Abstract
Background:
Tennis elbow is difficult to treat. The results of surgical treatments are not convincing. Treatment studies on Achilles and patellar tendinopathy targeting the richly innervated and vascularized soft tissues outside the tendon have shown promising outcomes. The innervation patterns in the fibrous/fatty tissues superficially to the elbow extensor origin have not been clarified.
Methods:
Nine tissue specimens from the fibrous/fatty tissue covering the extensor origin was taken from seven patients (mean age: 45 years) undergoing surgical treatment for chronic painful tennis elbow. The specimens were stained for morphology (haematoxylin & eosin, H&E) and immunohistochemically for general nerve marker protein gene product 9.5 (PGP 9.5) and markers for sympathetic (tyrosine hydroxylase, TH) and sensory nerve fibres (calcitonin gene-related peptide, CGRP).
Results:
All specimens contained multiple blood vessels and nerve structures indicated by morphology and immunoreactions. There was a frequent occurrence of TH reactions, especially peri-vascularly, but also in nerve fascicles. Immunoreactions for CGRP were seen in nerve fascicles and isolated nerve fibres.
Conclusion:
The results provide new information on the innervation patterns of the superficial tissues of the extensor origin and their potential as source of tennis elbow pain. Level of Evidence: IV.
Keywords: Innervation, Tennis Elbow, Lateral Epicondylitis, Superficial, Soft Tissue
Introduction
Chronic pain in the common extensor origin at the lateral epicondyle, commonly called tennis elbow, is a condition with unknown aetiology and pathogenesis, known to be troublesome to treat[1-3]. The origin of pain has not been clarified, but it’s commonly believed that the extensor carpi radialis brevis (ECRB) muscle origin is involved[4-6]. Examinations of tissue specimens from the ECRB origin have shown granulation like tissue, degenerative changes and occasionally micro-ruptures, but no inflammatory cell infiltrates[5-9].
Studies using ultrasound (US) and colour Doppler (CD) or power Doppler (PD) examinations have shown structural tendon changes in the origin (irregular structure, thickening, hypo-echogeneity, and calcifications), bone spurs and high blood flow inside and outside the extensor origin[10,11]. A possible relationship between high blood flow and pain has been suggested[10,11]. Colour Doppler (CD) sonography is an established technique to study blood flow, that has been demonstrated to be useful in the investigation, and to guide treatment.
Despite extensive research there is no consensus on an universally accepted treatment method for this painful condition[3]. A variety of non-surgical[12,13] and surgical[14,15] treatment methods are in use, but there is little scientific evidence for their effects. It is assumed that in up to 10 % of patients conservative methods fail and surgery is required[15,16]. Among the numerous surgical approaches that are available no technique has been found to be superior[17]. The majority of methods involve an intra-origin approach with debridement or release of the ECRB origin[15-18]. Furthermore, more recently, arthroscopic approaches targeting the articular (deep) surface of the extensors have been described[19,20].
Very little attention has been put on the superficial side of the ECRB origin and its potential as the source of pain, and as a target for treatments. In a recent study by Branson and co-workers it has been shown that polidocanol injections into this tissue can have superior effects on tendon structure and vascularity[21]. From previous studies on the innervation patterns in Achilles and patellar tendinopathy it is known that the majority of nerves are found outside the painful tendons in the peritendinous tissues[22,23]. The most frequent types of nerve structures found were sympathetic and sensory nerve fibres[22-24]. Minimally-invasive surgical treatment methods, targeting the nerve rich tissues outside these tendons have shown very good clinical results in short- and mid-term follow-ups[25-27]. Studies on the innervation patterns in tissue biopsies from tennis elbow patients have mainly focused on the inside of the origin and on the articular (deep) surface[28-31] both being target areas for previous surgical approaches. To our knowledge there is no information about the innervation patterns in the fibrous and fatty tissues covering the surface of the origin. However, based on the observations made by Branson et al.[21] – the positive outcome of polidocanol injections - it is very likely that this tissue is actively involved in the chronic pain mechanisms. The aim of this study was therefore to characterize this tissue and to clarify the innervation patterns in patients with chronic painful tennis elbow. For this purpose we have immmunohistochemically examined the expression for general nerve marker protein gene product 9.5 (PGP 9.5) and markers for sympathetic (tyrosine hydroxylase, TH) and sensory (calcitonin gene-related peptide, CGRP) innervation.
Material and methods
Patients
Seven patients (4 men and 3 women, mean age 45 years, range 40-48) with a long duration of pain (>6 months) diagnosed as tennis elbow in 9 elbows (5 unilateral, 2 bilateral), were included.
The diagnosis Tennis elbow was considered present if there was pain on palpation of the extensor origin, pain elicited from the region on resisted wrist extension and 3rd finger test, together with ultrasound and Doppler (CD) examination showing structural changes and high blood flow inside and outside the extensor origin. Patients with differential diagnoses such as cervico-brachialgia and radial nerve compression were excluded based on history and clinical findings. Patients previously having undergone surgical treatment were excluded. All patients had at least one cortisone injection given by other clinicians prior to this study.
Sonography
All tendons were examined with high resolution grey scale-ultrasound (US) and colour Doppler (CD), Acuson Sequoia 512, with 8-13 MHz frequency. The examinations were carried out in a sitting position, with the arm resting on a table, having 70-80 degrees of elbow flexion, and pronated wrist. CD was used to locate high blood flow. The same experienced doctor (HA) performed all US- and CD-examinations.
Biopsies - Surgical treatment
During pre-operative US+CD examination, skin markers were placed to localise the region with high blood flow outside the extensor origin. Under local anesthesia (3-4 ml of xyocain+Adreanaline) the tissue from the region with thickened fibrous tissue and high blood flow was released and removed from the surface of the extensor origin. This removed tissue represents the biopsies. This was followed by hemostasis and closure of the skin with non-resorbable sutures. Bandage with local compression over night. Postoperatively there was range of movement exercises and no heavy loading (not allowed to lift more than 2 kg) for 2 weeks. After 2 weeks there was suture removal and gradually increased loading, allowing free loading after 4 weeks.
Tissue processing and analysis of morphology
The taken biopsies were put in fixative solution containing 4% formaldehyde in 0.1 M phosphate buffer (pH 7.0) and incubated overnight at 4°C. After that tissue was washed three times (including one wash overnight) in Tyrode’s solution containing 10% sucrose (pH 7.2) and then mounted on a thin cardboard in OCT embedding medium (TissueTek, Miles Laboratories, Naperville, IL, USA). Finally, the specimens were frozen in liquid propane chilled with liquid nitrogen and stored at -80°C until use. Cryosections with a thickness of 7 µm were performed (Leica Microsystem CM 300, Heidelberg, Germany) and mounted on superfrost plus slides (Thermo Scientific, Braunschweig, Germany). Staining for morphology (H&E) was done according to an established protocol[32].
Immunohistochemistry
Antibodies used were directed towards general nerve marker protein gene product 9.5 (PGP 9.5; Serotec, code: 7863-0504; rabbit polyclonal), sympathetic nerve marker tyrosine hydroxylase (TH; Pel-Freez Arkansas; code: P40101; rabbit polyclonal) and sensory marker calcitonin related gene peptide (CGRP; Santa Cruz Biotechnologies, code: sc-8856; goat polyclonal).
The sections were initially pre-incubated in potassium permanganate for 2 min in order to enhance visualisation of specific immunofluorescence reaction sites. After that the slides were washed three times for 5 min each in 0.01 M phosphate-buffered saline (PBS, pH 7.4), containing 0.1% sodium azide as preservative, then kept in 1% Triton X-100 in 0.01 M PBS (pH 7.4) for 20 min followed by another washing step. Then sections were treated with 5% normal serum for 15 min and then incubated with the primary antibody (37°C, one hour). After that the slides were washed (three times) and again treated with 5% normal serum followed by incubation with the secondary antibody (37°C, 30 min). Eventually, the sections were washed and mounted with Vectashield mounting medium (code: H-1000). Microscopical evaluation was carried out using a Zeiss Axioscope 2 plus microscope equipped with epifluorescent technique and an Olympus DP70 digital camera.
Donkey normal serum (code: 017-000-121; Jackson Immune Research Laboratories Inc., West Grove, PA, USA) and FITC-conjugated donkey anti-goat secondary antibody (code: 705-095-147; Jackson Immune Research Inc.) were used for stainings for CGRP (goat polyclonal). When using rabbit primary antibodies (PGP 9.5, TH), swine normal serum (code: 014-000-121; Jackson Immune Research Inc.) and TRITC-conjugated swine anti-rabbit secondary antibody (code: R0156, DAKO) was used. For the rabbit antibodies (PGP 9.5, TH) normal serum (swine) and primary and secondary antibodies were diluted in 0.1% bovine serum albumin (BSA) in 0.01 M PBS (pH 7.4). When using goat antibodies (CGRP) all dilutions were made without BSA.
For all antibodies control stainings using PBS instead of the primary antibody were performed. The specificity of the antibodies was also tested in a previous study[33] on the plantaris tendon.
Ethics
The investigation was approved by the Ethical Committee of the Medical Faculty, University of Umeå.
Results
Sonography
All patients had structural changes inside the extensor origin and a thickened fibrous layer superficially, together with a high blood flow inside and superficial to the origin (Figure 1).
Figure 1.
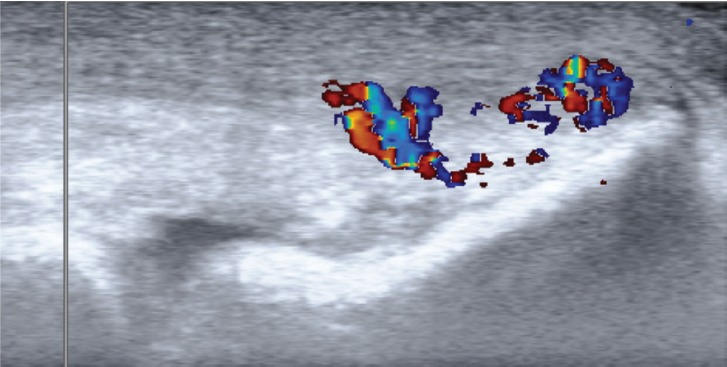
US+CD examination of the extensor origin in a patient with tennis Elbow. High blood flow can be seen in the tissues superficial to the extensor origin.
Morphology
The examined tissue specimens mainly consisted of loose peritendinous tissue and very minor pieces of tendinous-like tissue. The superficial loose connective tissues were highly vascularised and showed plenty of nerve structures that were even seen on sections stained for haematoxylin and eosin (Figure 2 B,C). To some extent, the numbers of blood vessels and nerve fascicles slightly varied between different specimens. Most specimens included fat tissue (not shown). The small tendinous parts of the tissue specimens showed a tendinosis-like tissue pattern including large numbers of tenocytes with a wavy and rounded appearance (Figure 2 A).
Figure 2.
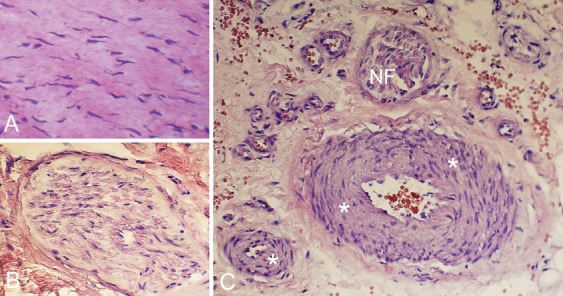
Sections stained for morphology (H&E). The tendinous tissue exhibits large numbers of tenocytes with different shapes including rounded and wavy appearance (A). There are nerve structures such as nerve fascicles (B) and also large numbers of blood vessels C). Asterisks point on blood vessel walls; NF indicates nerve fascicles.
General innervation
There was a marked expression of PGP 9.5 in the superficial soft tissues in all examined tissue specimens (Figure 3). Immunoreactions for PGP 9.5 were found in large and small nerve fascicles (Figure 3 A,B,C), in blood vessel walls (Figure 3 B,C) and in single nerve fibres mostly located close to the vessels (D). Overall, it could be observed that the nerve structures were located in close vicinity to the blood vessels (Figure 3 B,C,D). There was certain heterogeneity in the perivascular nerve fibre distribution. Some blood vessels exhibited a high degree of innervation and some negligible or no such innervation at all.
Figure 3.
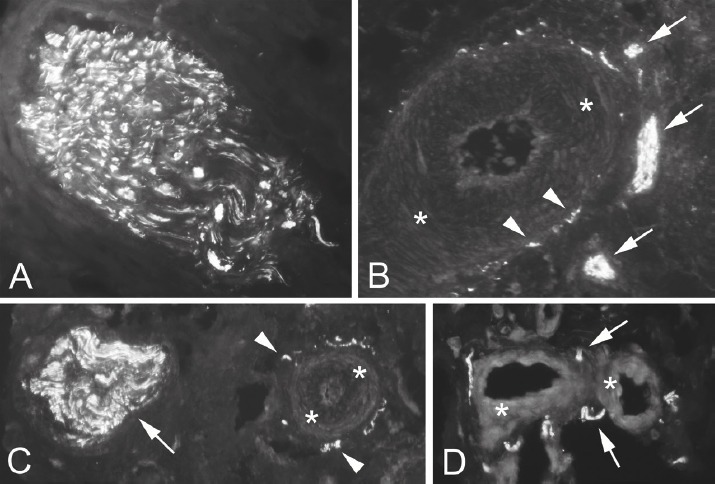
Sections stained for nerve marker PGP 9.5. Immunoreactions are seen in nerve fascicles (A,B,C), in blood vessel walls (B,C) and as single nerve fibres (D). PGP 9.5 is often located close to blood vessels (B,C,D). Arrows indicate nerve fibres and small nerve fascicles. Arrrowheads indicate reactions within the blood vessel wall. Asterisks point on blood vessel walls.
Nerve characterization
Immunoreactions for TH were frequent and mostly seen in nerve fascicles (Figure 4 A), in perivascular positions and in vessel walls (Figure 4 B,C). Reactions for CGRP were mainly seen in nerve fascicles and to some extent in freely coursing nerve fibres (Figure 5 A,B) often being located in vicinity of vessels. Overall there was a marked expression of both markers in this tissue. However, immunoreactions for TH were more frequent than those for CGRP.
Figure 4.
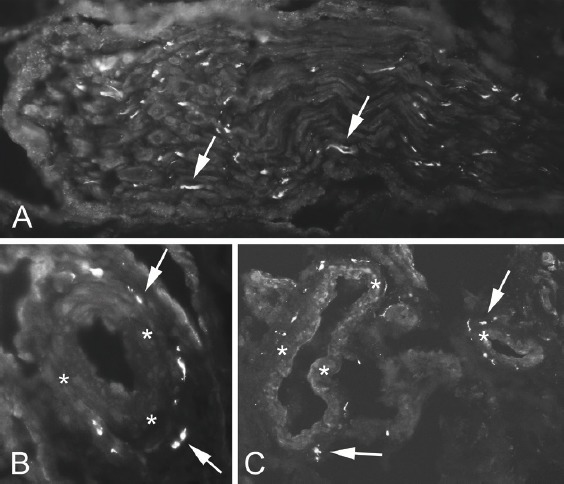
Sections stained for sympathetic nerve marker TH. Immunoreactions were observed in nerve fascicles (A), in blood vessel walls and in perivascular position (B,C). Arrows indicate positive immunoreactions. Asterisks point on blood vessel walls.
Figure 5.
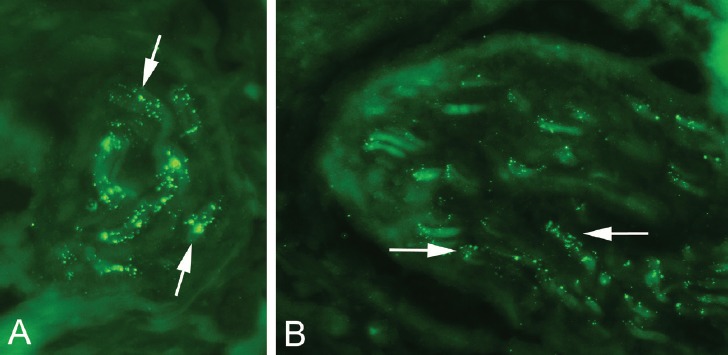
Sections stained for sensory nerve marker CGRP. Immunoreactions are seen in nerve fascicles (A,B). Arrows indicate positive reactions.
There were no adverse events related to the surgical treatment.
Discussion
The results of this study showed that there was a rich general innervation in close relation to blood vessels in the fibrous and fat tissues superficial to the extensor origin in all patients undergoing surgery for chronic painful tennis elbow. There were frequent both sympathetic and sensory nerve fibres.
To the best of our knowledge, there seems to be a lack of knowledge about innervation patterns in relation to the extensor origin in patients suffering from chronic painful tennis elbow. There seems to be no information about the superficial side of the origin. The main knowledge about the innervation patterns in this condition is from examinations on the inside and deep-articular side of the extensor origin[28-31]. On the inside both sympathetic and sensory innervation was found[28-30]. At the deep-articular side Sasaki and co-workers have detected increased levels of sympathetic but only limited sensory innervation[31]. In our study we have found a marked expression of both nerve markers suggesting crucial roles for sympathetic and sensory innervation in chronic pain in tennis elbow.
The sympathetic nervous system is traditionally known for its vasoconstrictive functions. However, recent studies have found out that it can also be involved in pain[34]. In tendinopathic tendons increased sympathetic markers have been found in peritendinous tissues but not in the tendon proper[24]. Furthermore, the tendon pain duration seems to be correlating with muscle sympathetic activity[35]. CGRP positive sensory nerve fibres are known to transmit pain but also to be involved in vasodilation[36]. Thus, it is very likely that both the sympathetic and sensory nerve fibres are involved in the pain and vascularisation in tendinopathies and tennis elbow.
The findings in the current study are very similar to the findings in patients with midportion Achilles tendinopathy and proximal patellar tendinopathy, were we also found a rich sympathetic and sensory innervation outside the tendon tissue proper[23,24,33]. For these tendons the findings have led to the invention of new ultrasound and Doppler-guided treatment methods, targeting the regions with blood vessels and nerves outside the tendons, methods that have shown very good clinical results[25,26,37]. Comparing the present results with specimens from the Achilles and patellar tendons we have observed that there seems to be even more nerve structures in the superficial tennis elbow specimens. Even though this observation is un-published and only descriptive, it further highlights the potential importance of this tissue in relation to the pain. This is supported by the study by Branson and co-workers who have observed pain reduction after polidocanol injections into this area. As tennis elbow belongs to the group of tendinopathies, it’s not unlikely that there are similarities also in the innervation patterns between the different tendons affected by this often chronic painful condition.
We used US+CD as part of the diagnostic procedure, and in all origins we found a localised high blood inside and outside the extensor origin. Doppler examination has been shown to have a high sensitivity[11]. Even though a direct correlation between the high blood flow seen on CD and the multiple blood vessels found in the tissue specimens cannot be established, it is an interesting observation. We believe it’s likely that the regions with high blood flow seen on CD examination represent the richly vascularized and nerve-rich tissue on the superficial side of the extensor origin and possibly is where the pain is elicited in tennis elbow.
Treatment of Tennis elbow is known to be difficult and there is no golden standard treatment methodology. In clinical praxis there are many opinions, and because of varying and sometimes poor treatment results some consider that no surgical treatment should be instituted. Among the most commonly used surgical treatments are debridement/release focusing on the inside of the ECRB origin and arthroscopic shaving techniques focusing on the (deep-articular) origin surface[14-20].
Clinical observations from a minor group of patients operated with this simple ultrasound and Doppler-guided scraping procedure, excising only the richly vascularized fatty fibrous tissue covering the extensor origin, has shown very good clinical outcomes in up to 3 years follow-ups. An interesting observation was that already after the local anaestetic was applied, 3-4 ml injected subcutaneously only, all patients were pain-free during loading.
Altogether the findings are interesting, but of course, robust clinical studies are needed for further evaluation of this new treatment approach. A weakness in the current study is the lack of control specimens from pain-free individuals in the same age group and having normal US and Doppler findings. This was due to ethical reasons. The clinical results after surgery should be seen as an observational finding of interest and needs to be verified in large material studies including control group. Future studies shall focus on the histological comparison of this superficial tissue in people with tennis elbow and those without clinical symptoms. Furthermore, a potential correlation of histological findings and the severity of clinical symptoms and/or the surgical outcome shall be investigated.
In conclusion, this study showed that the fibrous and fatty soft tissues superficial to the extensor origin in tennis elbow patients are highly vascularised and innervated with sympathetic and to some extent sensory nerve fibres. Preliminary results from surgical treatment targeting removal of this tissue have shown promising clinical results, but this type of treatment needs to be further evaluated in a robust clinical study.
Acknowledgement
The authors would like to thank Prof. Sture Forsgren, Assist. Prof. Ludvig Backman and Assist. Prof. Gustav Andersson for their input concerning the design of the study. We furthermore thank all the patients for their co-operation. Financial support was obtained from the Faculty of Medicine at Umeå University, the Swedish National Centre for Research in Sports (CIF) and Idrottshoügskolan, Umeå University. The funders had no role in study de- sign, data collection and analysis, decision to publish, or preparation of the manuscript.
Both authors were involved in the design, the analysis and interpretation of the results and the manuscript writing. CS performed the experiments, conducted the microscopical analyses and wrote the firs draft of the manuscript. HS conducted the operations and was responsible for all clinical data. Both authors approved the final version.
Footnotes
The authors have no conflict of interest.
Edited by: S. Warden
References
- 1.Boyer MI, Hastings H., 2nd Lateral tennis elbow: “Is there any science out there?”. J Shoulder Elbow Surg. 1999;8(5):481–91. doi: 10.1016/s1058-2746(99)90081-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad Z, Siddiqui N, Malik SS, Abdus-Samee M, Tytherleigh-Strong G, Rushton N. Lateral epicondylitis: a review of pathology and management. Bone Joint J. 2013;95-B(9):1158–64. doi: 10.1302/0301-620X.95B9.29285. [DOI] [PubMed] [Google Scholar]
- 3.Luk JK, Tsanb RC, Leung HB. Lateral epicondylalgia: midlife crisis of a tendon. Hong Kong Med J. 2014;20(2):145–51. doi: 10.12809/hkmj134110. [DOI] [PubMed] [Google Scholar]
- 4.Ljung BO, Lieber RL, Friden J. Wrist extensor muscle pathology in lateral epicondylitis. J Hand Surg Br. 1999;24(2):177–83. doi: 10.1054/jhsb.1998.0178. [DOI] [PubMed] [Google Scholar]
- 5.Potter HG, Hannafin JA, Morwessel RM, DiCarlo EF, O’Brien SJ, Altchek DW. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology. 1995;196(1):43–6. doi: 10.1148/radiology.196.1.7784585. [DOI] [PubMed] [Google Scholar]
- 6.Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20(6):746–9. doi: 10.1177/036354659202000618. [DOI] [PubMed] [Google Scholar]
- 7.Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177–82. [PubMed] [Google Scholar]
- 8.Nirschl RP. Elbow tendinosis/tennis elbow. Clin Sports Med. 1992;11(4):851–70. [PubMed] [Google Scholar]
- 9.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique - no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71(5):475–9. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 10.Zeisig E, Ohberg L, Alfredson H. Extensor origin vascularity related to pain in patients with Tennis elbow. Knee Surg Sports Traumatol Arthrosc. 2006;14(7):659–63. doi: 10.1007/s00167-006-0060-7. [DOI] [PubMed] [Google Scholar]
- 11.du Toit C, Stieler M, Saunders R, Bisset L, Vicenzino B. Diagnostic Accuracy Of Power-Doppler Ultrasound In Patients With Chronic Tennis Elbow. Br J Sports Med. 2008;42(11):872–6. doi: 10.1136/bjsm.2007.043901. [DOI] [PubMed] [Google Scholar]
- 12.Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med. 2005;39(7):411–22. doi: 10.1136/bjsm.2004.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims SE, Miller K, Elfar JC, Hammert WC. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY) 2014;9(4):419–46. doi: 10.1007/s11552-014-9642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder R, Green S, Bell S, Barnsley L, Smidt N, Assendelft WJ. Surgery for lateral elbow pain. Cochrane Database Syst Rev. 2002;1:CD003525. doi: 10.1002/14651858.CD003525. [DOI] [PubMed] [Google Scholar]
- 15.Lo MY, Safran MR. Surgical treatment of lateral epicondylitis: a systematic review. Clin Orthop Relat Res. 2007;463:98–106. doi: 10.1097/BLO.0b013e3181483dc4. [DOI] [PubMed] [Google Scholar]
- 16.Nirschl RP, Pettrone FA. Tennis elbow: the surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832–9. [PubMed] [Google Scholar]
- 17.Bosworth DM. Surgical treatment of tennis elbow;a follow-up study. J Bone Joint Surg Am. 1965;47:1533–1536. [PubMed] [Google Scholar]
- 18.Wittenberg RH, Schaal S, Muhr G. Surgical treatment of persistent elbow epicondylitis. Clin Orthop Relat Res. 1992;278:73–80. [PubMed] [Google Scholar]
- 19.Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582–7. doi: 10.1053/jars.2001.20098. [DOI] [PubMed] [Google Scholar]
- 20.Wada T, Moriya T, Iba K, Ozasa Y, Sonoda T, Aoki M, Yamashita T. Functional outcomes after arthroscopic treatment of lateral epicondylitis. J Orthop Sci. 2009;14(2):167–74. doi: 10.1007/s00776-008-1304-9. [DOI] [PubMed] [Google Scholar]
- 21.Branson R, Naidu K, du Toit C, Rotstein AH, Kiss R, McMillan D, et al. Comparison of corticosteroid, autologous blood or sclerosant injections for chronic tennis elbow. J Sci Med Sport. 2016 doi: 10.1016/j.jsams.2016.10.010. article in press. [DOI] [PubMed] [Google Scholar]
- 22.Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):125–32. doi: 10.1007/s00167-005-0636-7. [DOI] [PubMed] [Google Scholar]
- 23.Andersson G, Danielson P, Alfredson H, Forsgren S. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1272–9. doi: 10.1007/s00167-007-0364-2. [DOI] [PubMed] [Google Scholar]
- 24.Jewson JL, Lambert GW, Storr M, Gaida JE. The sympathetic nervous system and tendinopathy: a systematic review. Sports Med. 2015 May;45(5):727–43. doi: 10.1007/s40279-014-0300-9. [DOI] [PubMed] [Google Scholar]
- 25.Alfredson H, Ohberg L, Zeisig E, Lorentzon R. Treatment of midportion Achilles tendinosis: similar clinical results with US and CD-guided surgery outside the tendon and sclerosing polidocanol injections. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1504–9. doi: 10.1007/s00167-007-0415-8. [DOI] [PubMed] [Google Scholar]
- 26.Alfredson H. Ultrasound and Doppler-guided mini-surgery to treat midportion Achilles tendinosis: results of a large material and a randomised study comparing two scraping techniques. Br J Sports Med. 2011;45(5):407–10. doi: 10.1136/bjsm.2010.081216. [DOI] [PubMed] [Google Scholar]
- 27.Sunding K, Willberg L, Werner S, Alfredson H, Forssblad M, Fahlström M. Sclerosing injections and ultrasound-guided arthroscopic shaving for patellar tendinopathy: good clinical results and decreased tendon thickness after surgery-a medium-term follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2259–68. doi: 10.1007/s00167-014-3028-z. [DOI] [PubMed] [Google Scholar]
- 28.Ljung BO, Forsgren S, Friden J. Substance P and calcitonin gene-related peptide expression at the extensor carpi radialis brevis muscle origin: implications for the etiology of tennis elbow. J Orthop Res. 1999;17(4):554–9. doi: 10.1002/jor.1100170414. [DOI] [PubMed] [Google Scholar]
- 29.Ljung BO, Forsgren S, Fridén J. Sympathetic and sensory innervations are heterogeneously distributed in relation to the blood vessels at the extensor carpi radialis brevis muscle origin of man. Cells Tissues Organs. 1999;165(1):45–54. doi: 10.1159/000016673. [DOI] [PubMed] [Google Scholar]
- 30.Ljung BO, Alfredson H, Forsgren S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus. Studies on tennis elbow and medial epicondylalgia. J Orthop Res. 2004;22(2):321–7. doi: 10.1016/S0736-0266(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki K, Ohki G, Iba K, Kokai Y, Yamashita T, Wada T. Innervation pattern at the undersurface of the extensor carpi radialis brevis tendon in recalcitrant tennis elbow. J Orthop Sci. 2013;18(4):528–35. doi: 10.1007/s00776-013-0406-1. [DOI] [PubMed] [Google Scholar]
- 32.Spang C, Scott A, Danielson P, Lorentzon R, Forsgren S. GluT2 and NMDAR1 expression in cells in the inflammatory infiltrates in experimentally induced myositis: evidence of local glutamate signaling suggests autocrine/paracrine effects in an overuse injury model. Inflammation. 2012;35(1):39–48. doi: 10.1007/s10753-010-9287-z. [DOI] [PubMed] [Google Scholar]
- 33.Spang C, Harandi VM, Alfredson H, Forsgren S. Marked innervation but also signs of nerve degeneration in between the Achilles and plantaris tendons and presence of innervation within the plantaris tendon in midportion Achilles tendinopathy. J Musculoskelet Neuronal Interact. 2015;15(2):197–206. [PMC free article] [PubMed] [Google Scholar]
- 34.Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular Med. 2008;10(3):141–7. doi: 10.1007/s12017-007-8018-6. [DOI] [PubMed] [Google Scholar]
- 35.Jewson JL, Lambert EA, Docking S, Storr M, Lambert GW, Gaida JE. Pain duration is associated with increased muscle sympathetic nerve activity in patients with Achilles tendinopathy. Scand J Med Sci Sports. 2016 Dec 28; doi: 10.1111/sms.12820. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Fan T-P D, Hu D-E, Guard S, Gresham GA, Waitling KJ. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK-1 or interleukin-1 receptor. Br J Pharmacol. 1993;110:43–49. doi: 10.1111/j.1476-5381.1993.tb13769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lind B, Ohberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1327–32. doi: 10.1007/s00167-006-0161-3. [DOI] [PubMed] [Google Scholar]


