] | 69 (6.5) [4] | 71 (12) [11] | |
| 10 | 70 (13) [4] | 67 (24) [6] | 72 (12) [6] | 69 (217) [16] | ||
| 15 | 58 (18) [4] | 52 (13) [5] | 59 (29) [6] | 56 (21) [15] | ||
Values represent arithmetic means and standard deviations, except for AUC and Cmax, which are given as geometric means with percent coefficients of variation, and Tmax, for which the median and range are given. % UR is the percentage of the dose recovered in urine.
TABLE 2.
Summary of gatifloxacin pharmacokinetics following a 10-mg/kg dose of suspension and tablet formulations to children 6 to 16 years of agea
| Formulation | Mean (% CV) age (yr) [n] | Geometric mean (% CV) AUC (μg · h/ml) [n] | Geometric mean (% CV) Cmax (μg/ml) [n] | Median (% CV) Tmax (h) [n] | Mean (% CV) CLT/F (ml/min/kg) [n] | Mean (% CV) % UR [n] |
|---|---|---|---|---|---|---|
| Suspension | 11.0 (30) [16] | 38.6 (34) [16] | 3.76 (21) [16] | 3.0 (52) [16] | 4.52 (32) [16] | 69 (27) [12] |
| Tablet | 12.2 (28) [12] | 43.7 (24) [12] | 5.29 (22) [12] | 1.3 (64) [12] | 3.88 (25) [12] | 68 (15) [6] |
CV, coefficient of variation; % UR is the percentage of the dose recovered in urine.
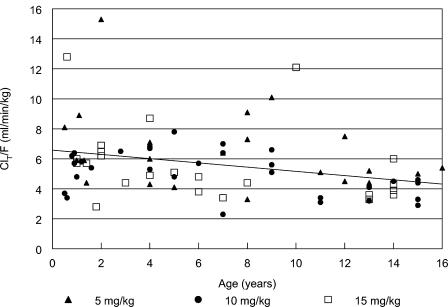
Apparent clearance, normalized to body weight, decreased slightly with increasing age (Fig. 2). The linear regression equation for CLT/F across all three dose levels and age was the following: CLT/F (in milliliters per minute per kilogram) = 6.6 to 0.15 × age (in years), r2 = 0.12, with the 95% confidence interval for the slope exclusive of 0 (−0.25 to −0.06). The inverse relationship between CLT/F and age suggests that younger children require a higher dose than older children and adults on a milligram-per-kilogram basis. However, restricting the 10-mg/kg dose to the usual adult daily dosage of 400 mg will compensate for this slightly lower clearance in the older children.
FIG. 2.
Apparent clearance of gatifloxacin following administration of suspension according to subject age. When apparent clearance was normalized by weight, a significant correlation exists with age (r = −0.36, P = 0.0015). This effect is not seen when apparent clearance is normalized by body surface area.
Complete urine collections were available from 61 of the older subjects (mean age, 8.3 ± 3.0 years). The mean renal clearance in these subjects was 4.5 ± 2.9 ml/h/kg. The total gatifloxacin collected in the urine during the 24-h period following drug administration averaged approximately 65% of the administered dose.
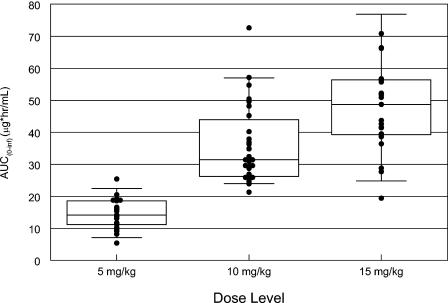
Individual gatifloxacin AUC for a given suspension dose are shown in Fig. 3. All subjects that received gatifloxacin at the 10-mg/kg dose level achieved predicted free (fE′) AUC/MIC ratios greater than 34 for typical S. pneumoniae isolates (MIC at which 90% of strains are inhibited [MIC90], 0.5 μg/ml) and greater than 250 for H. influenzae and Moraxella catarrhalis (MIC90 < 0.06 μg/ml).
FIG. 3.
Box plot of the AUC for gatifloxacin following administration of suspension at various dose levels.
DISCUSSION
This phase I trial to determine the pharmacokinetics of gatifloxacin in infants and children was undertaken to define optimal dosing for use in clinical trials involving invasive pediatric infections. Gatifloxacin demonstrates a broad spectrum of antibacterial activity against gram-positive and gram-negative aerobes and anaerobes (6, 10, 14, 22). In particular, the in vitro activity of gatifloxacin against S. pneumoniae is excellent, with similar activity against both penicillin-susceptible and nonsusceptible strains, including non-macrolide-susceptible strains (13, 14, 22).
While fluoroquinolones have gained widespread clinical use, there are toxicities that are of concern for some individual agents in this class of antibiotics. Gatifloxacin lacks a 2,4-difluorophenyl group at the N-1 position that is postulated to induce hepatic and hematologic toxicities observed with trovafloxacin and temafloxacin. In addition, gatifloxacin does not have a halide at C-8, which may also reduce its potential for phototoxicity (7).
Quinolone arthropathy in juvenile animals has been a particular concern for this class of agents and their use in pediatrics. A review of fluoroquinolone use in pediatrics estimated exposure in more than 7,000 skeletally immature patients and found that reversible episodes of arthralgia do not lead to long-term sequelae (4). Thus, prospective studies of gatifloxacin in children are justified.
In the present single-dose study, gatifloxacin was safe and, with the exception of some gastrointestinal disturbances, well tolerated. We did not encounter any adverse events to suggest connective tissue toxicity, but as a single-dose evaluation, this study was not designed to be sensitive in detection of these potential toxicities. There was formulation-specific vomiting with the suspension prototype formulation which was most prominent at the highest (15 mg/kg) dose level and may be dose-limiting. The bitter taste of the gatifloxacin suspension used in this trial prompted the development of an alternative formulation. The gatifloxacin oral suspension has been reformulated as a powder coprecipitated with stearic acid. This formulation is resistant to dissolution of gatifloxacin in the mouth, which may mask the bitter taste of the drug. This formulation has subsequently been included in phase II and III studies.
Increases in serum gamma glutamyl transferase, aspartate aminotransferase, and alanine aminotransferase were observed in 3 subjects within 24 h of gatifloxacin administration. Clinically relevant increases in liver function tests have been observed in fewer than 1% of adults treated with gatifloxacin (3). Of note, the one subject with the moderate liver enzyme elevation had received isoflurane and sevoflurane the day prior to study participation. Both of these agents have been implicated in acute hepatic injury. The liver function enzyme elevations seen in this study were transient and resolved without therapy. The clinical importance of these adverse events encountered in this study will be better delineated in multiple-dose phase II and III trials.
The pharmacokinetic profiles for gatifloxacin in pediatric subjects appeared to be generally comparable to those for disposition adult subjects, but the clearance of the drug was faster in pediatric subjects. Therefore, pediatric subjects require a higher dose, on a milligram per kilogram basis, than adults. A 10-mg/kg oral dose, to an absolute maximum of 600 mg, in pediatric subjects resulted in gatifloxacin exposure similar to that observed in adults given a 400-mg dose (5.7 mg/kg for 70 kg of body weight). Similar to adults, the absorption of gatifloxacin in pediatric subjects was rapid. In children, administration of a 10-mg/kg suspension dose resulted in comparable exposure to that seen following a 10-mg/kg dose given intravenously, suggesting that the oral bioavailability in children is high (M. Aguero, J. Blumer, J. Bradley, X. Saex-Llorens, M. O'Ryan, D. Grasela, F. Lacreta, M. Swingle, G. McCracken, and H. Jafri, 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A39, 2001). This is consistent with the high absolute oral bioavailability (96%) of gatifloxacin in adults.
Gatifloxacin clearance in adults is 206 ml/min, with a terminal half-life of 9.4 h and a volume of distribution (Vdss) of 1.5 liter/kg, resulting in an AUC of 28.6 μg · h/ml with a dose of 400 mg intravenously (11). Gatifloxacin apparent clearance in our pediatric subjects was 5.5 ml/min/kg, approximately ∼80% higher than adults after adjustment for body weight. The terminal elimination half-life in the older children was similar to that of adults, with a shorter half-life present in the younger cohorts (<6 years of age). The Cmax and AUC0-∞ in the present study after administration of gatifloxacin suspension at 10 mg/kg were similar to those achieved after administration of 400 mg of gatifloxacin to adults (4.0 versus 4.1 μg/ml and 36.0 versus 33.9 μg · h/ml).
In adults, renal gatifloxacin clearance is similar to total gatifloxacin clearance, with urinary excretion of unchanged drug accounting for more than 80% of the total dose in 48 h. The apparent clearance of gatifloxacin is linearly related to creatinine clearance values from 40 to 160 ml/min. Accordingly slightly higher gatifloxacin clearance values have been observed in younger adults and in men, consistent with greater creatinine clearances than in women and older adults (17). We observed similar pharmacokinetic behavior with our pediatric subjects. Renal clearance approached total apparent clearance, and unchanged gatifloxacin in the urine accounted for 60% of the dose in the urine over 24 h. Both of these findings suggest that gatifloxacin is predominantly eliminated in the urine in pediatric patients as well.
There was a minor effect of age on gatifloxacin clearance, with clearance higher in infants and children when normalized to body weight (correlation of age to CLT/F, r = −0.36, P = 0.0015). Similarly, the half-life was significantly correlated with age (r = 0.55, P < 0.0001). The large number of subjects in this study and relatively consistent pharmacokinetics among subjects allowed these effects to be detected. These observations are not unexpected, as renal function increases during childhood in proportion to body surface area after the first year of life (15). If the impact of age is evaluated with clearance that is scaled by body surface area rather than weight, the correlation between age and clearance disappears with an overall ClT/F of 146 ml/min/1.73 m2 (r = 0.13). Since gatifloxacin is dosed on a milligram-per-kilogram basis, some age-related differences in exposure are expected. However, these are probably not clinically significant and are mitigated by using the adult dose of 400 mg as a maximum dose (for 10 mg/kg) in older larger pediatric patients.
While the primary goal of this study was to determine the pharmacokinetics and dosing requirements of a gatifloxacin suspension formulation, a limited number of subjects over 6 years of age received the tablet formulation to assess the relative bioavailability of the two formulations. The tablet formulation appears to be absorbed more rapidly; however, the overall exposures from the two formulations are similar, allowing the use of a single standardized dose regardless of the formulation.
Guidelines for dosing gatifloxacin in pediatric subjects for further clinical studies were based upon achieving AUC that were reasonably comparable to those observed with adults given a therapeutic dose of 400 mg of gatifloxacin (mean AUC = 33 μg · h/ml). The 5- and 15-mg/kg doses resulted in mean AUC lower and higher than this value. The suspension dose of 10 mg/kg yielded a mean AUC nearly identical to this target adult value, and all of the pediatric subjects receiving this dose were within 33% of the adult AUC value. This suggests that a gatifloxacin dose of 10 mg/kg provides exposures in pediatric subjects comparable to those in adults.
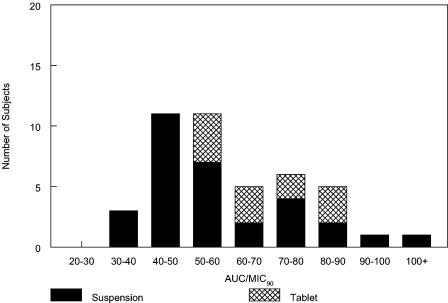
The selection of 10 mg/kg as the pediatric dose is also consistent with pharmacodynamic models of fluoroquinolones and common pediatric pathogens. The fE′ AUC/MIC ratio has been identified as the pharmacodynamically linked parameter for fluoroquinolones in adult respiratory tract infections (1, 9). For S. pneumoniae, a plasma ratio greater than 34 was associated with higher microbiological efficacy in adult community-acquired respiratory tract infections (1, 18, 20). Based on gatifloxacin's activity versus S. pneumoniae (MIC90 of 0.5 μg/ml) and its plasma protein binding of 20%, predicted fE′ AUC/MIC ratios in all study subjects were greater than 34 against this organism (Fig. 4). Higher fE′ AUC/MIC ratios of 125 to 250 are therapeutic for respiratory infections caused by various gram-negative bacilli (9). Gatifloxacin's potent activity against H. influenzae (MIC90 = 0.016 μg/ml) and M. catarrhalis (MIC90 = 0.06 μg/ml) translate into predicted fE′ AUC/MIC ratios greater than 250 for all pediatric subjects in this study that were administered a 10-mg/kg dose.
FIG. 4.
Estimated free AUC/MIC90 ratios following a 10-mg/kg daily dose of gatifloxacin to infants and children for S. pneumoniae or another pathogen for which the MIC90 was 0.5 μg/ml. All would have an estimated fE′ AUC/MIC ratio of ≥34.
The pharmacokinetics of gatifloxacin and its activity against a broad spectrum of pediatric pathogens support its development for infants and children. Good tissue penetration (2, 19; E. Leibovitz, C. Rubino. B. Damle, B. Cirincione, G. Duncan, D. Grasela, K. Hamed, R. Echols, and R. Dagan, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A14, 2003) and activity against S. pneumoniae (14) make it a potential candidate for the treatment of recurrent and refractory otitis media as well as other difficult-to-treat pediatric infections. This study suggests children and infants between 6 months and 16 years of age can receive similar weight-adjusted doses and achieve plasma concentration profiles that support once-daily dosing for many infections. Current pharmacokinetic and clinical data with gatifloxacin for adults also support further clinical trials for infants and children with this agent.
Gatifloxacin was safe as a single dose in infants and children. Low intersubject variability in concentrations in plasma was noted in this population. No important age- or formulation-related differences in gatifloxacin pharmacokinetics were noted, suggesting similar dose requirements among all pediatric populations. Gatifloxacin at a dose of 10 mg/kg every 24 h will achieve therapeutic exposures in infants and children.
Acknowledgments
This work was supported in part by NICHD grants 5U10HD31318 (San Diego), HD031323-11 (Cleveland), 2 U01 HD31313-12. 2U10 (Kansas City), and HD31324-11 (Arkansas) and with financial support from Bristol Myers Squibb.
The Pediatric Pharmacology Research Units are part of the NICHD Pediatric Pharmacology Research Network.
We also thank Mary Swingle and Nimish Vaccharajani for assistance in the development and analysis of this study.
REFERENCES
- 1.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bristol-Myers Squibb Pharmaceuticals. Data on file.
- 3.Bristol-Myers Squibb Pharmaceuticals. 1999. Tequin (gatifloxacin) package insert. Bristol-Myers Squibb Pharmaceuticals, Princeton, N.J.
- 4.Burkhardt, J. E., J. N. Walterspiel, and U. B. Schaad. 1997. Quinolone arthropathy in animals versus children. Clin. Infect. Dis. 25:1196-1204. [DOI] [PubMed] [Google Scholar]
- 5.Dellamonica, P., C. Pradier, B. Dunais, and H. Carsenti. 1998. New perspectives offered by a French study of antibiotic resistance in day-care centers. Chemotherapy 44(Suppl. 1):10-14. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V., R. N. Jones, M. A. Pfaller, and K. Kugler. 1999. Haemophilus influenza and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada 1997). Antimicrob. Agents Chemother. 43:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domagala, J. M. 1994. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 33:685-706. [DOI] [PubMed] [Google Scholar]
- 8.Fish, D. N., and D. S. North. 2001. Gatifloxacin, an advanced 8-methoxy fluoroquinolone. Pharmacotherapy 21:35-59. [DOI] [PubMed] [Google Scholar]
- 9.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Gross, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung-Tome, J., B. Minassian, B. Kolek, T. Waho, E. Huezko, and D. Bonner. 2000. In-vitro antibacterial spectrum of a new broad spectrum 8-methoxy fluoroquinolone, gatifloxacin. J. Antimicrob. Chemother. 45:437-446. [DOI] [PubMed] [Google Scholar]
- 11.Gajjar, D. A., F. P. LaCreta, H. D. Uderman, G. D. Kollia, G. Duncan, M. J. Birkhofer, and D. M. Grasela. 2000. A dose-escalation study of the safety, tolerability and pharmacokinetics of intravenous gatifloxacin in healthy adult men. Pharmacotherapy 20:49S-58S. [DOI] [PubMed] [Google Scholar]
- 12.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, and K. T. Luh. 1999. Extremely high incidence of macrolide and trimethoprim-sulfamethoxazole resistance among clinical isolates of Streptococcus pneumoniae in Taiwan. J. Clin. Microbiol. 37:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, C. N., W. H. Benjamin, B. M. Gray, M. C. Crain, K. M. Edwards, and K. B. Waites. 2001. In vitro activity of ABT-733, telithromycin and eight other antimicrobials against erythromycin-resistant Streptococcus pneumoniae respiratory isolates of children. Int. J. Antimicrob. Agents 18:531-535. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., M. L. Beach, M. A. Pfaller, and G. V. Doern. 1998. Antimicrobial activity of gatifloxacin tested against 1,676 gram-positive cocci isolated from patient infections in North and South America. Diagn. Microbiol. Infect. Dis. 32:247-252. [DOI] [PubMed] [Google Scholar]
- 15.Kon, V., and I. Ichikawa. 2004. Glomerular circulation and function, p. 25-44. In E. D. Avner, W. E. Harmon, and P. Niaudet (ed.), Pediatric nephrology, 5th ed. Lippincott, Williams & Wilkins, Baltimore, Md.15602664
- 16.LaCreta, F. P., S. Kaul, G. D. Kollia, G. Duncan, D. M. Randall, and D. M. Grasela. 2000. Interchangeability of 400-mg intravenous and oral gatifloxacin in healthy adults. Pharmacotherapy 20:59-66S. [DOI] [PubMed] [Google Scholar]
- 17.LaCreta, F. P., G. D. Kollia, G. Duncan, D. Behr, and D. M. Grasela. 2000. Age and gender effects on the pharmacokinetics of gatifloxacin. Pharmacotherapy 20:67S-75S. [DOI] [PubMed] [Google Scholar]
- 18.Lister, P. D. 2002. Pharmacodynamics of gatifloxacin against Streptococcus pneumoniae in an in vitro pharmacodynamic model: impact of area under the curve/MIC ratios on eradication. Antimicrob. Agents Chemother. 46:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutsar, I., I. R. Friedland, L. Wubbel, C. C. McCoig, H. S. Jafri, W. Ng, F. Ghaffar, and G. H. McCracken. 1998. Pharmacodynamics of gatifloxacin in cerebrospinal fluid in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob. Agents Chemother. 42:2650-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattoes, H. M., M. Banevicius, L. Dadong, C. Turley, D. Xuan, C. Nightengale, and D. P. Nicolau. 2001. Pharmacodynamic assessment of gatifloxacin against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2092-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha, S. K., N. Rikitomi, M. Ruhulamin, H. Masaki, M. Hanif, M. Islam, K. Watanabe, K. Ahmed, K. Matsumoto, R. B. Sack, and T. Nagatake. 1999. Antimicrobial resistance and serotype distribution of Streptococcus pneumoniae strains causing childhood infections in Bangladesh. J. Clin. Microbiol. 37:798-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White, R. L., K. A. Enzweiler, L. V. Friedrich, D. Wagner, D. Hoban, and J. A. Bosso. 2002. Comparative activity of gatifloxacin and other antibiotics against 4009 clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000. Diagn. Microbiol. Infect. Dis. 43:207-221. [DOI] [PubMed] [Google Scholar]
- 23.Wubbel, L., and G. H. McCracken, Jr. 1998. Management of bacterial meningitis: 1998. Pediatr. Rev. 19:78-84. [DOI] [PubMed] [Google Scholar]