Abstract
The tripeptide amide glycyl-prolyl-glycinamide (GPG-amide) is a new antiretroviral drug candidate, but its absorption mechanism is unknown. In this investigation, the transport and metabolism of GPG-amide were studied in a model of the human intestinal epithelium, Caco-2 cell monolayers. The results show that when the tripeptide amide came into contact with the apical enterocyte membrane, it was degraded by CD26 (dipeptidyl peptidase IV) to glycylproline and the antiretrovirally active metabolite glycinamide. Glycinamide retained antiretroviral activity in vitro after transport through the Caco-2 cell monolayers. The transport of glycinamide across Caco-2 cell monolayers occurred via passive diffusion with an apparent permeability coefficient of about 2 × 10−6 cm s−1, which suggests that it is absorbed by the oral route in sufficient amounts to be considered for oral administration. In conclusion, the tripeptide GPG-amide acts as a prodrug that is activated by CD26 to release the orally active antiretroviral compound glycinamide.
The market currently provides several drugs which counter the replication of human immunodeficiency virus (HIV) by inhibiting viral protease or reverse transcriptase (8). However, all these drugs face a common problem: the viral strains eventually develop resistance to them. The development of new anti-HIV drug candidates, especially those which attack the virus by alternative mechanisms, is therefore a continuing requirement.
One such new drug candidate that is effective against various HIV strains in vitro but that does not show cross-resistance and that does not select for HIV type 1 (HIV-1)-resistant mutants is the tripeptide amide glycyl-prolyl-glycinamide (GPG-amide) (Fig. 1) (2, 20). The glycine-proline-glycine motif occurs in the C-terminal region of the HIV capsid protein p24, and it was reported that viral capsid formation is disturbed in the presence of the tripeptide amide (10), although the exact mode of action has not yet been fully elucidated. Nonetheless, it seems clear that the mode of antiviral action of GPG-amide is not the same as those of other agents with activities against HIV (21).
FIG. 1.
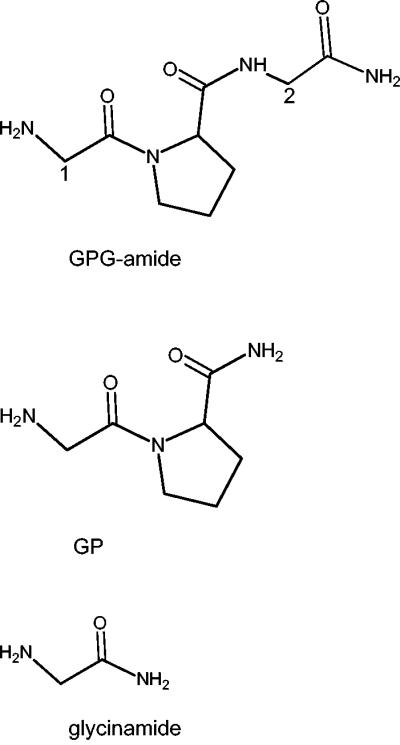
Structural formulas for GPG-amide, GP, and glycinamide. Position numbers 1 and 2 in GPG-amide (glycyl-prolyl-glycinamide) refer to the position of the 14C labeling in *GPG-amide (position 1) and GP*G-amide (position 2), respectively.
In order to be considered for development, it is generally required that a new antiviral drug be active after oral administration. Preliminary studies (15; J. Spira [Tripep AB], personal communication) with rats and human volunteers indicated that GPG-amide displays activity when it is administered orally. This is surprising, since GPG-amide has two inherent weaknesses that could limit its oral absorption: it is hydrophilic, which limits membrane permeation, and it is prone to degradation by either membrane-bound or intracellular proteases. However, the absorption would be facilitated if GPG-amide was recognized by intestinal transport proteins, which are able to shuttle hydrophilic compounds through the intestinal epithelial barrier. Since GPG-amide is a tripeptide, the oligopeptide transporter PepT1 could be involved in its absorption. This transporter is expressed in the human duodenum, jejunum, and ileum and cell lines such as Caco-2 cells (9) and is located in the apical membrane of intestinal epithelial cells (24). PepT1 has a broad substrate specificity, recognizing di- and tripeptides as well as some nonpeptide structures (6, 12), which makes it an interesting drug transport target.
In this investigation, the routes of transport of GPG-amide across a commonly used cell culture model of the intestinal epithelium (Caco-2 cells) were explored. In addition, we investigated the metabolism of GPG-amide in the model intestinal epithelium and the antiretroviral activity of the drug after transport across the model epithelium.
MATERIALS AND METHODS
Materials.
[14C]glycyl-prolyl-glycinamide (53 mCi/mmol; *GPG-amide) was purchased from the Isotope Laboratory at the Biological Research Centre, Szeged, Hungary, and glycyl-prolyl-[14C]glycinamide · HCl (58 mCi/mmol; GP*G-amide) and [14C]glycinamide (56 mCi/mmol; *G-amide) were obtained from Amersham Pharmacia Biotech, Little Chalfont, England.
Glycyl-prolyl-glycinamide hydrochloride was synthesized by Isochem, Gennevilliers, France. Glycinamide hydrochloride (G-amide), prolyl-glycinamide hydrochloride (PG-amide), glycyl-prolyl-glycine, and diprotin A were purchased from Bachem, Bubendorf, Switzerland. Glycylproline (GP), glycyl-sarcosine (Gly-Sar), glycylprolyl-p-nitroanilide (GP-pNA), and the buffer substances HEPES (biotechnology performance), morpholineethanesulfonic acid (MES; cell culture grade), and sodium carbonate were supplied by Sigma-Aldrich, Stockholm, Sweden. Ultrapure glycine was obtained from U.S. Biochemicals, Cleveland, Ohio. Formic acid and dansyl chloride were obtained from Fluka, Buchs, Switzerland. Acetonitrile and methanol of high-pressure liquid chromatography (HPLC) quality were supplied by Merck, Darmstadt, Germany.
Cell culture media and supplements were purchased from Gibco/Invitrogen, Täby, Sweden; scintillation liquid (Aquasafe 300+) was obtained from Zinsser Analytic (Frankfurt, Germany).
Cell culture.
Caco-2 cells (passages 96 to 106; American Type Culture Collection, Manassas, Va.) were maintained in Dulbecco's modified Eagle's medium, high glucose (DMEM), supplemented with 10% fetal calf serum (FCS) and 1% nonessential amino acids in an incubator at 37°C in a humidified atmosphere with 10% CO2; the medium was changed every second day, and the cells were passaged once a week, as described elsewhere (22). The transport and metabolism studies were performed on Caco-2 cell monolayers grown on cell culture inserts as a model system for the human intestine. Cells were seeded on polycarbonate cell culture inserts (4.4 × 105 cells per cm2; Transwell system; diameter, 12 mm; pore size, 0.4 μm; Corning Costar, Schiphol Rijk, The Netherlands) and were allowed to differentiate for 21 to 30 days in DMEM supplemented with 10% FCS, 1% nonessential amino acids, and PEST (100 U of penicillin per ml and 100 μg of streptomycin per ml). The medium was changed every second day.
The integrities of the Caco-2 cell monolayers from different passages were evaluated by measuring the permeation of the paracellular marker [14C]mannitol (apparent permeability coefficient [Papp] = 2.1 × 10−7± 1.1 × 10−7 cm s−1; n = 15).
Expression of the peptide transporter PepT1 has been shown at the mRNA level for our Caco-2 cells and passage numbers (18); the expression level was about 10 times lower than that found in the human jejunum (18). Vectorial transport of the PepT1-specific substrate Gly-Sar was confirmed for the Caco-2 cell monolayer passages that we used.
Transport experiments.
Bidirectional transport experiments were performed in Hank's balanced salt solution (HBSS) supplemented with 0.35 g of NaHCO3 and either 25 mM HEPES (pH 7.4) or 10 mM MES (pH 6.0); all solutions were pretempered. Before the transport experiment, the filters with the Caco-2 cell monolayers were washed with HBSS for 25 to 30 min (37°C, pH 6.0 or 7.4 in the apical chamber and pH 7.4 in the basolateral chamber). To start the experiment, the washing solutions were removed from the filters, drug solutions in HBSS were added to the donor chamber, and an adequate amount of HBSS was added to the receiver chamber (0.4 ml to the apical chamber and 1.2 ml to the basolateral chamber). The monolayers were incubated in a humidified atmosphere on a plate shaker (100 rpm; MTS4 [IKA-Schüttler]) at 37°C. Samples were withdrawn from the acceptor chambers at 15- to 30-min intervals for up to 120 min and replaced with the same volume of pretempered HBSS.
In order to obtain levels of radioactivity high enough for evaluation, it was necessary to use relatively high concentrations (20 μM) of the radioactive compounds *GPG-amide and GP*G-amide. Similarly, 20 μM *G-amide was used, since there were no differences in Papps at concentrations of 2.5 and 20 μM. All experiments were carried out in the presence of 20 μM cold substance in the donor solution. In the inhibition experiments, the inhibitor was added to the apical side as well as the basolateral side of the cell monolayers at a concentration of 5 mM (with the exception of tetraethylammonium bromide, which was used at 500 μM as a competitor for 10 μM *G-amide). The transport experiment with cold GP was carried out with 500 μM donor solution to accommodate the UV detection limits for the subsequent HPLC analysis.
Papps were calculated by the equation Papp = dQ/dt · [1/(A · C0)], where dQ/dt is the steady-state flux (in disintegrations per minute per second or micromolar per second), A is the surface area of the filter (in square centimeters), and C0 is the initial concentration in the donor chamber at each time interval (in disintegrations per minute per liter or micromolar). Retention of the mass balance (total level of radioactivity recovered from the apical and basolateral compartments compared with the initial level) was checked after each transport experiment.
Because transport of the tripeptide did not appear to be fully linear over the whole 120-min experimental period (the rate was lower in the first 30 min), the results were evaluated for the period from 30 to 90 min. Although only approximately 70% of the radioactivity of *GPG-amide was recovered from the apical and basolateral compartments after the transport experiment, indicating the possible accumulation of the substance or degradation products in the cells, this mass balance was considered sufficient to obtain reliable permeability data.
Degradation studies.
Caco-2 cell monolayers grown on filters (22 days) were prepared as described above for the transport experiments but were washed twice with HBSS (30 min total). The experiment was started by addition of 400 μM GPG-amide dissolved in HBSS (pH 6.0) to the apical side and addition of HBSS (pH 7.4) to the basolateral side of the monolayers. In a second set of cell monolayers, GPG-amide (400 μM; pH 7.4) was added to the basolateral side and HBSS (pH 6) was added to the apical side. In parallel, similar experiments were performed in the presence of 400 μM diprotin A in the GPG-amide solution. The filters were incubated on a shaker (100 rpm) at 37°C. The experiments were stopped at different time points, and samples from the apical and basolateral sides were taken and immediately frozen at −20°C. The filters with the cells were then washed three times with ice-cold HBSS, cut out, and frozen after addition of 400 μl of ice-cold MilliQ water. The filters were thawed and frozen twice (5 μl of 20% trifluoroacetic acid [TFA] was added after the first thawing), and the solution was drawn three times through a 27-gauge needle to disrupt the cells; the filter was removed, the suspension was centrifuged, and the supernatant was withdrawn and used for protein concentration determination and HPLC analysis of the metabolites. The experiments were repeated with HBSS (pH 7.4) on the apical side.
Expression of the brush border membrane peptidase CD26 (dipeptidyl peptidase IV) on our Caco-2 cell monolayers was examined by incubation with GP-pNA as the substrate both in the presence and in the absence of the CD26 inhibitor diprotin A (400 μM). Briefly, filters with Caco-2 cell monolayers were washed twice with HBSS (pH 7.4), and fresh HBSS (pH 7.4) was added apically (0.4 ml) and basolaterally (1.2 ml); GP-pNA at a final concentration of 0.5 mM was added to either the apical or the basolateral side, and the filters were incubated at 37°C. Samples of 100 μl were withdrawn from both sides after 3.5 and 9 min, and the absorption was monitored in an enzyme-linked immunosorbent assay plate reader at 405 nm. The experiment was repeated with cell monolayers washed six times to exclude contamination by the protease activity from the growth medium.
Determination of GPG-amide and metabolite concentrations.
The concentrations of GPG-amide, GP, and PG-amide were determined by HPLC at 210 nm on a Beckman Ultrasphere octadecyl silane column (4.6 by 250 mm; 5 μm) by performing isocratic runs with 0.1% TFA as the eluent at a flow rate of 1 ml/min and room temperature. Samples were acidified by addition of 2% TFA (5 μl/100-μl sample) before injection onto the HPLC column. The detection limits were 2 μM for GPG-amide and 4 μM for GP and PG-amide.
In order to detect glycinamide on the HPLC system, it was converted to its dansyl derivative by a protocol slightly modified from that supplied by Covance Laboratories (Harrogate, England). The following procedure was used: 200 μl of the sample was mixed with 80 μl of sodium carbonate (160 mM; pH 9.5) in polypropylene vials, 720 μl of acetonitrile was added, the solution was mixed, and the reaction was started by addition of 88 μl of dansyl chloride (2 mg/ml in CH3CN), followed by incubation at 37°C for 1 h and 10 min. The fluid was removed under a stream of nitrogen (the vials were at 45°C), and the precipitate was redissolved in 1 ml of water-methanol (90/10; vol/vol) and partially purified over Oasis HLB cartridges (Waters, Milford, Mass.); a water-methanol (60/40 vol/vol) washing step was included before elution with 100% methanol. The methanol was evaporated (under a nitrogen stream; the vials were at 40°C), and the residue was taken up by 200 μl of water-methanol (70/30; vol/vol). Depending on the sensitivity of the detector, the samples were diluted fivefold before injection onto the HPLC column. HPLC analysis was carried out with a Phenomenex Synergy Polar RP column (4.6 by 150 mm; 4 μm; 80 Å; Genetec, Gothenburg, Sweden) at 30°C with water-formic acid (100/0.2; vol/vol; eluent A) and methanol-formic acid (100/0.2; vol/vol; eluent B) in the following mobile phase composition: 0 to 8 min with 45% eluent B, 8.1 to 11 min with 95% eluent B, and 11.1 to 21 min with 45% eluent B. The derivative was detected by fluorescence (excitation wavelength, 335 nm; emission wavelength, 500 nm). Neither glycine nor proline interfered with this determination.
Antiviral activities of the substances after Caco-2 cell passage.
To achieve concentrations of the substances after Caco-2 cell passage high enough for the following antiviral tests, the transport experiments were carried out in RPMI 1640 medium, which was also used as the growth medium for the virus-infected cells. Sterile filtered 12 mM GPG-amide in RPMI 1640 medium (with l-glutamine and bicarbonate; Gibco) buffered with 25 mM HEPES (pH 7.4) was added apically to 23-day-old Caco-2 cell monolayers grown on Transwell filters (diameter, 24 mm); the basolateral compartment contained 1.3 ml of RPMI 1640 medium-HEPES. The cells were incubated for 90 min at 37°C while they were shaken at 100 rpm. After incubation, the basolateral fluid containing the substances that passed through the monolayers was collected and pooled. The same process was followed for glycinamide, glycine, and RPMI 1640 medium-HEPES. The concentration of glycinamide in the pooled basolateral RPMI 1640 medium was determined as described above. The pooled fluids were stored at −20°C and sterile filtered before they were added to the virus-infected H9 cell cultures.
Cultures of H9 cells (105 cells in each culture with 0.5 ml of RPMI 1640 medium with 10% FCS and penicillin-streptomycin) were incubated with HIV-1 (100 50% tissue culture infective doses of strain SF2) for 2 h; the virus was then removed by centrifugation. The cells were resuspended in 1 ml of RPMI 1640 medium (with 10% FCS and penicillin-streptomycin) alone or with the substances to be tested for their antiviral activities. The test substances were diluted to comparable concentrations, and three cell cultures were used for each substance and concentration. The cell cultures were maintained in 48-well plates at 37°C in a humidified atmosphere with 5% CO2. The cells were cultured for 11 days; the medium was changed on days 4 and 7 (0.5 ml of supernatant was discarded and 0.5 ml of fresh medium, with or without the test substance, was added to the culture). On day 11, the supernatant from each cell culture was collected and the virus content was quantified by a reverse transcriptase activity quantification assay (Cavidi Tech AB, Uppsala, Sweden).
Statistics.
Experiments were done in triplicate on each occasion. Values are expressed as means ± standard deviations. The differences were analyzed by Student's t test; a P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Transport of 14C-labeled GPG-amide through Caco-2 cell monolayers.
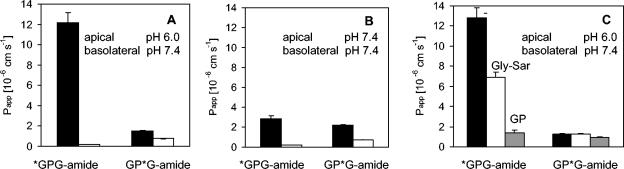
Initial experiments with GPG-amide, labeled at either the N-terminal or the C-terminal glycine, showed that the radioactivity transported through the Caco-2 cell monolayers represents the transport of different peptide degradation products. The difference between the Papp values for apical to basolateral (ap → bl) transport of *GPG-amide and GP*G-amide in the presence of a pH gradient (donor side, pH 6; receiver side, pH 7.4) was of particular interest (Fig. 2). Furthermore, Fig. 2 shows that only the ap → bl transport of the *GPG-amide-derived radioactivity was enhanced by a pH gradient (apical side, pH 6; basolateral side, pH 7.4). This transport enhancement in the presence of a pH gradient suggested that there may be an interaction between *GPG-amide and a pH-dependent transporter such as PepT1.
FIG. 2.
(A and B) Transport of differentially labeled GPG-amide (*GPG-amide andGP*G-amide) across Caco-2 cell monolayers either with a pH gradient (A) (apical side, pH 6.0; basolateral side, pH 7.4) or without a pH gradient (B) (pH 7.4 in both compartments); black bars, ap → bl transport; white bars, bl → ap transport. (C) Papp values for transport of differentially labeled GPG-amide in the apical (pH 6.0) to basolateral (pH 7.4) direction in the absence of PepT1 substrates (black bars) or the presence of PepT1 substrates (white bars, 5 mM Gly-Sar; grey bars, 5 mM GP). Values and error bars represent mean values (from triplicate experiments) ± standard deviations.
To test this hypothesis further, the transport experiments were repeated with the addition of substrates competitive for the PepT1 transporter (Gly-Sar and GP). The transport of *GPG-amide was decreased on addition of either Gly-Sar or GP, while the Papp value for GP*G-amide was not influenced by Gly-Sar and was only slightly decreased on addition of GP (Fig. 2C). Together, these results provide evidence that PepT1 is significantly involved in the transport of the *GPG-amide-derived radioactivity but has a smaller role in the transport of GP*G-amide-derived radioactivity. Thus, it appears that the tripeptide is metabolized before transport across the membrane and that the cleavage occurs after the proline, leaving [14C]GP, which is a substrate for PepT1 (17, 25). Cleavage after the first glycine would result in a 14C-labeled product, which, according to available knowledge, is not transported by PepT1.
Degradation of GPG-amide on Caco-2 cell monolayers.
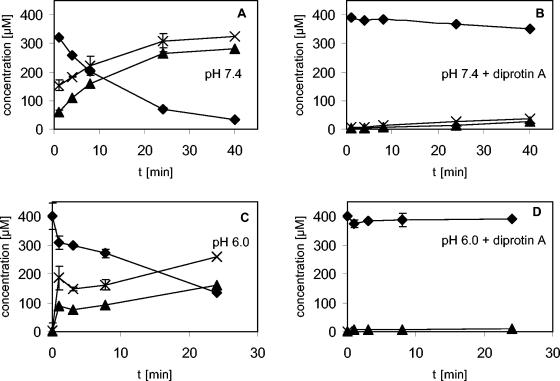
Figure 3 shows the time-dependent degradation of GPG-amide when it was incubated on the apical side of the Caco-2 cell monolayers. Degradation occurred at pH 7.4 as well as at pH 6.0. After 24 min, about 66% (pH 6.0) to 80% (pH 7.4) of the tripeptide disappeared from the apical solution without being detected inside the cells or in the basolateral compartment, while about 40% (pH 6.0) to 66% (pH 7.4) of the original peptide concentration was found as the dipeptide GP in the apical solution. In parallel with GP, glycinamide was identified (Fig. 3), which fits with the formation of a C-terminal cleavage product by proteolysis after the proline in GPG-amide (Fig. 3). Also in keeping with this theory, PG-amide, which would correspond to cleavage after the first glycine, was not detected. Calculation of the mass balance for GPG-amide and GP resulted in 95% recovery in the apical solution after 1 min, and this decreased to about 80% recovery after 40 min. This would fit if some of the metabolite was transported during this time, but the concentrations on the other side of the cell monolayer and in the cells were below the detection limit. In addition, the dipeptide GP, which is not degraded by membrane peptidases (17), can undergo degradation inside the cell: in transport experiments, GP disappeared in a time-dependent manner from the apical solution but was not detected on the other side of the cell monolayers or inside the cell (data not shown) (14).
FIG. 3.
Concentration of GPG-amide (solid diamonds), GP (solid triangles), and glycinamide (crosses) after incubation of 400 μM GPG-amide on the apical side of Caco-2 cell monolayers either in the absence (A and C) or in the presence (B and D) of 400 μM diprotin A. (A and B) Apical and basolateral sides, pH 7.4; (C and D) apical side, pH 6.0; basolateral side, pH 7.4. Values and error bars represent mean values (from triplicate experiments) ± standard deviations.
In the presence of the protease inhibitor diprotin A, less than 10% of GPG-amide was degraded within 24 min (Fig. 3). Diprotin A competitively inhibits CD26 (dipeptidyl peptidase IV; EC 3.4.14.5), an enzyme which removes N-terminal dipeptides, with a preference for peptides which have proline as the second residue (see http://cn.expasy.org/enzyme/). CD26 is expressed in the apical brush border membrane of the intestinal epithelium (13) and is one of several proteases that are expressed in Caco-2 cells (11). The presence of CD26 on the Caco-2 cells used in the present study was confirmed by monitoring the turnover of the CD26 substrate GP-pNA. As expected, a rapid turnover of GP-pNA was observed on the apical side but not the basolateral side of the Caco-2 cell monolayers; the enzyme activity was reduced by 90% when the CD26 inhibitor diprotin A was added. No change in enzyme activity was observed when a more extensive washing procedure was used. It is therefore concluded that apically localized CD26 is the protease responsible for the GPG-amide degradation observed in this study.
When GPG-amide was incubated on the basolateral side of the Caco-2 cell monolayers, only 10% of the original GPG-amide disappeared from the solution and as little as 4.5% was detected as GP in the basolateral solution after 24 min. This strengthens our conclusion that the main metabolic degradation of GPG-amide is accomplished by CD26 in the apical brush border membrane.
The pattern of GPG-amide degradation in our studies was also in agreement with the observed permeability coefficients: the Papp for glycinamide transport (ap → bl) compares well with that for GP*G-amide transport. Furthermore, our results confirm and extend the findings of Balzarini et al. (4), who recently demonstrated that isolated lymphocyte surface glycoprotein CD26 (dipeptidyl peptidase IV) takes GPG-amide as a substrate and that human CD26-active lymphatic cells convert GPG-amide to glycinamide.
Since diprotin A is itself a peptide, which means that it interacts with cellular transporters, it was impossible to study the PepT1-mediated transport, if any, of undegraded GPG-amide.
Transport of glycinamide.
The apparent Papp values for the ap → bl and bl → ap transport of glycinamide are given in Table 1. The extent of transport in the bl → ap direction slightly exceeded that in the ap → bl direction. Without a pH gradient over the cell monolayer, the extent of ap → bl transport was increased compared to that in the presence of a pH gradient. This result reflects the ionization properties of glycinamide, which has a pKa value of 8.1. This means that less than 1% of the glycinamide is not charged at pH 6.0, whereas 16.6% is not charged at pH 7.4. Because the rate of glycinamide transport increased with the fraction of uncharged drug and because passive permeation of the cell is possible only for uncharged drug, at least part of the glycinamide must be transported transcellularly. However, the low molecular mass (74 Da) and the hydrophilicity (calculated partition coefficient between octanol and water [clogP] is equal to −1.98) makes this drug sufficiently membrane impermeant and sufficiently small to be transported mainly via the passive paracellular route (23). Somewhat surprisingly for such a small and hydrophilic substance, the Papp values decreased more than 10-fold if the temperature was lowered from 37 to 4°C. Note, however, that this finding does not necessarily point to an active transport mechanism, as even the well-established paracellular permeability marker polyethylene glycol 900 showed a sevenfold reduction in transport rate across Caco-2 cell monolayers when the temperature was changed from 37 to 4°C (7). Importantly, the transport of glycinamide was not inhibited by addition of 5 mM glycine, Gly-Sar, or GPG-amide, nor was it inhibited by addition of 500 μM tetraethylammonium bromide, indicating that the transport was not mediated by a glycine transporter, PepT1, or an organic cation transporter (data not shown). Moreover, addition of up to 2 mM cold glycinamide to the labeled compound did not influence ap → bl transport, independently of the pH at the apical side. This finding gives further support for a predominantly passive mechanism of glycinamide transport. From these results, it may be tentatively concluded that the main fraction of glycinamide passes the cell monolayer by the paracellular route, as shown in Fig. 4.
TABLE 1.
Papp values for ap → bl and bl → ap transport of glycinamide (40 μM) across Caco-2 cell monolayers
| Temp and pH (ap/bl) | Mean Papp (10−6 cm s−1) ± SDa
|
|
|---|---|---|
| ap → bl | bl → ap | |
| 37°C | ||
| 6.0/7.4 | 1.5 ± 0.1 | 2.3 ± 0.1 |
| 7.4/7.4 | 2.0 ± 0.3 | 2.7 ± 0.1 |
| 4°C | ||
| 6.0/7.4 | 0.13 ± 0.02 | 0.12 ± 0.01 |
| 7.4/7.4 | 0.15 ± 0.03 | 0.12 ± 0.01 |
n = 3 to 11 for each experiment. Differences between the values at 37°C (ap → bl compared to bl → ap and pH gradient compared to no pH gradient) are statistically significant (P < 0.05).
FIG. 4.
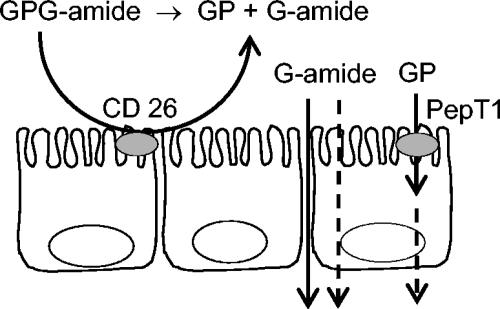
Model of the metabolism and transport of the prodrug GPG-amide in the intestinal epithelium. The tripeptide is degraded by CD26 (dipeptidyl peptidase IV) in the apical membrane, and the inactive metabolite GP is transported into the cells via the peptide transporter PepT1 (14), while the active antiviral metabolite, glycinamide, is transported across the cell monolayer by passive diffusion via paracellular and transcellular pathways.
Antiviral activities of glycinamide and GPG-amide after transport through Caco-2 cell monolayers.
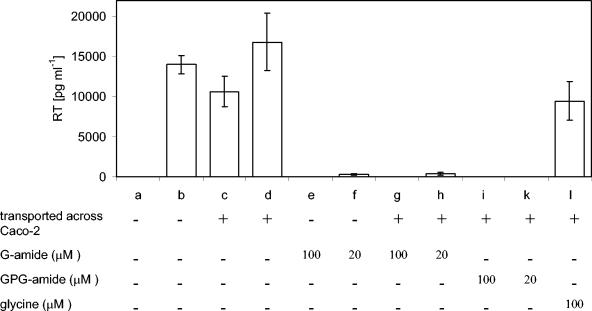
As the tripeptide was degraded by contact with the apical side of the Caco-2 cell monolayers, the antiviral activities of GPG-amide and its metabolite, glycinamide, were studied after transport through Caco-2 cell monolayers. Since GP has no antiretroviral activity (21), it was not further studied. Figure 5 shows the amount of the viral reverse transcriptase in HIV-1-infected H9 cells after various treatments. Both GPG-amide and glycinamide exhibited antiviral activities after transport through Caco-2 cell monolayers; these antiviral activities were not mediated by the glycine transported through Caco-2 cell monolayers or by the RPMI 1640 medium. The antiviral activity of the transported glycinamide was comparable to that displayed by untreated glycinamide. Consequently, the tripeptide GPG-amide may be considered a prodrug, which releases the new antiviral compound glycinamide after hydrolysis by CD26. Since GPG-amide is degraded rapidly after apical incubation with Caco-2 cells (Fig. 3), it is unlikely that the antiviral activity of GPG-amide after transport through the cell monolayer originates only from GPG-amide, which leaks through the tight junctions between cells and which is subsequently activated by the culture of infected H9 cells. Preliminary results from a recently performed transport experiment, in which we used detection by mass spectrometry, gave an estimated Papp value of about 2 × 10−8 cm s−1; extrapolation gives an estimated concentration in the nanomolar range for the experiment whose results are shown in Fig. 5. This is at least 50-fold lower than the 50% inhibitory concentration of GPG-amide (5 μM) (20); therefore, it is unlikely that the antiviral effect originated from undegraded GPG-amide.
FIG. 5.
Antiviral activities of substances after transport across Caco-2 cell monolayers. The viral load of the cell cultures is expressed as the amount of reverse transcriptase (RT) in HIV-1-infected H9 cells (bars b to l) or uninfected cells (bar a). Bars b to d are negative controls, in which RPMI 1640 medium (b) or RPMI 1640 medium from the basolateral side of the Caco-2 cell monolayers diluted with fresh RPMI 1640 medium in the same proportions as the transported 100 μM (c) or 20 μM (d) solutions were used. Additional treatments are indicated. Values and error bars represent mean values (from triplicate experiments) ± standard deviations.
Although the exact mechanism by which G-amide inhibits HIV replication is not clear, it has been shown by transmission electron microscopy that in the presence of glycinamide in HIV-infected cultures, the virus particles produced have defective core structures (1).
Finally, if glycinamide is to be further developed as an antiretroviral drug candidate, it must be systemically available after oral administration. Several studies correlated the passive drug permeation obtained in studies with Caco-2 cells and the absorption of the test drug after oral administration to humans (see, for example, references 3, 5, and 19). In different laboratories, the lowest Papp values that predicted the complete absorption of passively transported compounds such as glycinamide range from 1 × 10−6 cm s−1 (3) to 7 × 10−5 cm s−1 (16). For comparison, the Papp of glycinamide observed in the present study was about 2 × 10−6 cm s−1. Thus, if glycinamide is found to be sufficiently soluble and stable in the intestinal lumen and does not undergo presystemic metabolism, this Papp value indicates that glycinamide is likely to be significantly absorbed by the oral route and that further development of glycinamide as a drug candidate is warranted.
Acknowledgments
This work was financially supported by the Swedish Agency for Innovation Systems (Vinnova), Tripep AB, Huddinge, Sweden, and the Swedish Research Council (Vetenskapsrådet; grant 9478). A. Vahlne is stock owner and responsible for research at Tripep AB.
We thank Pia Österwall, Huddinge University Hospital, for the experimental work with the HIV-infected cell cultures.
REFERENCES
- 1.Andersson, E., P. Horal, A. Jejcic, S. Hoglund, J. Balzarini, A. Vahlne, and B. Svennerholm. 2005. Glycine-amide is an active metabolite of the antiretroviral tripeptide glycyl-prolyl-glycine-amide. Antimicrob. Agents Chemother. 49:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, E., P. Horal, A. Vahlne, and B. Svennerholm. 2004. No cross-resistance or selection of HIV-1 resistant mutants in vitro to the antiretroviral tripeptide glycyl-prolyl-glycine-amide. Antivir. Res. 61:119-124. [DOI] [PubMed] [Google Scholar]
- 3.Artursson, P., and J. Karlsson. 1991. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 175:880-885. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., E. Andersson, D. Schols, P. Proost, J. van Damme, B. Svennerholm, P. Horal, and A. Vahlne. 2004. Obligatory involvement of CD26/dipeptidyl peptidase IV in the activation of the antiretroviral tripeptide glycylprolylglycinamide (GPG-NH2). Int. J. Biochem. Cell Biol. 36:1848-1859. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom, C. A., M. Strafford, L. Lazorova, A. Avdeef, K. Luthman, and P. Artursson. 2003. Absorption classification of oral drugs based on molecular surface properties. J. Med. Chem. 46:558-570. [DOI] [PubMed] [Google Scholar]
- 6.Brodin, B., C. U. Nielsen, B. Steffansen, and S. Frokjaer. 2002. Transport of peptidomimetic drugs by the intestinal di/tri-peptide transporter, PepT1. Pharmacol. Toxicol. 90:285-296. [DOI] [PubMed] [Google Scholar]
- 7.Cogburn, J. N., M. G. Donovan, and C. S. Schasteen. 1991. A model of human small intestinal absorptive cells. 1. Transport barrier. Pharm. Res. 8:210-216. [DOI] [PubMed] [Google Scholar]
- 8.Gulick, R. M. 2003. New antiretroviral drugs. Clin. Microbiol. Infect. 9:186-193. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Ruiz, D., Q. Wang, O. S. Gudmundsson, T. J. Cook, R. L. Smith, T. N. Faria, and G. T. Knipp. 2001. Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS PharmSci. 3:E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoglund, S., J. Su, S. S. Reneby, A. Vegvari, S. Hjerten, I. M. Sintorn, H. Foster, Y. P. Wu, I. Nystrom, and A. Vahlne. 2002. Tripeptide interference with human immunodeficiency virus type 1 morphogenesis. Antimicrob. Agents Chemother. 46:3597-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell, S., A. J. Kenny, and A. J. Turner. 1992. A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem. J. 284(Pt 2):595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, V. H. 2000. Membrane transporters. Eur. J. Pharm. Sci. 11(Suppl. 2):S41-S50. [DOI] [PubMed] [Google Scholar]
- 13.Matter, K., M. Brauchbar, K. Bucher, and H. P. Hauri. 1990. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell 60:429-437. [DOI] [PubMed] [Google Scholar]
- 14.Myara, I., C. Charpentier, and A. Lemonnier. 1984. Prolidase and prolidase deficiency. Life Sci. 34:1985-1998. [DOI] [PubMed] [Google Scholar]
- 15.Pehrson, P. O., A. Sonnerborg, A. Vahlne, and J. Spira. 2000. An initial safety and efficacy clinical trial of GPG-NH2, a tripeptide with a new antiretroviral made of action. AIDS 14:S17-S18. [Google Scholar]
- 16.Rubas, W., N. Jezyk, and G. M. Grass. 1993. Comparison of the permeability characteristics of a human colonic epithelial (Caco-2) cell line to colon of rabbit, monkey, and dog intestine and human drug absorption. Pharm. Res. 10:113-118. [DOI] [PubMed] [Google Scholar]
- 17.Rubino, A., and S. Guandalini. 1977. Dipeptide transport in the intestinal mucosa of developing rabbits. Ciba Found. Symp. 1977:61-77. [DOI] [PubMed] [Google Scholar]
- 18.Sai, Y., Y. Kato, I. Tamai, A. Tsuji, A.-C. Svensson, and P. Artursson. 2003. Quantification of expression levels of intestinal transporters and application to oral drug delivery. Annu. Meet. Japanese Soc. Study Xenobiotics, abstr. 18.
- 19.Stenberg, P., U. Norinder, K. Luthman, and P. Artursson. 2001. Experimental and computational screening models for the prediction of intestinal drug absorption. J. Med. Chem. 44:1927-1937. [DOI] [PubMed] [Google Scholar]
- 20.Su, J., E. Andersson, P. Horal, M. H. Naghavi, A. Palm, Y. P. Wu, K. Eriksson, M. Jansson, H. Wigzell, B. Svennerholm, and A. Vahlne. 2001. The nontoxic tripeptide glycyl-prolyl-glycine amide inhibits the replication of human immunodeficiency virus type 1. J. Hum. Virol. 4:1-7. [PubMed] [Google Scholar]
- 21.Su, J., M. H. Naghavi, A. Jejcic, P. Horal, Y. Furuta, Y. P. Wu, S. L. Li, W. W. Hall, L. Goobar-Larsson, B. Svennerholm, and A. Vahlne. 2001. The tripeptide glycyl-prolyl-glycine amide does not affect the early steps of the human immunodeficiency virus type 1 replication. J. Hum. Virol. 4:8-15. [PubMed] [Google Scholar]
- 22.Tavelin, S., J. Grasjo, J. Taipalensuu, G. Ocklind, and P. Artursson. 2002. Applications of epithelial cell culture in studies of drug transport. Methods Mol. Biol. 188:233-272. [DOI] [PubMed] [Google Scholar]
- 23.Tavelin, S., J. Taipalensuu, L. Soderberg, R. Morrison, S. Chong, and P. Artursson. 2003. Prediction of the oral absorption of low-permeability drugs using small intestine-like 2/4/A1 cell monolayers. Pharm. Res. 20:397-405. [DOI] [PubMed] [Google Scholar]
- 24.Walker, D., D. T. Thwaites, N. L. Simmons, H. J. Gilbert, and B. H. Hirst. 1998. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. 507(Pt 3):697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter, E., T. Kissel, and G. L. Amidon. 1996. The intestinal peptide carrier: a potential transport system for small peptide derived drugs. Adv. Drug Deliv. Rev. 20:33-58. [Google Scholar]