Abstract
The in vitro activity of tebipenem (TBM), a new oral carbapenem antibiotic, against Streptococcus pneumoniae clinical isolates (n = 202) was compared with those of 15 reference agents. The isolates were classified into five genotypic classes after PCR identification of abnormal pbp1a, pbp2x, and pbp2b genes: (i) penicillin-susceptible S. pneumoniae (PSSP) isolates with no abnormal pbp genes (n = 34; 16.8%), (ii) genotypic penicillin-intermediate S. pneumoniae (gPISP) isolates with only an abnormal pbp2x gene [gPISP (2x)] (n = 48; 23.8%), (iii) gPISP isolates with abnormal pbp1a and pbp2x genes (n = 32; 15.8%), (iv) gPISP isolates with abnormal pbp2x and pbp2b genes (n = 16; 7.9%), and (v) genotypic penicillin-resistant S. pneumoniae (gPRSP) isolates with three abnormal pbp genes (n = 72; 35.6%). The majority of the strains tested had mefA (n = 59; 29.2%) or ermB (n = 91; 45%) gene-mediating macrolide resistance. For these isolates the MIC at which 90% of isolates are inhibited was significantly lower for TBM than for the reference oral antibiotics, as follows: 0.002 μg/ml for PSSP, 0.004 μg/ml for gPISP (2x), 0.016 μg/ml for gPISP (isolates with abnormal pbp1a and pbp2x genes and isolates with abnormal pbp2x and pbp2b genes), and 0.063 μg/ml for gPRSP. In addition, TBM showed excellent bactericidal activity against gPRSP isolates, which exhibited a 3-log10 decrease within 2 h when they were incubated with a concentration greater than or equal to the MIC. Inhibition of cell wall synthesis toward the long axis and subsequent cell lysis were observed by scanning electron microscopy after a short-term exposure to TBM, unlike the effects seen with cephalosporins. These data suggest that TBM has potent activity against multidrug-resistant S. pneumoniae, the causative pathogen of community-acquired respiratory tract infections.
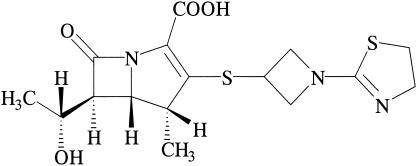
It is well known that carbapenem antibiotics have broad-spectrum activities and strong bactericidal actions against members of the family Enterobacteriaceae, Pseudomonas aeruginosa, and gram-positive cocci except methicillin-resistant Staphylococcus aureus and metallo-β-lactamase-producing pathogens. Four parenteral carbapenem agents, imipenem (14, 17), panipenem (24), meropenem (8, 28, 37), and biapenem (12), are used clinically in Japan as chemotherapeutic agents for the treatment of severe bacterial infections. However, no oral carbapenem antibiotic has yet been marketed. Tebipenem (TBM)-pivoxil (PI), a novel oral carbapenem agent with a 1-(1,3-thiazolin-2-yl) azetidin-3-ylthio group at the C-2 position, was developed by Wyeth Lederle Japan, Co. Ltd. (Tokyo, Japan) in 1994. The active metabolite of TBM was previously reported to be LJC11,036 (11) (Fig. 1), which in vitro shows broad-spectrum and potent activity against microorganisms that cause respiratory tract infections (RTIs) and urinary tract infections (UTIs) (11, 20). The agent also shows a high degree of stability to dehydropeptidase-I; and absorption of the active metabolite, which is converted by esterase, into blood from the intestine has been shown to be good in phase I clinical studies (M. Yokokawa, M. Yano, and M. Nakashima, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F388, 1999). Phase II clinical studies of TBM-PI are now being conducted by Meiji Seika Kaisha, Ltd. (Tokyo, Japan), in Japan.
FIG. 1.
Chemical structure of TBM.
In this study, we evaluated the in vitro antibacterial and bactericidal activities of TBM against penicillin (PEN)-nonsusceptible Streptococcus pneumoniae isolates in comparison with those of 15 reference agents. The damage of PEN-resistant S. pneumoniae (PRSP) cells after exposure to TBM was also observed by scanning electron microscopy (SEM).
MATERIALS AND METHODS
Microorganisms and growth conditions.
S. pneumoniae strains (n = 202) from clinical samples collected from pediatric patients with RTIs (n = 173), acute otitis media (n = 10), sepsis (n = 9), and meningitis (n = 10) were isolated in our laboratory between April 2002 and December 2002. The isolates were routinely grown on sheep blood agar II plates (Nippon Becton Dickinson, Tokyo, Japan) at 37°C in a 5% CO2 atmosphere. After several purifications, they were stored at −80°C in 10% skim milk (Difco Laboratories, Detroit, Mich.) for subsequent use. These isolates were identified as S. pneumoniae by PCR for the lytA gene (9).
MIC determination.
The MIC of each antibiotic was determined by the agar dilution method with cation-adjusted Mueller-Hinton (MH) agar (Difco Laboratories) supplemented with 5% defibrinated sheep blood (23). The size of the bacterial inoculum was adjusted to 105 CFU/spot by using bacteria precultured on blood agar II plates at 37°C in a 5% CO2 atmosphere for 18 h. The MIC was defined as the lowest antibiotic concentration which inhibited visible growth after 18 to 24 h of incubation. S. pneumoniae ATCC 49619 was used as a quality control strain for susceptibility testing.
Antibiotics.
An active metabolite of TBM-PI synthesized at the Medical Research Laboratories, Wyeth Lederle Japan Co., Ltd., was supplied through Meiji Seika Kaisha, Ltd. Fifteen reference agents were obtained from the respective pharmaceutical companies; PEN, ampicillin (AMP), amoxicillin (AMX), and cefditoren (CDN) from Meiji Seika Kaisha, Ltd.; cefaclor (CEC) and cefcapene (CFN) from Shionogi Co., Ltd. (Osaka, Japan); cefdinir (CDR) from Fujisawa Pharmaceutical Co., Ltd. (Osaka, Japan); cefpodoxime (CPD) from Sankyo Co., Ltd. (Tokyo, Japan); faropenem (FRM) from Suntory Ltd. (Osaka, Japan); clarithromycin (CLR) from Taisho-Toyama Pharmaceutical Co., Ltd. (Tokyo, Japan); azithromycin (AZM) from Pfizer Japan Inc. (Tokyo, Japan); telithromycin (TEL) from Aventis Pharma Ltd. (Tokyo, Japan); clindamycin (CLI) from Pharmacia & Upjohn Co., Ltd. (Tokyo, Japan); and levofloxacin (LVX) from Daiichi Pharmaceutical Co., Ltd. (Tokyo, Japan).
Cefotaxime (CTX) was obtained from Chugai Pharmaceutical Co., Ltd., and was used as the standard reference for intravenous agents.
PCR conditions.
Abnormal pbp genes encoding PBP 1A (3, 19, 27), PBP 2X (4, 18, 25), and PBP 2B (6, 36) were detected by PCR. The conditions and primers used in this study are those described previously (22, 33). Each PEN-binding protein (PBP) gene was amplified only from strains with the same pbp1a, pbp2x, and pbp2b gene sequences as susceptible strain R6 at the primer binding sites. These sites were positioned in blocks of highly diverged sequences and in the sequence of conserved amino acids motifs, such as Ser-Thr-Met-Lys of the pbp1a gene in PRSP strains. On the basis of the PCR results, the strains tested were classified into five groups according to their pbp genotypes, as follows: (i) PEN-susceptible S. pneumoniae (PSSP) isolates with three normal pbp genes, (ii) genotypic PEN-intermediate S. pneumoniae (gPISP) isolates with abnormal pbp2x genes [gPISP (2x)], (iii) gPISP isolates with abnormal pbp1a and pbp2x genes, (iv) gPISP isolates with abnormal pbp2x and pbp2b genes, and (v) genotypic PRSP (gPRSP) isolates with three abnormal pbp genes.
In addition, the ermB (30) and mefA (26, 29) genes, which mediate macrolide resistance, were detected by PCR, together with the pbp and the lytA genes (31, 32).
Killing kinetics.
Time-kill curves of TBM and reference antibiotics for JPS240 (serotype 6B) and ME19 (serotype 19F) strains of PRSP were determined at concentrations corresponding to the MIC, two times the MIC, and four times the MIC for each strain. A bacterial suspension (500 μl) in MH broth supplemented with 5% defibrinated sheep blood was grown at 37°C for 3 h and was then inoculated into 9.5 ml of fresh MH broth containing each of the antibiotics to be tested and 5% sheep blood. The tubes were then incubated without shaking, and the cultures were sampled at predetermined intervals. The samples were serially diluted 10-fold with MH broth, and 100 μl of each diluted sample was plated in triplicate on blood agar plates. The numbers of colonies that grew on the blood agar plates were counted after incubation at 37°C in a 5% CO2 atmosphere for 20 h.
SEM.
PRSP strain ME19 was grown in Todd-Hewitt broth, to avoid contamination with blood, at 37°C in a 5% CO2 atmosphere for 18 h. Three milliliters of the culture was inoculated to 100 ml of fresh Todd-Hewitt broth and was cultured continuously at 37°C for 3 h. Subsequently, antibiotics were added to the broth and the incubation was continued at 37°C for 2 h. After fixation with 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 6.8) at room temperature for 20 min, the cells were harvested by centrifugation at 1,400 × g for 5 min and fixed with 2% glutaraldehyde at 4°C for 2 h and then with 1% osmium tetroxide at 4°C overnight. After the specimens were dried, they were coated with a 2-nm layer of platinum-carbon (16) and were observed under an ultra-high-resolution, low-voltage (LV) scanning electron microscope (S-900LV; commercially available as model S-900H [Hitachi, Tokyo, Japan]) at 2 kV.
RESULTS
Activity of TBM against S. pneumoniae.
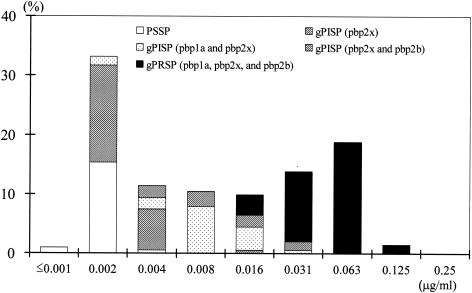
Table 1 shows the activities of TBM and 10 reference β-lactam antibiotics against S. pneumoniae isolates obtained from pediatric patients with RTIs, acute otitis media, sepsis, and meningitis. Figure 2 shows the distributions of the susceptibilities of these isolates to TBM according to their genotypes. All strains were classified into five genotypic classes according to the presence of abnormal PBP genes, namely, pbp1a, pbp2x, and pbp2b, as identified by PCR: (i) PSSP isolates with no abnormal PBP gene (n = 34; 16.8%); (ii) gPISP (2x) isolates with an abnormal pbp2x gene (n = 48; 23.8%); (iii) gPISP isolates with abnormal pbp1a and pbp2x genes (n = 32; 15.8%); (iv) gPISP isolates with abnormal pbp2x and pbp2b genes (n = 16; 7.9%); and (v) gPRSP isolates with abnormal pbp1a, pbp2x, and pbp2b genes (n = 72; 35.6%).
TABLE 1.
Comparative in vitro activities of tebipenem and reference β-lactam antibiotics against S. pneumoniae isolates classified genotypically according to abnormal PBP genes by PCR
| Antibiotic | MIC (μg/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSSP (n = 34)
|
gPISP (n = 48)a
|
gPISP (n = 48)b
|
gPRSP (n = 72)c
|
|||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |
| Tebipenem | <0.001-0.004 | 0.002 | 0.002 | 0.002-0.016 | 0.002 | 0.004 | 0.002-0.031 | 0.008 | 0.016 | 0.016-0.125 | 0.063 | 0.063 |
| Penicillin G | 0.008-0.063 | 0.016 | 0.031 | 0.031-0.25 | 0.063 | 0.063 | 0.063-0.5 | 0.25 | 0.25 | 0.5-4 | 2 | 2 |
| Ampicillin | 0.008-0.031 | 0.016 | 0.031 | 0.031-0.5 | 0.031 | 0.063 | 0.063-1 | 0.25 | 0.5 | 0.5-4 | 2 | 2 |
| Amoxicillin | 0.008-0.063 | 0.016 | 0.031 | 0.016-0.25 | 0.063 | 0.063 | 0.063-0.5 | 0.125 | 0.5 | 0.25-4 | 1 | 2 |
| Cefotaxime | 0.008-0.25 | 0.016 | 0.125 | 0.031-0.5 | 0.125 | 0.25 | 0.063-2 | 0.5 | 1 | 0.25-8 | 0.5 | 1 |
| Cefaclor | 0.25-1 | 0.5 | 1 | 0.5-8 | 1 | 1 | 0.5-16 | 8 | 8 | 16-64 | 64 | 64 |
| Cefpodoxime | 0.016-0.5 | 0.031 | 0.25 | 0.063-2 | 0.5 | 1 | 0.125-8 | 2 | 4 | 1-32 | 2 | 4 |
| Cefdinir | 0.031-0.25 | 0.063 | 0.25 | 0.063-1 | 0.25 | 0.5 | 0.125-4 | 2 | 2 | 2-32 | 4 | 8 |
| Cefditoren | 0.004-0.063 | 0.008 | 0.031 | 0.016-0.25 | 0.063 | 0.125 | 0.063-1 | 0.5 | 0.5 | 0.25-8 | 0.5 | 1 |
| Cefcapene | 0.004-0.25 | 0.008 | 0.125 | 0.031-0.5 | 0.125 | 0.5 | 0.063-2 | 0.5 | 1 | 0.25-8 | 0.5 | 1 |
| Faropenem | 0.008-0.016 | 0.008 | 0.008 | 0.008-0.063 | 0.008 | 0.016 | 0.016-0.25 | 0.031 | 0.125 | 0.063-1 | 0.25 | 0.5 |
Clinical isolates of S. pneumoniae that have an abnormal pbp2x gene.
Clinical isolates that have abnormal pbp2x and pbp2b or pbp1a and pbp2x genes.
Clinical isolates that have three abnormal PBP genes: pbp1a, pbp2x, and pbp2b.
FIG. 2.
Distribution of MICs of TBM for clinical isolates of S. pneumoniae (n = 202) classified into groups PSSP, gPISP, and gPRSP according to their susceptibilities to TBM.
The MIC range, the MIC at which 50% of isolates are inhibited (MIC50), and the MIC90 of TBM for the PSSP, gPISP (2x), gPISP, and gPRSP isolates indicated that TBM had stronger antibacterial activities than the other antibiotics. The MIC90s for the gPRSP isolates were as follows: TBM, 0.063 μg/ml; FRM, 0.5 μg/ml; CTX, CDN, and CFN, 1 μg/ml; PEN, AMP, and AMX, 2 μg/ml; CPD, 4 μg/ml; CDR, 8 μg/ml; and CEC, 64 μg/ml. Of these β-lactam agents, CPD, CDR, FRM, CDN, and CFN were developed by Japanese pharmaceutical companies and are marketed mainly in Japan.
Namely, TBM was very active against the isolates tested, with MICs of 0.002 to 0.031 μg/ml for the gPISP isolates and 0.016 to 0.125 μg/ml for the gPRSP isolates. The MICs of TBM ranged from ≤0.001 to 0.004 μg/ml for all PSSP and gPISP (2x) isolates except one strain that had two amino acid substitutions in Ser-Thr-Met-Lys (STMK) of the conserved motif.
Table 2 shows the MIC ranges, MIC50S, and MIC90s of CLR, AZM, TEL, CLI, and LVX for S. pneumoniae strains. The frequencies at which strains possessed the mefA and the ermB genes were 29.2% (n = 59) and 45% (n = 91), respectively. The MIC90s for all strains were as follows: TEL, 0.125 μg/ml; LVX, 2 μg/ml; and CLR, AZM, and CLI, 64 μg/ml.
TABLE 2.
In vitro activities of macrolide antibiotics, telithromycin, and levofloxacin against S. pneumoniae isolatesa
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Clarithromycin | 0.031-64 | 4 | 64 |
| Azithromycin | 0.031-64 | 16 | 64 |
| Clindamycin | 0.016-64 | 0.063 | 64 |
| Telithromycin | 0.016-4 | 0.063 | 0.125 |
| Levofloxacin | 0.5-4 | 1 | 2 |
A total of 202 isolates were tested.
Killing kinetics.
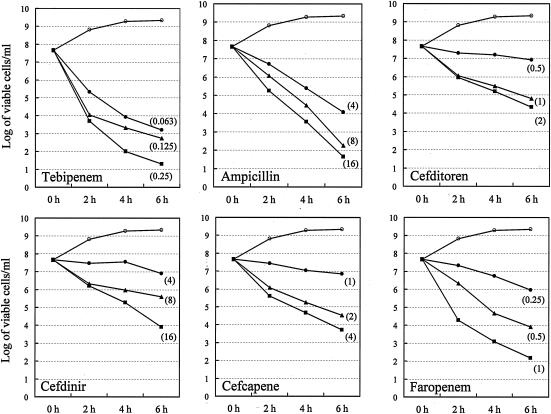
Figure 3 shows the time-kill curves for TBM and the reference β-lactam antibiotics (AMP, CDN, CDR, CFN, and FRM) at concentrations corresponding to the MIC, two times the MIC, and four times the MIC for PRSP strain JPS240 (serotype 6B). This strain was isolated from the cerebrospinal fluid of a pediatric patient with meningitis.
FIG. 3.
Kinetic curves of bacterial killing by TBM and the other five β-lactam antibiotics at the MICs (•), two times the MICs (▴), and four times the MICs (▪) for PRSP strain JPS240 (serotype 6B). ○, control (no drug).
Unlike the five reference antibiotics, TBM exhibited an apparent bactericidal effect at concentrations higher than the MIC. TBM showed the strongest bactericidal activity as early as 2 h after exposure at two times the MIC, followed by AMP and FRM (4 h at two times the MIC), CDN and CFN (6 h at two times the MIC), and CDR (>6 h at two times the MIC), with the reduction being on the order of 3 log units of viable cells.
The bactericidal activity of TBM against PRSP strain ME19 (serotype 19F) was similar to that against PRSP strain JPS240 (data not shown).
Morphological changes.
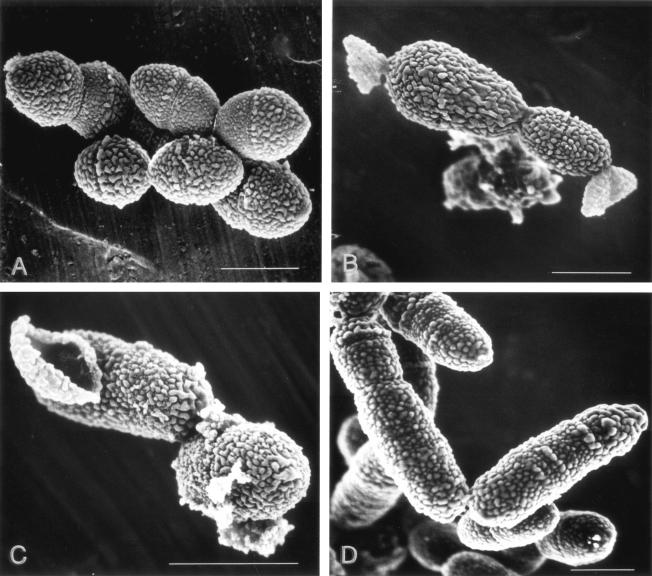
Figure 4 shows the morphological changes of PRSP ME19 cells following exposure to TBM at the MIC and two times the MIC and reference antibiotic CDN at the MIC for 2 h. These observations were made under an LV scanning electron microscope. As shown for the control cells (Fig. 4A), we presumed that the homogeneous protrusions on the spongiform surface were part of the cell wall. We also considered that the cell wall synthesis toward the long axis occurred in the shape of a coil paralleling a septum, because protrusions were observed in a parallel arrangement with the septum.
FIG. 4.
Morphological changes to S. pneumoniae ME19 (serotype 19F) cells (A) after exposure to TBM at its MIC (0.063 μg/ml) (B) and two times its MIC (0.125 μg/ml) (C) and CDN at its MIC (0.5 μg/ml) (D). Cells were observed with a scanning electron microscope (model S-900H; Hitachi). Each bar indicates 1 μm.
Cell wall synthesis stopped in the pneumococcal cells exposed to TBM at 0.063 μg/ml (the MIC) and 0.125 μg/ml (two times the MIC) for 2 h, and the protrusions were pulled in the direction of the long axis with the swelling of the cell (Fig. 4B). After that, cell lysis was observed from the sites considered to be fragile (Fig. 4B and C). The effect of exposure for more than 4 h could not be examined by SEM because of complete cell lysis.
With exposure to cephalosporins, such as CDN (Fig. 4D), cell lysis occurred following marked cell elongation due to the inhibition of septum formation.
DISCUSSION
Severe infections caused by PEN-nonsusceptible S. pneumoniae and/or macrolide-antibiotic resistant isolates have been a matter of concern worldwide (1, 13, 15). In Japan, much attention has been given to the increased incidence of PEN-nonsusceptible S. pneumoniae since the late 1980s (2). Now, the incidence of these isolates is higher in Japan than in the United States and Europe (31, 34).
One conceivable reason for this may be differences in the rates of use of oral antibiotics by outpatients in different countries. In the 1980s in Japan, attention was focused on β-lactamase-producing pathogens; therefore, many oral cephalosporin antibiotics with an amino-thiazol side chain at position 7 were developed. Regrettably, the development of those agents took place before PEN-nonsusceptible S. pneumoniae isolates became prevalent (10, 35). Consequently, their clinical efficacies for the treatment of RTIs caused by PISP and PRSP isolates were not evaluated correctly. Although the clinical efficacies of cephalosporin antibiotics for pneumococcal infections refer to the parameter time above the MIC (7), the concentrations of new oral cephalosporins in serum are usually low, ranging from 0.5 to 1.5 μg/ml after the administration of a single dose of 100 mg in adults.
From the background presented above, the development of new oral β-lactam antibiotics with excellent bactericidal activities against PRSP and high degrees of bioavailability has been expected in Japan. TBM, which is under development in Japan, is one of the new carbapenem antibiotics expected to fulfill such requirements. The prominent feature of TBM is its potent activity against the main causative microorganisms, except metallo-β-lactamase-producing pathogens and methicillin-resistant S. aureus strains, in outpatients with RTIs and UTIs (11, 20).
As for PBPs related to β-lactam resistance in S. pneumoniae, TBM shows higher affinities for PBP 1A and PBP 2B, high-molecular-weight enzymes, and for PBP 3, a low-molecular-weight enzyme, than for PBP 2X (11).
As shown in Results, in addition to the excellent MIC90 of TBM for PISP and PRSP isolates, strong bactericidal activity accompanied by cell lysis was observed within a short time after exposure to TBM at a concentration equal to or greater than the MIC. These results may be caused by its unique affinity for PBPs. Moreover, this bactericidal action might be reflected in the efficacy of TBM observed in a murine pneumonia model (20).
As Craig (5) stated, the time above the MIC is generally believed to be the major pharmacokinetic-pharmacodynamic parameter determining the in vivo efficacies of β-lactams, unlike aminoglycosides and fluoroquinolones. However, in the TBM study using a mouse PRSP infection model, a high correlation was observed between the in vivo efficacy and the maximum concentration of drug in plasma/MIC or the area under the concentration-time curve/MIC rather than the time above the MIC as a pharmacokinetic-pharmacodynamic parameter (Meiji Seika Kaisha, Ltd., personal communication). The great difference between these results and common knowledge about β-lactam antibiotics is probably due to the strong bactericidal activity of TBM described in the study (21).
Phase I clinical studies have already been conducted with TBM. The values of the pharmacokinetic parameters for TBM after the administration of a single dose of 150 mg to healthy volunteers were as follows: 5.85 ± 2.35 μg/ml for the maximum concentration of drug in plasma, 0.58 ± 0.42 h for the time to the maximum concentration of drug in plasma, 0.50 ± 0.26 h for the half-life, 5.57 ± 1.08 μg · h/ml for the area under the concentration-time curve, and 73.4% ± 6.7% for urinary excretion within 6 h.
Phase II clinical studies with adult patients are in progress. The bacteriological effects of TBM against PRSP presented in this study will be clarified through the analysis of data from phase II clinical studies in the near future.
Acknowledgments
We thank Akiko Ono, Naoko Chiba, and Somey Y. Murayama for technical assistance.
We thank Meiji Seika Kaisha, Ltd., for financial support.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Arimasu, O., H. Meguro, H. Shiraishi, K. Sugamata, and F. Hiruma. 1988. A case of pneumococcal meningitis resistant to beta-lactam antibiotic treatment. Kansenshogaku Zasshi 62:682-683. (In Japanese.) [PubMed] [Google Scholar]
- 3.Asahi, Y., and K. Ubukata. 1998. Association of a Thr-371 substitution in a conserved amino acid motif of penicillin-binding protein 1A with penicillin resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahi, Y., Y. Takeuchi, and K. Ubukata. 1999. Diversity of substitutions within or adjacent to conserved amino acid motifs of penicillin-binding protein 2X in cephalosporin-resistant Streptococcus pneumoniae isolates. Antimicrob. Agents Chemother. 43:1252-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 6.Dowson, C. G., A. Hutchison, and B. G. Spratt. 1989. Nucleotide sequence of the penicillin-binding protein 2B gene of Streptococcus pneumoniae strain R6. Nucleic Acids Res. 17:7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl.):38-44. [PubMed] [Google Scholar]
- 8.Edwards, J. R. 1995. Meropenem: a microbiological overview. J. Antimicrob. Chemother. 36(Suppl. A):1-17. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, P., J. L. Garcia, E. Garcia, and R. Lopez. 1986. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promotor in Escherichia coli. Gene 43:265-272. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa, K., K. Yamamoto, N. Chiba, R. Kobayashi, K. Nagai, M. R. Jacobs, P. C. Appelbaum, K. Sunakawa, and K. Ubukata. 2003. Diversity of amoxicillin-resistance genes in Haemophilus influenzae in Japan and the United States. Microbiol. Drug Resist. 9:39-46. [DOI] [PubMed] [Google Scholar]
- 11.Hikida, M., K. Itahashi, A. Igarashi, T. Shiba, and M. Kitamura. 1999. In vitro antibacterial activity of LJC 11,036, an active metabolite of L-084, a new oral carbapenem antibiotic with potent antipneumococcal activity. Antimicrob. Agents Chemother. 43:2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hikida, M., K. Kawashima, K. Nishiki, Y. Furukawa, K. Nishizawa, I. Saito, and S. Kuwao. 1992. Renal dehydropeptidase-I stability of LJC 10,627, a new carbapenem antibiotic. Antimicrob. Agents Chemother. 36:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebner, R. E., A. D. Wasas, and K. P. Klugman. 2000. Trends in antimicrobial resistance and serotype distribution of blood and cerebrospinal fluid isolates of Streptococcus pneumoniae in South Africa, 1991-1998. Int. J. Infect. Dis. 4:214-218. [DOI] [PubMed] [Google Scholar]
- 14.Kesado, T., T. Hashizume, and Y. Asahi. 1980. Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics. Antimicrob. Agents Chemother. 17:912-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klugman, K. P. 1990. Pneumococcal resistance to antibiotics. Clin. Microbiol. Rev. 3:171-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konomi, M., K. Fujimoto, T. Toda, and M. Osumi. 2003. Characterization and behaviour of α-glucan synthase in Schizosaccharomyces pombe as revealed by electron microscopy. Yeast 20:427-438. [DOI] [PubMed] [Google Scholar]
- 17.Kropp, H., J. G. Sundelof, J. S. Kahan, F. M. Kahan, and J. Birnbaum. 1980. MK0787 (N-formimidoyl thienamycin): evaluation of in vitro and in vivo activities. Antimicrob. Agents Chemother. 17:993-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laible, G., B. G. Spratt, and R. Hakenbeck. 1991. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993-2002. [DOI] [PubMed] [Google Scholar]
- 19.Martin, C., B. Thomas, and R. Hakenbeck. 1992. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding proteins 1A and 1B. J. Bacteriol. 174:4517-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki, S., T. Hosoyama, N. Furuya, Y. Ishii, T. Matsumoto, A. Ohno, K. Tateda, and K. Yamaguchi. 2001. In vitro and in vivo antibacterial activities of L-084, a novel oral carbapenem, against causative organisms of respiratory tract infections. Antimicrob. Agents Chemother. 45:203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller, M., A. de la Peńa, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai, K., Y. Shibasaki, K. Hasegawa, T. A. Davies, M. R. Jacobs, K. Ubukata, and P. C. Appelbaum. 2001. Evaluations of the primers for PCR to screen Streptococcus pneumoniae isolates, β-lactam resistance and to detect common macrolide resistance determinants. J. Antimicrob. Chemother. 48:915-918. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Fifth informational supplement M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Neu, H. C., N. X. Chin, G. Saha, and P. Labthavikul. 1986. In vitro activity against aerobic and anaerobic gram-positive and gram-negative bacteria and beta-lactamase stability of RS-533, a novel carbapenem. Antimicrob. Agents Chemother. 30:828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pares, S., N. Mouz, Y. Petillot, R. Hakenbeck, and O. Dideberg. 1996. X-ray structure of Streptococcus pneumoniae PBP2x, a primary target enzyme. Nat. Struct. Biol. 3:284-289. [DOI] [PubMed] [Google Scholar]
- 26.Shortridge, V. D., R. K. Flamm, N. Ramer, J. Beyer, and S. K. Tanaka. 1996. Novel mechanism of macrolide resistance in Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 26:73-78. [DOI] [PubMed] [Google Scholar]
- 27.Smith, A. M., and K. P. Klugman. 1998. Alterations in PBP 1A essential for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunagawa, M., H. Matsumura, A. Sasaki, H. Yamaga, Y. Sumita, and H. Nouda. 1996. Structure-activity relationship of 1β-methyl-carbapenem to its antibacterial activity: effect of the C-2 side chain and the 1β-methyl group. J. Antibiot. 49:1175-1178. [DOI] [PubMed] [Google Scholar]
- 29.Tait-Kamradt, A., J. Clancy, M. Cronan, F. Dib-Hajj, L. Wondrack, W. Yuan, and J. Sutcliffe. 1997. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trieu-Cuot, P., C. Poyart-Salmeron, C. Carlier, and P. Courvalin. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 18:3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubukata, K. 2003. Problems associated with high prevalence of multidrug-resistant bacteria in patients with community-acquired infections. J. Infect. Chemother. 9:285-291. [DOI] [PubMed] [Google Scholar]
- 32.Ubukata, K., S. Iwata, and K. Sunakawa. 2003. In vitro activities of new ketolide, telithromycin, and eight other macrolide antibiotics against Streptococcus pneumoniae having mefA and ermB genes that mediate macrolide resistances. J. Infect. Chemother. 9:221-226. [DOI] [PubMed] [Google Scholar]
- 33.Ubukata, K., T. Muraki, A. Igarashi, Y. Asahi, and M. Konno. 1997. Identification of penicillin and other β-lactam resistance in Streptococcus pneumoniae by PCR. J. Infect. Chemother. 3:190-197. [DOI] [PubMed] [Google Scholar]
- 34.Ubukata, K., Y. Asahi, K. Okuzumi, and M. Konno. 1996. Incidence of penicillin-resistant Streptococcus pneumoniae in Japan, 1993-1995. J. Infect. Chemother. 1:177-184. [DOI] [PubMed] [Google Scholar]
- 35.Ubukata, K., Y. Shibasaki, K. Yamamoto, N. Chiba, K. Hasegawa, Y. Takeuchi, K. Sunakawa, and M. Inoue. 2001. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 45:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamane, A., H. Nakano, Y. Asahi, K. Ubukata, and M. Konno. 1996. Directly repeated insertion of 9-nucleotide sequence detected in penicillin-binding protein 2B gene of penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:1257-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, Y., N. Bhachech, and K. Bush. 1995. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by β-lactamases. J. Antimirob. Chemother. 35:75-84. [DOI] [PubMed] [Google Scholar]