Abstract
A lack of σB activity reduces methicillin resistance in heterogeneous Staphylococcus epidermidis 1057, whereas inactivation of the anti-sigma factor RsbW switched the phenotype to homogeneous expression of resistance. Oxacillin induction of mecA transcription is reduced in a σB-negative strain. However, mecA is not involved in the switch of expression phenotype.
Staphylococcus epidermidis is the predominant cause of foreign-body-associated infections (19, 21). A major problem with these organisms is the widespread methicillin resistance of clinical isolates (1, 2, 5), which is often linked to the presence of the icaADBC operon responsible for biofilm formation in S. epidermidis (3, 4). Additionally, a population of attached S. epidermidis single cells is able to persist even under extremely high antibiotic concentrations, as demonstrated for methicillin-resistant S. epidermidis 1057 (10).
Recently, we demonstrated that the inactivation of rsbU (encoding a positive regulator of the alternative sigma factor σB) reduced methicillin resistance and decreased biofilm formation in S. epidermidis (6, 13). In methicillin-resistant Staphylococcus aureus (MRSA), it was shown that σB activity is required for the expression of high-level methicillin resistance (23), indicating a similar regulation. Interestingly, the role of σB in the regulation of biofilm formation in S. aureus seems to be different from that in S. epidermidis. Recently, Valle et al. (22) demonstrated that the regulatory protein SarA and not σB is essential for biofilm formation in S. aureus, whereas an influence of σB could be observed by Rachid et al. only under osmotic stress conditions for a single isolate of a collection of S. aureus strains (17). Additionally, the environmental conditions required for biofilm formation by S. aureus differ significantly from those required by S. epidermidis (8).
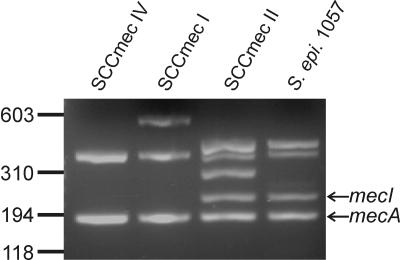
In staphylococci, methicillin resistance as well as biofilm formation are influenced by a variety of environmental factors, such as available nutrients and increased osmolarity or the presence of antibiotics (7, 8, 12, 18). By generation of S. epidermidis 1457 mutants with a deletion of the σB operon, we could demonstrate that the rsbU-dependent regulation of biofilm formation is mediated by the regulation of σB activity (9). To investigate the influence of σB activity on methicillin resistance in S. epidermidis, we transduced the deletions of the σB operon of S. epidermidis in this study from the methicillin-susceptible genetic background of S. epidermidis 1457 (Table 1) into heterogeneous methicillin- and penicillin-resistant S. epidermidis strain 1057 as described previously (9), resulting in the rsbU, rsbV, rsbW, sigB, rsbUVW, and rsbUVWsigB mutants (Table 1). In order to specify the structural type of the SCCmec cassette of S. epidermidis 1057, we performed a multiplex PCR assay (16). Except for the fragment specific for the kdp locus, S. epidermidis 1057 displayed the fragment pattern which is characteristic for the type II SCCmec cassette (Fig. 1), including the mecI-specific fragment. Additionally, the mecR gene could be detected by PCR in S. epidermidis 1057 (data not shown). These data indicate that S. epidermidis 1057 harbors an intact mecI/mecR regulatory system.
TABLE 1.
S. epidermidis strains used in this study
| Strain | Reference or source | Comments |
|---|---|---|
| 1457 | 14 | mecA-negative isolate from infected central venous catheter |
| 1457rsbU | 9 | rsbU::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; strongly repressed σB activity |
| 1457rsbV | 9 | rsbV::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; strongly repressed σB activity |
| 1457rsbW | 9 | rsbW::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; constitutive σB activity |
| 1457sigB | 9 | sigB::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; lack of σB activity |
| 1457rsbUVW | 9 | rsbUVW::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; constitutive σB activity; lack of autoinduction from the internal σB-dependent promoter |
| 1457rsbUVWsigB | 9 | rsbUVWsigB::erm derivate from S. epidermidis 1457 derived by allelic gene replacement; lack of σB activity |
| 1057 | 15 | mecA- and blaZ-positive isolate from infected central venous catheter |
| 1057rsbU | This study | Transductant of S. epidermidis 1057 from the 1457rsbU |
| 1057rsbV | This study | Transductant of S. epidermidis 1057 from the 1457rsbV |
| 1057rsbW | This study | Transductant of S. epidermidis 1057 from the 1457rsbW |
| 1057sigB | This study | Transductant of S. epidermidis 1057 from the 1457sigB |
| 1057rsbUVW | This study | Transductant of S. epidermidis 1057 from the 1457rsbUVW |
| 1057rsbUVWsigB | This study | Transductant of S. epidermidis 1057 from the 1457rsbUVWsigB |
FIG. 1.
Characterization of the SCCmec cassette in S. epidermidis 1057. The SCCmec element was characterized by a multiplex PCR (16). Typical representatives of the SCCmec types IV, I, and II of MRSA are displayed in lanes 1 to 3. Except for the fragment specific for the kdp locus, S. epidermidis 1057 displayed the fragment pattern which is characteristic for the type II SCCmec cassette. The fragments representing the genes mecA and mecI are indicated.
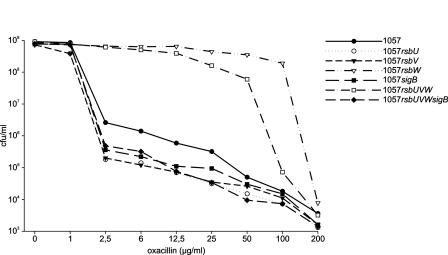
The expressed phenotype of methicillin resistance by the generated mutants was investigated by population analysis on Mueller-Hinton (MH) agar supplemented with 2% NaCl and increasing concentrations of oxacillin, as described previously (13). Wild-type S. epidermidis 1057 displayed heterogeneous expression of methicillin resistance with an already >2-log-fold reduction of growing colonies at an oxacillin concentration of 2.5 μg/ml (Fig. 2), whereas a small subpopulation was able to grow even at 200 μg of oxacillin/ml. Mutants with dysfunctional σB activity displayed an even more sensitive distribution of the population, with an additional ∼10-fold reduction of growing colonies at oxacillin concentrations between 2.5 and 50 μg/ml. However, a similar fraction of the population was still able to grow at 200 μg of oxacillin/ml (Fig. 2). Interestingly, in mutants with deletions of rsbW, the heterogeneous expression phenotype of the wild type was shifted to a homogeneous expression of methicillin resistance, with MICs for about 90% of the population of at least 50 μg/ml (Fig. 2). These data indicate that the alternative sigma factor σB is required for the modulation of the phenotypic expression of methicillin resistance in S. epidermidis, as was observed in S. aureus (23). We could thereby demonstrate for the first time that the inactivation of the negative regulator of σB activity RsbW causes the phenotypic switch from a heterogeneous to a homogeneous expression of methicillin resistance. This finding is additionally corroborated by the observation of reduced oxacillin resistance in the rsbU and rsbV mutants, in which the activity of the anti-RsbW factor RsbV is suppressed or lacking completely, whereas the sigB gene is still intact. These data indicate that the rsbW gene could be a locus of so-called chr* mutations (20), which are responsible for phenotypic switches observed with heterogeneously resistant methicillin-resistant S. epidermidis and MRSA strains in vivo during therapy.
FIG. 2.
Phenotypic characterization of methicillin resistance by population analysis. Wild-type S. epidermidis 1057 displayed a heterogeneous expression of methicillin resistance, with a more than 2-log-fold reduction at an oxacillin concentration of 2.5 μg/ml. Mutants with dysfunctional σB (the rsbU, rsbV, sigB, and rsbUVWsigB strains) displayed a more sensitive distribution, with about an additional 1-log-fold reduction of cells at lower oxacillin concentrations. In mutants with inactivation of the anti-sigma factor RsbW (the rsbW and rsbUVW strains), the heterogeneous expression phenotype of the wild type was shifted to a homogeneous expression of methicillin resistance.
For further transcriptional analysis, the rsbUVWsigB and rsbUVW strains were characterized. To investigate σB activity and its relevance for the transcription of mecA in these mutants, transcriptional analyses were performed by real-time reverse transcription (RT)-PCR. As a marker for σB activity, the asp23 gene of S. epidermidis, which is transcribed from at least two different σB-dependent promoters (9), was used. The strains were cultivated prior to RNA extraction in MH broth supplemented with 2% NaCl (MHNaCl) as well as in MHNaCl supplemented with a subinhibitory concentration of 1 μg of oxacillin/ml (MHoxa). In both media, the investigated strains displayed almost identical growth curves, except for the rsbUVWsigB strain, which displayed a slight delay of growth (25 to 45 min during exponential growth) in MHoxa. However, the cell densities during stationary phase were almost identical for all strains and media (data not shown). RNA was extracted at a time point when all strains were in the mid-exponential growth phase (9 h in both media) as well as during stationary phase (17 h) by using a modified protocol of the RNeasy bacteria kit (QIAGEN, Hilden, Germany) as described previously (9). The cutoff for significant differences in regulation was defined as 2.5-fold up- or down-regulation of the respective genes (9). RT-PCR was performed in an iCycler thermal cycler using the oligonucleotides gyrB-real1 (5′-CTGACAATGGCCGTGGTATTC-3′), gyrB-real2 (5′-GAAGATCCAACACCGTGAAGAC-3′), asp23-real1 (5′-TCCAACTTCTACAGATACGCC-3′), asp23-real2 (5′-AAAATTGCAGGTATTGCAGC-3′), mecA-real1 (5′-ATTATGGCTCAGGTACTGCTATC-3′), and mecA-real2 (5′-CTGGTGAAGTTGTAATCTGGAAC-3′) as described previously (9). Relative transcriptional levels within distinct experiments were determined by using the 2−ΔΔCT method (11) and compared to the wild type with gyrB as the reference housekeeping gene. RT-PCR was performed in triplicate in each of three independent experiments. As observed for the S. epidermidis 1457 mutants, transcription of the σB-dependent gene asp23 was significantly reduced in the rsbUVWsigB strain (Table 2). The observed differences were more pronounced under conditions of oxacillin induction. In the rsbUVW mutant, only marginal differences of asp23 transcription were observed (Table 2). Thereby, in MHNaCl, σB activity was increased during exponential phase and decreased during stationary phase, whereas in MHoxa, σB activity was decreased during exponential growth phase but similar to that of the wild type in stationary phase. The decrease of σB activity in the rsbUVW strain compared to the wild type could be explained by the lack of transcription from the internal σB-dependent promoter preceding rsbV in the σB operon of S. epidermidis (6) and thereby the lack of σB autoinduction under the investigated conditions.
TABLE 2.
Transcriptional differences between mutants and the wild type
| Strain | Gene | Transcriptional difference compared to the wild-type straina
|
|||
|---|---|---|---|---|---|
| MHNaCl
|
MHoxa
|
||||
| Exponential | Stationary | Exponential | Stationary | ||
| 1057rsbUVW | asp23 | +3 | −5 | −5 | +2 |
| 1057rsbUVW | mecA | +1 | +1 | −2 | +1 |
| 1057rsbUVWsigB | asp23 | −24 | −42 | −71 | −604 |
| 1057rsbUVWsigB | mecA | +6 | +5 | +2 | −2 |
The differences of transcription (n-fold) between mutants and wild-type S. epidermidis 1057 were calculated by the 2−ΔΔCT method. Values represent the mean of results from three independent experiments. Significant differences are displayed in bold.
Interestingly, the homogeneously resistant rsbUVW mutant displayed no significant differences from the wild type in mecA transcription under all investigated conditions, whereas in the less resistant rsbUVWsigB mutant, mecA transcription was slightly induced in MHNaCl (Table 2). However, investigating the induction of mecA transcription during growth in medium supplemented with a subinhibitory concentration of oxacillin (1 μg/ml) revealed that in the σB-negative mutant, the effect of oxacillin induction was smaller than in the wild type and the rsbUVW mutant. During exponential growth, oxacillin induction led to 102-fold and 66-fold increases of mecA transcription in the wild type and the rsbUVW mutant, whereas in the rsbUVWsigB mutant, a 25-fold increase was observed. The differences in oxacillin induction were more prominent during stationary phase, in which only a 3-fold induction could be observed for the rsbUVWsigB strain, whereas 1057 and the rsbUVW strain displayed 27- and 32-fold increases, respectively.
Transcriptional analysis revealed that a lack of σB activity leads to a dysfunctional regulation of mecA transcription with increased transcriptional activity without oxacillin induction and on the other hand a reduced induction by oxacillin, especially during the stationary phase. Interestingly, despite the phenotypic switch of the rsbUVW mutant to a homogeneous expression of oxacillin resistance, no significant differences in mecA transcription compared to the heterogeneous wild type could be observed, indicating that besides the expected inducibility by oxacillin, the σB regulation of genes other than mecA must be responsible for differences in the phenotypic expression of oxacillin resistance in S. epidermidis.
Acknowledgments
We thank Rainer Laufs for his continuous support.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Werner-Otto-Stiftung, and the Forschungsförderungsfonds Medizin des Universitätsklinikums Hamburg-Eppendorf, Hamburg, Germany, given to J.K.-M.K. and D.M.
REFERENCES
- 1.Archer, G. L., and M. W. Climo. 1994. Antimicrobial susceptibility of coagulase-negative staphylococci. Antimicrob. Agents Chemother. 38:2231-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frebourg, N. B., S. Lefebvre, S. Baert, and J. F. Lemeland. 2000. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J. Clin. Microbiol. 38:877-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridkin, S. K., J. R. Edwards, F. C. Tenover, R. P. Gaynes, and J. E. McGowan, Jr. 2001. Antimicrobial resistance prevalence rates in hospital antibiograms reflect prevalence rates among pathogens associated with hospital-acquired infections. Clin. Infect. Dis. 33:324-330. [DOI] [PubMed] [Google Scholar]
- 6.Knobloch, J. K. M., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, P. M. Kaulfers, and D. Mack. 2002. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J. Antimicrob. Chemother. 49:683-687. [DOI] [PubMed] [Google Scholar]
- 8.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 191:101-106. [DOI] [PubMed] [Google Scholar]
- 9.Knobloch, J. K.-M., S. Jäger, M. A. Horstkotte, H. Rohde, and D. Mack. 2004. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor σB by repression of the negative regulator gene icaR. Infect. Immun. 72:3838-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knobloch, J. K. M., H. von Osten, M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med. Microbiol. Immunol. 191:107-114. [DOI] [PubMed] [Google Scholar]
- 11.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 12.Mack, D., P. Becker, I. Chatterjee, S. Dobinsky, J. K. M. Knobloch, G. Peters, H. Rohde, and M. Herrmann. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294:203-212. [DOI] [PubMed] [Google Scholar]
- 13.Mack, D., A. Sabottke, S. Dobinsky, H. Rohde, M. A. Horstkotte, and J. K. M. Knobloch. 2002. Differential expression of methicillin resistance by different biofilm-negative Staphylococcus epidermidis transposon mutant classes. Antimicrob. Agents Chemother. 46:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedelmann, M., A. Sabottke, R. Laufs, and D. Mack. 1998. Generalized transduction for genetic linkage analysis and transfer of transposon insertions in different Staphylococcus epidermidis strains. Zentbl. Bakteriol. 287:85-92. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231-243. [DOI] [PubMed] [Google Scholar]
- 20.Ryffel, C., A. Strassle, F. H. Kayser, and B. Berger-Bachi. 1994. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 38:724-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thylefors, J. D., S. Harbarth, and D. Pittet. 1998. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect. Control Hosp. Epidemiol. 19:581-589. [DOI] [PubMed] [Google Scholar]
- 22.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 23.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]