Abstract
Varicella-zoster virus (VZV) is responsible for primary infections as well as reactivations after latency in the dorsal root ganglia. The treatment of such infections is mandatory for immunocompromised patients and highly recommended for elderly patients with herpes zoster infections (also called zona or shingles). The treatment of choice is presently based on four molecules, acyclovir (ACV), valaciclovir, famciclovir, and (in Europe) brivudine (BVDU). We present here our data on the antiviral activity of a new class of potent and selective anti-VZV compounds, bicylic pyrimidine nucleoside analogues (BCNAs), against a broad variety of clinical isolates and different drug-resistant virus strains. The results show that the BCNAs are far more potent inhibitors than ACV and BVDU against clinical VZV isolates as well as the VZV reference strains Oka and YS. The BCNAs were not active against ACV- and BVDU-resistant VZV strains bearing mutations in the viral thymidine kinase gene but kept their inhibitory potential against virus strains with mutations in the VZV DNA polymerase gene. Mutant virus strains selected in the presence of the BCNAs were solely cross-resistant to drugs, such as ACV and BVDU, that depend for their antiviral action on metabolic activation by the viral thymidine kinase.
Varicella-zoster virus (VZV) is an alphaherpesvirus that is known to be responsible for varicella in young children and for localized recurrent lesions (called zoster) later in life, due to reactivation in the ganglia. The disease can be severe in immunocompromised patients such as AIDS patients or transplant recipients. The reference drug for the treatment of immunocompromised patients presenting with VZV infections and of patients presenting with zoster is acyclovir (ACV) (7). Recently, brivudine (BVDU), another nucleoside analog, has been marketed for the treatment of varicella and zoster (15, 23).
We have demonstrated that several members of a new class of antivirals, the bicyclic pyrimidine nucleoside analogues (BCNAs), are highly potent and selective inhibitors of VZV in vitro (3-5, 12, 13, 16-18). This new class of compounds exhibits exclusive specificity for VZV and obligatorily requires an alkyl or alkylaryl side chain at the furan moiety for potent anti-VZV activity.
ACV-resistant VZV strains have been reported to emerge with increasing frequency in immunocompromised patients after long-term therapy with ACV (1). These observations justify drug sensitivity evaluation of resistant viral strains obtained after exposure to molecules belonging to different classes of antiviral agents.
The present work demonstrates the exquisite antiviral activity of a selection of the most potent BCNAs against a broad range of clinical VZV isolates. These BCNA molecules were also investigated for their activity against VZV strains that were selected in the presence of several of the BCNAs, demonstrating that the BCNAs select for drug-resistant VZV phenotypes resulting from mutations in the virus-encoded thymidine kinase (TK).
MATERIALS AND METHODS
Viruses.
The VZV reference strains Oka and YS were used as control viruses in the different assays, and the Oka strain was used to select drug-resistant virus strains. Seventeen clinical VZV isolates, some of which are resistant to ACV, were included (2, 19). Virus stocks were prepared as described previously (2).
Cells.
All the experiments were performed on confluent human embryonic lung fibroblast (HEL) cell cultures.
Compounds.
The synthesis of the different BCNAs used in the present study has been described elsewhere (12, 13). 5-Furo-6-octyl-dUrd (Cf 1368), 5-furo-(6- hexylphenyl)-dUrd (Cf 1742), 5-furo-(6-pentylphenyl)-dUrd (Cf 1743), and 5-(6-thiono-decyl)-dUrd (Cf 1603) (Fig. 1) were compared to the reference drugs ACV [9-(2-hydroxyethoxymethyl)guanine] (GlaxoSmithKline, Stevenage, United Kingdom), penciclovir (PCV) [9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine] (Novartis Pharmaceuticals Corp., East Hanover, N.J.), ganciclovir (GCV) [9-(1,3-dihydroxy-2-propoxymethyl)guanine] (Roche, Basel, Switzerland), foscarnet (PFA) (phosphonoformate sodium salt) and its predecessor phosphonoacetic acid (PAA) (Sigma Chemical Co., St. Louis, Mo), BVDU [(E)-5-(2- bromovinyl)-1-(β-D-2′-deoxyribofuranos-1-yl-uracil) (originally prepared at Searle, United Kingdom), sorivudine (BVaraU) [1-β-d-arabinofuranosyl-(E)-5-(2-bromovinyl)uracil] (Yamasa Shoyu, Choshi, Japan), the acyclic nucleoside phosphonate analogues, (S)-HPMPC (cidofovir [CDV]) [(S)-1-(3-hydroxy-2-phosphonomethoxypropyl)cytosine] and PMEA (adefovir) [9-[2- (phosphonylmethoxyethyl)adenine]] (Gilead Sciences, Foster City, Calif.), and (S)-HPMPA [(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine] and PMEDAP [9-(2-phosphonomethoxyethyl)-2,6-diaminopurine] (A. Holý, Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Prague, Czech Republic).
FIG. 1.
Structural formulae of BVDU and BCNAs.
Antiviral assays.
VZV-drug susceptibility assays were performed as previously described (2). Briefly, confluent HEL cells in 96-well microtiter plates were infected with 20 PFU of cell-associated virus per well. After 2 h of incubation, the inoculum was removed and replaced by the different dilutions (in duplicate) of the tested molecules. After 5 days of incubation, the cells were fixed and stained with Giemsa. The activity was determined by counting the number of plaques for each dilution. The activity is expressed as EC50, the effective compound concentration required to reduce virus-induced cytopathicity (CPE) by 50% compared to the untreated control. The data were statistically analyzed by Student's t test.
The toxicity of the compounds is expressed as CC50, the compound concentration required to reduce cell growth by 50% compared to an untreated control.
Selection of drug-resistant VZV strains by multiple step selection.
The drug-resistant virus strains were obtained by serial passage of cell-associated VZV (Oka strain) in the presence of increasing concentrations of the compounds, starting at the EC50 (1). The initial multiplicity of infection used to start the procedure of selection with the different drugs was 0.05. The cell cultures were incubated until virus CPE was about 70%, and then the drug concentration was increased by twofold with every subsequent passage of the virus.
After the drugs had reached concentrations of 100 μg/ml for PMEA, PMEDAP, and PFA and 10 μg/ml for ACV, PCV, GCV, BVDU, BVaraU, HPMPC, HPMPA, and the BCNAs, a last passage was performed in drug-free medium to obtain a virus stock.
The various drug-resistant VZV strains, denoted ACVr, BVDUr, BVaraUr, Cf 1368r, Cf 1603r, Cf 1742r, Cf 1743r, PFAr, PMEAr, PMEDAPr, and PCVr, were subjected to titer determination and subsequently evaluated for their drug sensitivity in vitro. For PCV and PFA, the selection of drug-resistant virus strains was performed in duplicate, leading to two independent mutants for each drug, denoted PCVr/A, PCVr/B, PFAr/A, and PFAr/B.
RESULTS
Activity of BCNAs against clinical VZV isolates in HEL cell cultures.
To determine whether clinical VZV strains were as sensitive as the laboratory VZV strains Oka and YS to the antiviral effects of the BCNAs, we evaluated the activity of the most active BCNAs against 17 clinical isolates in a plaque reduction assay with HEL cell cultures. The clinical VZV strains were all low-passage viruses isolated from the skin of patients with either varicella or zoster infection, who did not undergo antiviral treatment.
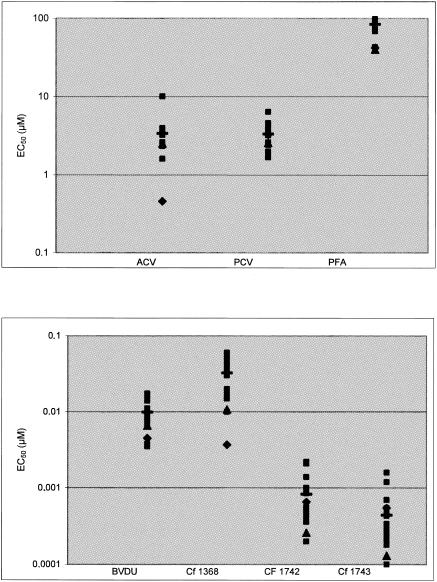
The most potent BCNAs, Cf 1368, Cf 1742, and Cf 1743, inhibited the replication of wild-type VZV clinical isolates with average EC50 of 0.032 ± 0.017, 0.00083 ± 0.00058, and 0.00043 ± 0.00039 μM, respectively, whereas the EC50 of the reference drugs ACV, PCV, BVDU, and PFA were 3.38 ± 1.87, 3.34 ± 1.20, 0.0098 ± 0.0040, and 84.4 ± 13.6 μM, respectively (Table 1). The EC50 obtained for the wild-type clinical VZV isolates were comparable to those obtained for the laboratory VZV strains YS and Oka (Fig. 2).
TABLE 1.
Activity of BCNAs against VZV clinical isolates in HEL cells
| Clinical isolate | EC50 (μM) ofa:
|
||||||
|---|---|---|---|---|---|---|---|
| ACV | PCV | BVDU | PFA | Cf 1368 | Cf 1742 | Cf 1743 | |
| VZV-5 | 2.38 ± 0.22 | 2.41 ± 1.45 | 0.0068 ± 0.0045 | 91.3 ± 5.1 | 0.020 ± 0.004 | 0.00039 ± 0.00002 | 0.00025 ± 0.00001 |
| VZV-12 | 3.78 ± 0.50 | 4.27 ± 0.62 | 0.0111 ± 0.0055 | 93.6 ± 2.0 | 0.039 ± 0.016 | 0.0010 ± 0.00009 | 0.00043 ± 0.00025 |
| VZV-13 | 3.31 ± 0.40 | 3.54 ± 0.15 | 0.0099 ± 0.0030 | 88.4 ± 1.9 | 0.030 ± 0.003 | 0.00043 ± 0.00011 | 0.00029 ± 0.00012 |
| VZV-16 | 3.51 ± 0.62 | 3.65 ± 0.57 | 0.0140 ± 0.0113 | 97.9 ± 1.1 | 0.044 ± 0.004 | 0.00090 ± 0.00014 | 0.00032 ± 0.00010 |
| VZV-20 | 3.24 ± 0.62 | 3.22 ± 0.42 | 0.0108 ± 0.0046 | 95.3 ± 4.7 | 0.040 ± 0.028 | 0.00056 ± 0.00051 | 0.00034 ± 0.00008 |
| VZV-21 | 2.47 ± 1.10 | 1.90 ± 0.90 | 0.0074 ± 0.0049 | 43.2 ± 1.6 | 0.015 ± 0.004 | 0.00036 ± 0.00009 | 0.00010 ± 0.00009 |
| VZV-22 | 3.47 ± 0.81 | 6.44 ± 2.07 | 0.0096 ± 0.0017 | 76.9 ± 14.5 | 0.020 ± 0.004 | 0.00050 ± 0.00034 | 0.00026 ± 0 |
| VZV-23 | 1.60 ± 0 | 2.62 ± 1.41 | 0.0038 ± 0.0001 | 98.5 ± 3.4 | 0.015 ± 0.014 | 0.00052 ± 0.00068 | 0.00018 ± 0.00013 |
| VZV-24 | 1.61 ± 0.08 | 1.67 ± 0.88 | 0.0035 ± 0.0008 | 69.0 ± 14.5 | 0.010 ± 0.008 | 0.00020 ± 0.00013 | 0.00008 ± 0.00004 |
| VZV-25 | 2.64 ± 0.98 | 3.54 ± 0.30 | 0.0067 ± 0.0047 | 81.0 ± 9.5 | 0.017 ± 0.005 | 0.00087 ± 0.00018 | 0.00021 ± 0.00015 |
| VZV-26 | 3.49 ± 0.72 | 3.54 ± 0.26 | 0.0090 ± 0.0042 | 85.5 ± 6.8 | 0.016 ± 0.002 | 0.00051 ± 0.00018 | 0.00022 ± 0.00014 |
| VZV-27 | 2.33 ± 1.55 | 4.03 ± 0.45 | 0.015 ± 0.017 | 81.5 ± 17.5 | 0.059 ± 0.063 | 0.0021 ± 0.0015 | 0.0016 ± 0.0013 |
| VZV-28 | 3.98 ± 2.30 | 4.35 ± 1.73 | 0.016 ± 0.012 | 84.6 ± 8.0 | 0.051 ± 0.035 | 0.00085 ± 0.00008 | 0.00046 ± 0.00050 |
| VZV-30 | 10.1 ± 1.7 | 4.61 ± 1.76 | 0.017 ± 0.011 | 93.8 ± 4.7 | 0.059 ± 0.044 | 0.0022 ± 0.0001 | 0.00070 ± 0.00047 |
| VZV-31 | 3.51 ± 0.32 | 2.0 ± 0.70 | 0.0084 ± 0.0004 | 72.9 ± 21.2 | 0.034 ± 0.005 | 0.00055 ± 0.00058 | 0.00055 ± 0.00032 |
| VZV-32 | 3.64 ± 0.31 | 2.55 ± 0.64 | 0.0083 ± 0.0032 | 91.8 ± 7.4 | 0.053 ± 0.037 | 0.00014 ± 0.0015 | 0.0012 ± 0.0013 |
| VZV-33 | 2.53 ± 0.88 | 2.49 ± 1.17 | 0.0095 ± 0.0015 | 89.7 ± 4.5 | 0.026 ± 0.022 | 0.00076 ± 0.00063 | 0.00027 ± 0.00022 |
| Mean | 3.38 | 3.34 | 0.0098 | 84.4 | 0.032 | 0.00083 | 0.00043 |
| SD | 1.87 | 1.2 | 0.004 | 13.6 | 0.017 | 0.00058 | 0.00039 |
| Cytotoxicity (CC50) | >888 | >790 | >600 | >666 | 138 | 105 | 108 |
| Selectivity index | >263 | >237 | >61,224 | >7.9 | 4,313 | 126,506 | 251,163 |
EC50s represent means of at least two independent experiments.
FIG. 2.
Activity of BCNAs and anti-VZV compounds against clinical VZV isolates (▪) and VZV reference strains Oka (▴) and YS (♦). Mean values for clinical isolates are indicated by horizontal bars.
Cf 1742 and Cf 1743 were markedly more potent than other established anti-VZV drugs in vitro. Thus, Cf 1742 and Cf 1743 showed 10- to 25-fold higher activity than BVDU and 4,000- to 7,800-fold higher potency than ACV and PCV in cell culture (P < 0.001). Furthermore, when the selectivity index (ratio of CC50 to EC50) was calculated for clinical VZV isolates, Cf 1742 and Cf 1743 showed an unprecedentedly high selectivity against VZV (>100,000).
Susceptibilities of several drug-resistant VZV strains to BCNAs.
The activity of the BCNAs against a variety of VZV (Oka strain) mutants that were isolated after exposure to different classes of antiviral compounds was evaluated. The development of resistance to BCNAs (Cf1368, Cf1742, Cf1743, and Cf1603) was also determined to investigate the potential cross-resistance of these virus isolates to other anti-VZV drugs.
As shown in Table 2, the ACV-, BVDU- and BVaraU-resistant VZV strains that were resistant to drugs that depend for their activation (phosphorylation) on the viral thymidine kinase (TK) (i.e., ACV, BVDU, BVaraU, GCV, and PCV) were also (cross) resistant to the BCNAs. A similar pattern of drug resistance was observed when VZV strains selected for resistance against BCNAs were evaluated for their sensitivities to TK-dependent-drugs (Table 2). The BCNAs also lost activity (Table 3) against two TK-deficient VZV strains isolated from an AIDS patient who had undergone prolonged treatment with ACV (19). These results pointed to an alteration in the viral TK as the molecular basis for the drug-resistant phenotype selected by the BCNAs. In contrast, the BCNAs remained fully active against virus strains that were selected by exposure to PCV, PFA, PMEA, and PMEDAP (Table 4). The resistant phenotype of these VZV mutants could be attributed to mutations in the viral DNA polymerase gene, which evidently did not affect the antiviral potency of the BCNAs (our unpublished data).
TABLE 2.
Drug susceptibility profiles of drug-resistant VZV Oka strains obtained in vitro
| Mutant virus straina | EC50 (μM)b of:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cf 1368 | Cf 1603 | Cf 1742 | Cf 1743 | ACV | PCV | GCV | BVDU | BVaraU | PFA | PAA | PMEA | PMEDAP | HPMPC | HPMPA | |
| Wild type | 0.011 ± 0.004 | 0.0083 ± 0.0081 | 0.00019 ± 0.00016 | 0.00007 ± 0.00013 | 2.5 ± 1.6 | 2.2 ± 1.2 | 1.3 ± 1.3 | 0.0066 ± 0.0045 | 0.00015 ± 0.00008 | 39.8 ± 13.6 | 50.7 ± 17.9 | 17.8 ± 9.6 | 2.3 ± 1.1 | 0.21 ± 0.35 | 0.046 ± 0.071 |
| ACVr | >5 ± 0 | ≥3.56 ± 1.48 | >5 ± 0 | >5 ± 0 | 54.6 ± 21.3 | 26.9 ± 22.1 | 9.9 ± 13.4 | 42.0 ± 31.2 | ≥37.5 ± 28.0 | 36.0 ± 12.7 | 45.7 ± 25.7 | 18.7 ± 17.9 | 3.0 ± 3.2 | 0.09 ± 0.10 | 0.014 ± 0.009 |
| BVDUr | >5 ± 0 | ≥4.8 ± 0.4 | >5 ± 0 | >5 ± 0 | 47.5 ± 27.5 | 23.0 ± 11.1 | 5.8 ± 5.5 | ≥48.6 ± 15.6 | >57 ± 0 | 49.3 ± 17.3 | 59.3 ± 5.4 | 27.5 ± 10.5 | 4.3 ± 3.4 | 0.17 ± 0.11 | 0.080 ± 0.090 |
| BVaraUr | >5 ± 0 | >5 ± 0 | >5 ± 0 | >5 ± 0 | 68.4 ± 25.8 | 64.8 ± 17.0 | 6.7 ± 5.5 | ≥55.5 ± 9.0 | >57 ± 0 | 42.0 ± 6.0 | 41.4 ± 13.6 | 26.4 ± 7.0 | 3.0 ± 0.6 | 0.10 ± 0.13 | 0.010 ± 0.008 |
| cf 1368r | >5 ± 0 | >5 ± 0 | >5 ± 0 | >5 ± 0 | ≥72.2 ± 35.1 | 18.2 ± 6.7 | 2.6 ± 0.7 | ≥40.8 ± 24.9 | ≥35.5 ± 25.2 | 38.6 ± 17.3 | 50.0 ± 24.2 | 14.3 ± 8.6 | 3.2 ± 2.4 | 0.06 ± 0.01 | 0.043 ± 0.080 |
| cf 1603r | >5 ± 0 | >5 ± 0 | >5 ± 0 | >5 ± 0 | ≥76.8 ± 25.3 | ≥70.3 ± 13.8 | 9.1 ± 7.5 | >60 ± 0 | >57 ± 0 | 27.8 ± 8.5 | 42.4 ± 14.1 | 21.7 ± 6.8 | 2.3 ± 0.8 | 0.15 ± 0.13 | 0.028 ± 0.037 |
| cf 1742r | >5 ± 0 | >5 ± 0 | >5 ± 0 | >5 ± 0 | ≥57.9 ± 43.5 | 65.0 ± 19.4 | 6.0 ± 5.9 | 38.0 ± 31.2 | >57 ± 0 | 35.3 ± 2.8 | 51.7 ± 8.6 | 13.7 ± 3.9 | 2.6 ± 0.4 | 0.29 ± 0.13 | 0.025 ± 0.021 |
| cf 1743r | >5 ± 0 | ≥4.8 ± 0.3 | >5 ± 0 | >5 ± 0 | 50.0 ± 14.1 | 43.6 ± 23.2 | 2.8 ± 1.5 | ≥43.8 ± 28.2 | >57 ± 0 | 25.5 ± 10.2 | 28.8 ± 4.4 | 8.5 ± 2.5 | 1.3 ± 0.7 | 0.05 ± 0.06 | 0.018 ± 0.030 |
Selected for resistance against each of the indicated compounds.
EC50s represent means of at least two independent experiments.
TABLE 3.
Activity of BCNAs against VZV isolates recovered from an AIDS patient before (NCLU-II) and after (NCLU-III and NCLU-IV) treatment with ACV
| Clinical isolate | EC50 (μM)a of:
|
||||||
|---|---|---|---|---|---|---|---|
| ACV | PCV | BVDU | PFA | Cf 1368 | Cf 1742 | Cf 1743 | |
| NCLU-II (wt)b | 7.4 ± 4.8 | 5.5 ± 3.4 | 0.024 ± 0.002 | 86.6 ± 3.1 | 0.060 ± 0.004 | 0.0022 ± 0.0003 | 0.00053 ± 0.00042 |
| NCLU-III (TK−) | 66.7 ± 31.5 | 62.2 ± 23.8 | >15 ± 0 | 51.8 ± 1.8 | >2 ± 0 | >2 ± 0 | >2 ± 0 |
| NCLU-IV (TK−) | 81.9 ± 23.4 | >80 ± 0 | >15 ± 0 | 83.4 ± 2.5 | >2 ± 0 | >2 ± 0 | >2 ± 0 |
EC50s represent means of two independent experiments.
wt, wild type.
TABLE 4.
Drug susceptibility profiles of drug-resistant VZV Oka strains obtained in vitro
| Mutant virus straina | EC50 (μM)b of:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cf 1368 | Cf 1603 | Cf 1742 | Cf 1743 | ACV | PCV | GCV | BVDU | BVaraU | PFA | PAA | PMEA | PMEDAP | HPMPC | HPMPA | |
| Wild type | 0.011 ± 0.004 | 0.0083 ± 0.0081 | 0.00019 ± 0.00016 | 0.00007 ± 0.00013 | 2.5 ± 1.6 | 2.2 ± 1.2 | 1.3 ± 1.3 | 0.0066 ± 0.0045 | 0.00015 ± 0.00008 | 39.8 ± 13.6 | 50.7 ± 17.9 | 17.8 ± 9.6 | 2.3 ± 1.1 | 0.21 ± 0.35 | 0.046 ± 0.071 |
| PFAr/A | 0.039 ± 0.026 | 0.024 ± 0.012 | 0.00029 ± 0.00027 | 0.00015 ± 0.00002 | 15.1 ± 12.5 | 6.6 ± 3.3 | 1.3 ± 1.6 | 0.0117 ± 0.0087 | 0.00037 ± 0.00003 | 240.0 ± 68.9 | 254.2 ± 37.1 | 86.7 ± 37.3 | 14.5 ± 9.0 | 0.44 ± 0.25 | 0.148 ± 0.169 |
| PFAr/B | 0.020 ± 0.022 | 0.011 ± 0.009 | 0.00005 ± 0.00004 | 0.00002 ± 0 | 11.5 ± 13.4 | 5.8 ± 3.2 | 1.2 ± 2.2 | 0.0117 ± 0.0084 | 0.00027 ± 0.00014 | 274.0 ± 28.3 | 231.3 ± 41.4 | 79.8 ± 51.2 | 13.2 ± 8.5 | 0.25 ± 0.13 | 0.148 ± 0.129 |
| PCVr/A | 0.040 ± 0.031 | 0.0043 ± 0.0039 | 0.00046 ± 0.00064 | 0.00015 ± 0.00022 | 18.9 ± 14.8 | 12.5 ± 10.3 | 0.39 ± 0.28 | 0.0057 ± 0.0027 | 0.00034 ± 0.0005 | 326.3 ± 122.9 | 236.3 ± 112.8 | 131.8 ± 73.2 | 21.9 ± 13.0 | 0.20 ± 0.16 | 0.011 ± 0.017 |
| PCVr/B | 0.012 ± 0.008 | 0.0053 ± 0.0053 | 0.00051 ± 0.00051 | 0.00025 ± 0.00022 | 13.3 ± 4.9 | 7.9 ± 3.2 | 0.63 ± 0.24 | 0.0042 ± 0.0018 | 0.00054 ± 0.00014 | 128.5 ± 13.7 | 123.5 ± 22.8 | 112.7 ± 7.3 | 11.3 ± 1.9 | 0.48 ± 0.10 | 0.017 ± 0.016 |
| PMEAr | 0.013 ± 0.007 | 0.0073 ± 0.0038 | 0.00026 ± 0.00042 | 0.00008 ± 0.00010 | 35.1 ± 14.4 | 19.5 ± 15.0 | 1.4 ± 1.3 | 0.0120 ± 0.0096 | 0.00031 ± 0.00017 | 234.1 ± 27.6 | 153.5 ± 41.0 | ≥183 ± 0 | 41.3 ± 26.2 | 0.76 ± 0.29 | 0.083 ± 0.059 |
| PMEDAPr | 0.024 ± 0.010 | 0.0062 ± 0.0051 | 0.00027 ± 0.00028 | 0.00017 ± 0.00017 | 7.4 ± 3.2 | 2.0 ± 1.3 | 0.17 ± 0.16 | 0.0017 ± 0.0008 | 0.00014 ± 0.00006 | 141.2 ± 68.9 | 118.5 ± 106.4 | 120.4 ± 53.1 | 24.5 ± 10.0 | 0.26 ± 0.24 | 0.013 ± 0.014 |
Selected for resistance to each of the indicated compounds.
EC50s represent means of at least two independent experiments.
Thus, as with ACV, BVDU, and BVaraU, but not PCV and the acyclic nucleoside phosphonates PMEA and PMEDAP, development of resistance of VZV to BCNAs occurred preferentially and consistently at the level of the VZV-encoded TK and not at the level of the viral DNA polymerase.
DISCUSSION
Active immunization (vaccination) against VZV is now possible by using a live-attenuated vaccine obtained from the reference strain Oka. The vaccine is highly protective in healthy children, and an immune response can still be observed in individuals who are not fully protected by the vaccine. However, a recent report (10) describes a disseminated varicella infection following vaccination of a child with a profound deficiency of natural killer T cells. This and other reports (6) express concern about the development of life-threatening infections postvaccination that might need antiviral treatment. Another report mentions that chronic herpes zoster lesions that were due to the vaccine VZV strain and were treated with ACV led to the emergence of resistant virus with the genetic background of the vaccine Oka strain (9). The recent description of several distinct varicella virus strains raises the question of the possibility that the present Oka vaccine may not be protective against all clinical wild-type VZV strains (6). In addition, an increasing number of patients, particularly transplanted individuals, have iatrogenically induced immunodeficiency. Finally, a variety of reports stress the importance of VZV as the agent of opportunistic infection (8, 14, 20) The use of certain potent immunosuppressive drugs, such as mycophenolate mofetil, seems to increase the incidence of VZV infections, reinforcing the need for prompt and better control of these infections (8). Also, the treatment of chronic myelogenous leukemia may be associated with a low incidence of VZV infections (11). All these observations stress the need for new potent antiviral molecules effective against VZV.
The present study demonstrates that BCNAs, a new class of bicyclic pyrimidine nucleoside analogues with specific anti-VZV activity (3-5, 12, 13, 16-18), are virtually equipotent against both laboratory VZV strains and clinical VZV isolates in vitro. Their activity against VZV is far superior to that of the reference compounds ACV and PCV and is also superior to that of BVDU, the drugs presently marketed for the treatment of VZV infections in humans (7, 20, 21). Unfortunately, since the BCNAs are solely inhibitors of VZV and not of any other herpesviruses, including simian varicella virus, no animal model is available to evaluate the antiviral efficacy of the BCNAs in vivo (18). Despite their unique structure and antiviral selectivity, the BCNAs are not active against VZV strains that are resistant to ACV and BVDU due to amino acid mutations in the viral TK. Also, VZV strains selected by exposure to different BCNAs were cross-resistant to other BCNAs as well as to ACV and BVDU. In contrast, drug-resistant VZV strains obtained under the selective pressure of PFA, PCV, or PMEA, which are known to generate mutations at the level of the viral DNA polymerase, remained sensitive to the different BCNAs tested. Interestingly, although both ACV and PCV are activated by the viral TK, they select for drug-resistant virus strains that exhibit a different genotype (i.e., mutations in TK and DNA polymerase, respectively) (G. Andrei, P. Fiten, G. Opdenakker, J. Balzarini, E. De Clercq, C. McGuigan; and R. Snoeck, Program Abstr 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-289, 2003; G. Andrei, P. Fiten, G. Opdenakker, E. De Clercq, C and R. Snoeck, Program Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. V-770, 2004). Although the underlying mechanism is not well understood, it explains the observations that PCV-resistant virus retains its sensitivity to TK-dependent drugs including BCNAs (Table 4) whereas ACV-resistantvirus is cross-resistant to all TK-dependent drugs (Table 2). When all virus resistance data are taken together, they confirm that the VZV-encoded TK is essential for the phosphorylation of the BCNAs and for their eventual anti-VZV activity (16). Indeed, the BCNAs used in this study have also been evaluated for their affinity for purified VZV and herpes simplex virus type 1 (HSV-1) TK (4). The 50% inhibitory concentration (IC50) of the VZV TK enzyme activity ranged between 1.5 and 4.5 μM for Cf 1742 and Cf 1743 and the reference compounds BVDU and BVaraU. HSV-1 TK showed a similar affinity for BVDU and BVaraU but lacked any measurable activity against Cf 1742 and Cf 1743 (4). Both Cf 1368 and Cf 1603 were also recognized by VZV TK, although to a lesser extent than were their phenylalkyl-substituted counterparts (IC50, 37 to 43 μM), but were again not inhibitory to HSV-1 TK (IC50, >500 μM). As expected, the guanine analogues were not markedly recognized by VZV TK. Thus, these data are in agreement with the drug resistance data obtained in this study and point to the crucial role of VZV-encoded TK in the viral specificity and antiviral activity of the BCNAs.
The antiviral drugs with activity against VZV (i.e., ACV, PCV, and BVDU) are also active against the two other human alpha-herpesviruses (HSV-1 and HSV-2), and for some (e.g., GCV) the antiviral spectrum is even extended to other herpesviruses, i.e., beta-herpesviruses such as the human cytomegalovirus. As a rule, these molecules are (i) either purine nucleoside analogues such as ACV, PCV, and GCV or pyrimidine nucleoside analogues such as BVDU, which require phosphorylation by the viral TK for their activation; (ii) nucleotide analogues such as CDV, or (iii) pyrophosphate analogues such as PFA, which do not require TK for their activation. The two last classes of molecules are active against resistant viruses harboring mutations in the thymidine kinase gene. The BCNAs differ from the other known anti-VZV drugs in that they are highly selective for VZV and do not inhibit the closely related HSV-1 and HSV-2. At least one of the reasons for this unprecedented selectivity is the lack of substrate affinity for HSV-1 TK (4), making the BCNAs unique in this respect. BCNA resistance development is situated predominantly at the level of VZV TK; in addition, cross-resistance to other TK-depending drugs (i.e., BVDU and ACV) was observed. It would be interesting to identify the nature of the mutations that are present in the VZV TK of the BCNA-resistant VZV isolates and to compare them with those occurring in the BVDU- or ACV-resistant virus strains.
Recently, other classes of molecules have been described in which the antiviral activity is restricted to certain herpesviruses from different subfamilies. This is the case for the series of benzimidazole d- and l-ribonucleosides showing specific and significant activity against both human cytomegalovirus and Epstein-Barr virus (24). Similarly to BCNAs, a series of nonnucleosides, i.e., N′-α-methylbenzyl-N′-arylthiourea analogs, have demonstrated selective activity against VZV, being inactive against any other human herpesviruses. Drug-resistant viruses selected by exposure to one of these molecules were also cross-resistant to other members of the series. However, these compounds retained activity against ACV-resistant strains of VZV. The presence of mutations at the level of the VZV-encoded ORF54 suggests that the products of that gene would play a role in the mechanism of antiviral activity of these nonnucleoside derivatives (22).
In conclusion, the BCNAs were demonstrated to be exquisitely potent and selective inhibitors of clinical VZV isolates. Despite their unique preference for VZV, they selected for drug-resistant virus strains that showed cross-resistance to other VZV TK-dependent drugs.
Acknowledgments
We thank Anita Camps, Lies Van den Heurck, Steven Carmans, and Lizette van Berckelaer for excellent technical assistance and Christiane Callebaut for fine editorial help.
This research was supported by grants from the Belgian Fonds voor Geneeskundig Wetenschappelijk Onderzoek (FWO No. G.0267.04) and the Belgian Geconcerteerde Onderzoeksacties (GOA No. 00/12).
REFERENCES
- 1.Andrei, G., E. De Clercq, and R. Snoeck. 2004. In vitro selection of drug-resistant varicella-zoster virus (VZV) mutants (OKA strain): differences between acyclovir and penciclovir? Antiviral Res. 61:181-187. [DOI] [PubMed] [Google Scholar]
- 2.Andrei, G., R. Snoeck, D. Reymen, C. Liesnard, P. Goubau, J. Desmyter, and E. De Clercq. 1995. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur. J. Clin. Microbiol. Infect. Dis. 14:318-328. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J., R. Sienaert, S. Liekens, A. Van Kuilenburg, A. Carangio, R. Esnouf, E. De Clercq, and C. McGuigan. 2002. Lack of susceptibility of bicyclic nucleoside analogs, highly potent inhibitors of varicella-zoster virus, to the catabolic action of thymidine phosphorylase and dihydropyrimidine dehydrogenase. Mol. Pharmacol. 61:1140-1145. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini, J., and C. McGuigan. 2002. Chemotherapy of varicella-zoster virus by a novel class of highly specific anti-VZV bicyclic pyrimidine nucleosides. Biochim. Biophys. Acta 1587:287-295. [DOI] [PubMed] [Google Scholar]
- 5.Balzarini, J., and C. McGuigan. 2002. Bicyclic pyrimidine nucleoside analogues (BCNAs) as highly selective and potent inhibitors of varicella-zoster virus replication J. Antimicrob. Chemother. 50:5-9. [DOI] [PubMed] [Google Scholar]
- 6.Breuer, J. 2003. Monitoring virus strain variation following infection with VZV: is there a need and what are the implications of introducing the Oka vaccine? Commun. Dis. Public Health 6:59-62. [PubMed] [Google Scholar]
- 7.Gross, G., H. Schöfer, S. Wassilew, K. Friese, A. Timm, R. Guthoff, H. W. Pau, J. P. Malin, P. Wutlzer, and H. W. Doerr. 2003. Herpes zoster guideline of the German Dermatology Society. J. Clin. Virol. 26:277-289. [DOI] [PubMed] [Google Scholar]
- 8.Lauzurica, R., B. Bayés, C. Frias, N. Fontseré, A. Hernandez, L. Matas, A. Jimenez, J. Bonet, and R. Romero. 2003. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant. Proc. 35:1758-1759. [DOI] [PubMed] [Google Scholar]
- 9.Levin, M. J., K. M. Dahl, A. Weinberg, R. Giller, A. Patel, and P. R. Krause. 2003. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus, in an immunosuppressed child. J. Infect. Dis. 188:954-959. [DOI] [PubMed] [Google Scholar]
- 10.Levy, O., J. S. Orange, P. Hibberd, S. Steinberg, P. LaRussa, A. Weinberg, S. B. Wilson, A. Shaulov, G. Fleisher, R. S. Geha, F. A. Bonilla, and M. Exley. 2003. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 188:948-953. [DOI] [PubMed] [Google Scholar]
- 11.Mattiuzzi, G. N., J. E. Cortes, M. Talpaz, J. Rèuben, M. B. Rios, J. Shan, D. Kontoyiannis, F. J. Giles, I. Raad, S. Verstovsek, A. Ferrajoli, and H. M. Kantarjian. 2003. Development of varicella-zoster virus infection in patients with chronic myelogenous leukemia treated with imatinib mesylate. Clin. Cancer Res. 9:976-980. [PubMed] [Google Scholar]
- 12.McGuigan, C., C. J. Yarnold, G. Jones, S. Velazquez, H. Barucki, A. Brancale, G. Andrei, R. Snoeck, E. De Clercq, and J. Balzarini. 1999. Potent and selective inhibition of varicella-zoster virus (VZV) by nucleoside analogues with an unusual bicyclic base. J. Med. Chem. 42:4479-4484. [DOI] [PubMed] [Google Scholar]
- 13.McGuigan, C., H. Barucki, S. Blewett, A. Carangio, J. T. Erichsen, G. Andrei, R. Snoeck, E. De Clercq, and J. Balzarini. 2000. Highly potent and selective inhibition of varicella-zoster virus by bicyclic furopyrimidine nucleosides bearing an aryl side-chain. J. Med. Chem. 43:4993-4997. [DOI] [PubMed] [Google Scholar]
- 14.Pacini-Edelstein, S. J., M. Mehra, M. E. Ament, J. H. Vargas, M. G. Martin, and S. V. McDiarmid. 2003. Varicella in pediatric liver transplant patients: a retrospective analysis of treatment and outcome. J. Pediatr. Gastroenterol. Nutr. 37:183-186. [DOI] [PubMed] [Google Scholar]
- 15.Rabasseda, X. 2003. Brivudine: a herpes virostatic with rapid antiviral activity and once-daily dosing. Drugs Today 39:359-371. [DOI] [PubMed] [Google Scholar]
- 16.Sienaert, R., L. Naesens, A. Brancale, E. De Clercq, C. McGuigan, and J. Balzarini. 2002. Specific recognition of the bicyclic pyrimidine nucleoside analogs, a new class of highly potent and selective inhibitors of varicella-zoster virus (VZV), by the VZV-encoded thymidine kinase. Mol. Pharmacol. 61:249-254. [DOI] [PubMed] [Google Scholar]
- 17.Sienaert, R., L. Naesens, A. Brancale, A. Carangio, G. Andrei, R. Snoeck, A. Van Kuilenburg, E. De Clercq, C. McGuigan, and J. Balzarini. 2003. Metabolic and pharmacological characteristics of the bicyclic nucleoside analogues (BCNAs) as highly selective inhibitors of varicella-zoster virus. Nucleosides Nucleotides Nucleic Acids 22:995-997. [DOI] [PubMed] [Google Scholar]
- 18.Sienaert, R., G. Andrei, R. Snoeck, E. De Clercq, C. McGuigan, and J. Balzarini. 2004. Inactivity of the bicyclic pyrimidine nucleoside analogues (BCNAs) against simian varicella virus (SVV) does not correlate with their substrate activity for SVV-encoded thymidine kinase. Biochem. Biophys. Res. Commun. 315:877-883. [DOI] [PubMed] [Google Scholar]
- 19.Snoeck, R., M. Gerard, C. Sadzot-Delvaux, G. Andrei, J. Balzarini, D. Reymen, N. Ahadi, J. M. De Bruyn, J. Piette, B. Rentier, N. Clumeck, and E. De Clercq. 1994. Meningoradiculoneuritis due to acyclovir-resistant varicella zoster virus in an acquired immune deficiency syndrome patient. J. Med. Virol. 42:338-347. [DOI] [PubMed] [Google Scholar]
- 20.Snoeck, R., G. Andrei, and E. De Clercq. 1999. Current pharmacological approaches to the therapy of varicella zoster virus infections. A guide to treatment. Drugs 57:187-206. [DOI] [PubMed] [Google Scholar]
- 21.Snoeck, R., G. Andrei, and E. De Clercq. 2000. Novel agents for the therapy of varicella-zoster virus infections. Exp. Opin. Investig. Drugs 9:1743-1751. [DOI] [PubMed] [Google Scholar]
- 22.Visalli, R. J., J. Fairhurst, S. Srinivas, W. Hu, B. Feld, M. DiGrandi, K. Curran, A. Ross, J. D. Bloom, M. van Zeijl, T. R. Jones, J. O'Connel, and J. I. Cohen. 2003. Identification of small molecule compounds that selectively inhibit varicella-zoster virus replication. J. Virol. 77:2349-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wassilew, S. W., and P. Wutzler. on behalf of the Brivudin Herpes Zoster Study Group. 2003. Oral brivudin in comparison with acyclovir for improved therapy of herpes zoster in immunocompetent patients: results of a randomized, double-blind, multicultured study. Antiviral Res. 59:49-56. [DOI] [PubMed] [Google Scholar]
- 24.Williams, S. L., C. B. Hartline, N. L. Kushner, E. A. Harden, D. J. Bidanset, Dr. J. C. Drach, L. B. Townsend, M. R. Underwood, K. K. Biron, and E. R. Kern. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]