Abstract
T-705, a substituted pyrazine compound, has been found to exhibit potent anti-influenza virus activity in vitro and in vivo. In a time-of-addition study, it was indicated that T-705 targeted an early to middle stage of the viral replication cycle but had no effect on the adsorption or release stage. The anti-influenza virus activity of T-705 was attenuated by addition of purines and purine nucleosides, including adenosine, guanosine, inosine, and hypoxanthine, whereas pyrimidines did not affect its activity. T-705-4-ribofuranosyl-5′-triphosphate (T-705RTP) and T-705-4-ribofuranosyl-5′-monophosphate (T-705RMP) were detected in MDCK cells treated with T-705. T-705RTP inhibited influenza virus RNA polymerase activity in a dose-dependent and a GTP-competitive manner. Unlike ribavirin, T-705 did not have an influence on cellular DNA or RNA synthesis. Inhibition of cellular IMP dehydrogenase by T-705RMP was about 150-fold weaker than that by ribavirin monophosphate, indicating the specificity of the anti-influenza virus activity and lower level of cytotoxicity of T-705. These results suggest that T-705RTP, which is generated in infected cells, may function as a specific inhibitor of influenza virus RNA polymerase and contributes to the selective anti-influenza virus activity of T-705.
Influenza is responsible for much morbidity and mortality in the world (7), and effective treatment is required. We now have two classes of drugs for the treatment of influenza, the ion channel blockers and the neuraminidase inhibitors. The ion channel blockers amantadine and rimantadine are of limited use because of a lack of activity against influenza B virus, side effects, and the rapid emergence of resistant virus strains (9). Neuraminidase inhibitors are effective against both influenza A and B viruses, and their usefulness in clinical treatment has been reported (2, 11). Ribavirin is a guanosine analogue and inhibits various RNA and DNA viruses, including influenza viruses (19). Its clinical efficacy against influenza virus after aerosol treatment of infected individuals has been reported (5), but it has been approved for use in only a few countries. Influenza viruses are able to undergo rapid antigenic changes, especially in the surface glycoproteins, and novel influenza virus variants which have high levels of virulence may emerge in the human population and cause severe disease. Therefore, exploration for novel anti-influenza virus agents is of most importance.
T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) has been found to have potent inhibitory activity against RNA viruses in vitro, especially influenza A, B, and C viruses. The selectivity index (the ratio of the 50% cell-inhibitory concentration [CC50]/50% influenza virus-inhibitory concentration [IC50]) was more than 6,000 (Table 1) (6). T-705 showed therapeutic efficacy in mouse infection models and had a profile different from those of ribavirin and oseltamivir (16).
TABLE 1.
Anti-influenza virus activities and cytotoxicities of T-705 and ribavirina
The values are means ± standard deviations for three independent experiments.
Activity against influenza A PR/8/34 virus.
Cytotoxicity in MDCK cells.
The present study describes an experimental approach to clarifying the mode of action of T-705, and the data indicate that T-705-4-ribofuranosyl-5′-triphosphate (T-705RTP) is the active form that contributes to anti-influenza virus activity.
MATERIALS AND METHODS
Reagents and compounds.
T-705 and its related compounds, T-705-4-ribofuranosyl-5′-monophosphate [T-705RMP; 6-fluoro-3,4-dihydro-3-oxo-4-(5-O-phosphono-β-d-ribofuranosyl)-2-pyrazinecarboxamide] and T-705RTP (6-fluoro-3,4-dihydro-4-[5-O-(hydroxy{[hydroxy (phosphonooxy) phosphinyl]oxy}phosphinyl)-β-d-ribofuranosyl]-3-oxo-2-pyrazinecarboxamide) (see Fig. 5), were synthesized at Toyama Chemical Co., Ltd. 6-Fluoro-3-oxo-3,4-dihydro-2-[2-14C]pyrazinecarboxamide ([14C]T-705; specific activity 8.38MBq/mg) was purchased from Daiichi Pure Chemicals Co., Ltd. (Ibaraki, Japan). Ribavirin (1-d-ribofuranosyl-1,2,4-triazole-3-carboxamide), adenine, guanine, adenosine, guanosine, inosine, 2′-deoxyadenosine, 2′-deoxyguanosine, cytosine, thymine, uracil, hypoxanthine, xanthine, uric acid, and reference compounds aphidicolin and actinomycin D were purchased from Sigma Chemical Co. (St. Louis, Mo.). [3H]thymidine, [3H]uridine, [32P]GTP, and [32P]UTP (specific activities, 86.0, 20.0, 236, and 3,000 Ci/mmol, respectively) were purchased from Amersham Co. (Piscataway, N.J.). [8-14C]IMP (specific activity, 0.053 Ci/mmol), ribavirin 5′-monophosphate (ribavirin MP) and ribavirin 5′-triphosphate (ribavirin TP) were purchased from Moravek (Brea, Calif.).
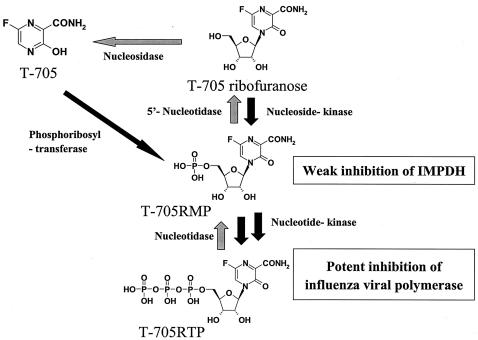
FIG. 5.
Hypothetic mechanism of action of T-705. T-705 may be converted to T-705 ribofuranosyl phosphates by host cell enzymes. The triphosphate form, T-705RTP, strongly inhibited by influenza virus RNA polymerase activity. Meanwhile, T-705 and its phosphates showed little inhibitory effect on the replication of the host cell.
Virus.
Influenza A/PR/8/34 (H1N1) virus was a kind gifted from Y. Okuno (Osaka Prefectural Institute of Public Health) and was propagated in MDCK cells.
Time-of-addition experiment.
A time-of-addition experiment was carried out with MDCK cells. The confluent monolayers of MDCK cells in 24-well tissue culture plates were inoculated on ice (approximately 4°C) with 140 PFU of virus, corresponding to a multiplicity of infection of 0.001 PFU/cell. After adsorption for 60 min, the monolayers were washed three times with phosphate-buffered saline (PBS) and incubated in Eagle minimal essential medium (EMEM) containing 1% bovine serum albumin and 2 μg of trypsin per ml with 100% humidity and 5% CO2 at 35°C (time zero). The test medium containing T-705 at 10 μg/ml (63.7 μM, approximately 60 times higher than the IC50; Table 1) was added at −1 to 0 (adsorption), 0 to 2, 2 to 4, 4 to 6, 6 to 8, or 8 to 10 h. After each incubation period, the monolayers were washed three times with PBS and incubated with fresh medium until 10 h postinfection. Then, the monolayers were frozen at −80°C and were subjected to two freeze-thaw cycles prior to determination of the viral yield by a plaque assay.
Reversal of antiviral activity.
The in vitro antiviral activities of T-705 and ribavirin and reversal of their activities by nucleic acids and nucleosides were studied in MDCK cells infected with influenza A/PR/8/34 virus. Six-well tissue culture plates containing MDCK cells grown to a confluent state were inoculated with 70 PFU of influenza virus. After adsorption of virus for 1 h at 35°C, the inoculum was removed and the cell monolayers were overlaid with EMEM containing 0.5% agarose, 0.001% DEAE-dextran, 2 μg of tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin per ml, and the test compound, with and without the reversal agents. The concentration of T-705 was 1 μg/ml (6.4 μM, approximately 6 times higher than the IC50; Table 1), and that of ribavirin was 10 μg/ml (41.0 μM, 1.3 times higher than the IC50 and half the CC50; Table 1). Nucleic acids and nucleosides, except for guanosine and 2′-deoxyguanosine for experiments with ribavirin, were added at 10-fold the molarity of the IC50 of each antiviral agent (i.e., 63.7 μM for experiments with T-705 and 410 μM for experiments with ribavirin). Guanosine and 2′-deoxyguanosine were added at concentrations of twofold the molarity of ribavirin (82.0 μM) for experiments with ribavirin. After the monolayers were incubated at 35°C in a 5% CO2 atmosphere for 2 days, the plates were fixed with 3% formaldehyde and stained with 0.005% amido black. The number of plaques was counted, and the percent inhibition of antiviral activity was calculated.
HPLC analysis of intracellular metabolites.
The intracellular phosphorylation profile of T-705 in MDCK cells was evaluated by radiochromatogram analysis by high-performance liquid chromatography (HPLC). MDCK cells were incubated with EMEM containing [14C]T-705 at 100 μg/ml (637 μM) for 24 h. Then, the medium was removed and the cell layers were washed with ice-cold PBS. T-705 and its metabolites were extracted by treatment with 70% ice-cold methanol. This extract was warmed at 20°C for 1 h and then loaded onto a SAX column (Mega Bond Elut; GL Sciences Inc., Tokyo, Japan). The fraction from the loaded column was eluted with 0.5 M triethylammonium hydrogen carbonate solution, dried, and stored at −30°C until it was analyzed. The dried samples were dissolved in 0.002 M phosphate buffer (pH 7.0) and analyzed by anion-exchange HPLC on a Partisil-10 SAX analytical column (4.6 by 250 mm; model L-6200; Hitachi Ltd., Tokyo, Japan). The mobile phase consisted of two solvents: solvent A (0.002 M phosphate buffer [pH 7.0]) and solvent B (0.2 M phosphate buffer [pH 7.0]). The mobile phase was pumped through the column at 1.0 ml/min. Elution was performed by using the following solvent gradient program (expressed as a percentage of solvent): 100% solution A from 0 to 15 min and 0 to 100% solution B from 15 to 65 min, with a hold with 100% solution B from 65 to 70 min. The column elute was monitored continuously at 350 nm by using an L-4000 UV detector (Hitachi Ltd.). Radioactivity was analyzed by use of a radiochemical detector (Radiomatic 525TR; Perkin-Elmer Life Sciences, Boston, Mass.). Under these conditions, the retention times for pure standards of T-705, T-705RMP, and T-705RTP were about 8, 27, and 62 min, respectively.
Inhibitory effect on influenza RNA polymerase.
Egg allantoic fluid was harvested from eggs infected with influenza virus for 60 h, and the virus was pelleted by ultracentrifugation (100,000 × g at 4°C for 90 min). Isolated virions were disrupted by exposure to detergent (0.25% Triton N-101) and were used as a source of influenza virus RNA polymerase. The enzyme reaction was started by addition of 5 μl of enzyme solution into the reaction mixture (final volume, 50 μl) containing 100 mM Tris-HCl (pH 8.0), 100 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.25% Triton N-101, 5 μCi of [α-32P]GTP, 100 μM ATP, 50 μM each CTP and UTP, 1 μM GTP, and 200 μM adenyl (3′-5′) guanosine with or without a test compound (T-705, T-705RMP, T-705RTP, or ribavirin TP). After incubation at 30°C for 1 h, 10% trichloroacetic acid (TCA) was added and the mixtures were left on ice for 1 h. The precipitate was collected through a glass fiber filter (GF/C; Whatman), and the filter was washed sufficiently with 5% TCA. After the filter was dried, the radioactivity was counted in a liquid scintillation counter. For the kinetic study, the Ki value was obtained by same assay, except that the concentrations of the nucleoside triphosphates were 5 μCi for [α-32P]UTP, 1 μM UTP, 100 μM ATP, 50 μM CTP, and 12.5 to 200 μM GTP.
Inhibitory effects on cellular DNA and RNA synthesis.
Semiconfluent monolayers of MDCK cells in 24-well tissue culture plates were incubated with EMEM containing T-705 at 10 and 100 μg/ml (63.7 and 637 μM, respectively) or ribavirin at 0.1, 1, and 10 μg/ml (0.4, 4.1, and 41.0 μM, respectively) for 6 h prior to the addition of the labeled precursors, [3H]thymidine and [3H]uridine (1 μCi/well). The incubations were continued for another 3 h. The cells were washed with ice-cold PBS, and then the cells in each well were spotted onto a GF/C filter. The filters were washed sufficiently with 5% TCA, and the filter-bound radioactivity was counted with a liquid scintillation counter. Aphidicolin at 5 μM was used as the positive control for the DNA synthesis study, and actinomycin D at 5 μM was used as the positive control for the RNA synthesis study.
Inhibition of IMPDH activity.
IMP dehydrogenase (IMPDH) activity was determined by measuring the conversion of [14C]IMP to [14C]XMP by a modified method reported previously (1). The reaction (final volume, 30 μl) was performed in a test tube containing 100 mM Tris-HCl (pH 8.0), 100 mM KCl, 3 mM EDTA, 500 μM NAD, 50 μg of bovine serum albumin per ml, 40 μM [14C]IMP, 22 μg of protein prepared from MDCK cell lysates (4 × 107 cells/ml), and a test compound (T-705RMP or ribavirin MP). See Fig. 4 for the concentrations of the test compounds. After incubation at 37°C for 60 min, the reaction was stopped, and the reaction mixture was deproteinized by adding a twofold volume of acetonitrile. The supernatant obtained after centrifugation at 3,000 × g was evaporated off of the acetonitrile fraction. The radioactivities of [14C]IMP and [14C]XMP in the aqueous supernatants were detected with a radiochemical detector (Radiomatic 525TR HPLC system with a Deverosil ODS-MG-5 column; 4.6 mm [inner diameter] by 250 mm). The mobile phase consisted of 20% acetonitrile, 0.02 M potassium phosphate (pH 7.0), and 5 mM tetrabutylammonium bromide. The rate of XMP formation to the total count was determined for each sample.
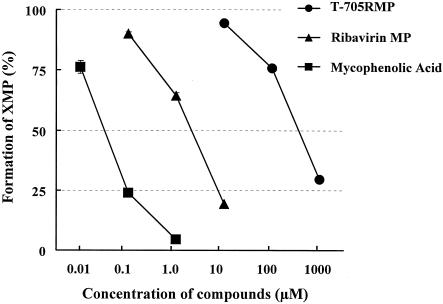
FIG. 4.
Inhibitory effects of T-705RMP, ribavirin MP, and mycophenolic acid on cellular IMPDH activity. The formation of [14C]XMP from [14C]IMP was determined with a radiometric 525TR HPLC system and is given as the percentage of that for the vehicle-treated control. The data are means ± standard deviations (n = 3). Two independent experiments were done, and representative data are shown.
RESULTS
Time-of-addition experiment.
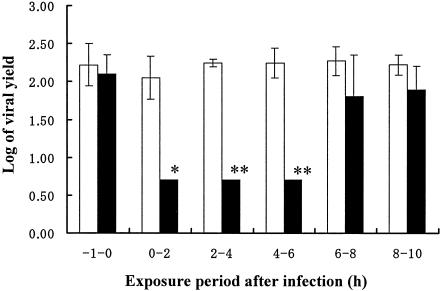
A time-of-addition experiment was conducted to determine the effects of varying the time of T-705 addition on the replication of influenza virus. About 8 to 10 h was usually required to detect the progeny virus after inoculation of influenza A/PR/8/34 virus in our experiments. Significant inhibition of virus replication was observed when T-705 was added to the cells within 6 h after viral infection. T-705 did not exert its antiviral activity when it was added 1 h before infection or 6 to 10 h after infection (Fig. 1). This result indicates that T-705 interferes with an early to middle stage of influenza virus replication but not the adsorption or the release stage.
FIG. 1.
Time-of-addition experiment. MDCK cells were inoculated with influenza A/PR/8/34 virus at a multiplicity of infection of 0.001. T-705 was added at the indicated times. Viral yields were determined at 10 h postinfection by plaque assays. Open columns, the mean viral yield for control cells; closed columns, the mean viral yield for cells treated with T-705 (10 μg/ml); vertical lines, standard deviations (n = 3). Two independent experiments were done, and representative data are shown. *, results significantly different from that for each control by Student's t test (P < 0.05), **, results significantly different from that for each control by Student's t test (P < 0.01).
Reversal of antiviral activity by nucleosides and nucleic acids.
The inhibition of antiviral activities of T-705 and ribavirin by nucleosides and nucleic acids was assayed by plaque assay in MDCK cells (Table 2). Viral plaque numbers were reduced to 7.5 and 6.8% in the presence of T-705 at 6.4 μM (1 μg/ml) and ribavirin at 41 μM (10 μg/ml), respectively. The antiviral activity of T-705 was significantly inhibited when adenine, guanine, adenosine, guanosine, inosine, 2′-deoxyadenosine, 2′-deoxyguanosine, or hypoxanthine was added at a 10-fold higher molarity. Xanthine partially inhibited the antiviral activity of T-705. Addition of cytosine, thymine, uracil, and uric acid had no effect on the activity of T-705. A reversal of the antiviral activity of ribavirin was observed by addition of guanine, adenosine, or 2′-deoxyadenosine at a 10-fold higher molarity and by addition of guanosine and 2′-deoxyguanosine at a 2-fold higher molarity. Addition of adenine, inosine, or hypoxanthine to the culture did not inhibit the activity of ribavirin. None of these nucleosides or nucleic acids by itself inhibited plaque formation at each concentration tested (data not shown).
TABLE 2.
Reversal of anti-influenza virus activities of T-705 and ribavirin by nucleic acids and nucleosides
| Compound combined | % of control activitya
|
|
|---|---|---|
| T-705 | Ribavirin | |
| Vehicle (alone) | 7.5 ± 10.9 | 6.8 |
| Adenine | 106.3 ± 14.9b | 12.4 |
| Guanine | 101.8 ± 12.7b | 76.3 |
| Adenosine | 96.6 ± 10.8b | 91.1 |
| Guanosine | 95.0 ± 18.3b | 70.2 |
| Inosine | 104.4 ± 11.1b | 1.7 |
| 2′-Deoxyadenosine | 114.2 ± 2.3b | 73.4 |
| 2′-Deoxyguanosine | 95.3 ± 11.3b | 61.9 |
| Cytosine | 12.5 ± 15.8 | 2.1 |
| Thymine | 5.5 ± 8.1 | 7.1 |
| Uracil | 8.6 ± 12.5 | 5.8 |
| Hypoxanthine | 105.7 ± 9.8b | 0.0 |
| Xanthine | 36.8 ± 57.6 | 14.1 |
| Uric acid | 8.3 ± 12.1 | 5.0 |
The number of plaques was counted and is given as a percentage of the number of plaques for the nontreated controls. Except for the concentrations of guanosine and 2′-deoxyguanosine, which were twice the IC50s of ribavirin, all nucleosides and nucleic acids were added at a 10-fold molarity of the IC50 of each compound. The values are means ± standard deviations for three (T-705) or two (ribavirin) independent experiments.
Results significantly different from the results for the controls treated with T-705 alone by Dunnett's test (P < 0.01).
HPLC analysis of intracellular metabolites.
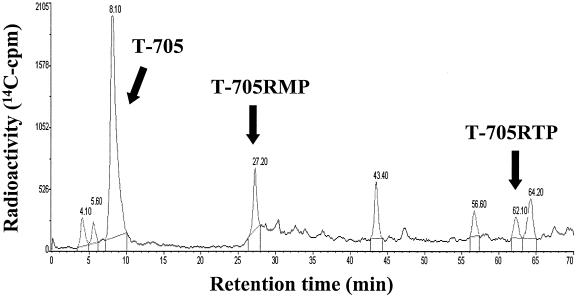
A typical HPLC radiochromatogram of intracellular extracts from MDCK cells treated with [14C]T-705 at a concentration of 100 μg/ml for 24 h is shown in Fig. 2. The retention times of the T-705 metabolites identified were confirmed by coinjection of each pure standard. Unchanged T-705 and two other metabolites, T-705RMP (retention time, 27 min) and T-705RTP (retention time, 62 min), were detected.
FIG. 2.
HPLC radiochromatogram of intracellular extracts from MDCK cells incubated with [14C]T-705 for 24 h. Extracts were analyzed by HPLC on a SAX column, as described in Materials and Methods.
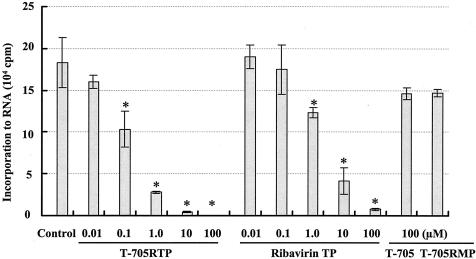
Inhibitory effect on influenza virus RNA polymerase.
The influenza virus RNA polymerase activity in the presence of T-705RTP, T-705, T-705RMP, or ribavirin TP is shown in Fig. 3. T-705RTP and ribavirin TP inhibited the enzyme activity in a dose-dependent manner, with IC50s of 0.14 and 2.4 μM, respectively. T-705 and T-705RMP did not show any significant inhibitory effect at a concentration of 100 μM. Kinetic analyses were performed with GTP as the substrate. In Lineweaver-Burk plots, all straight lines crossed the vertical axis at the same point, and it was shown that inhibition of influenza virus RNA polymerase by T-705RTP was competitive with that by the GTP substrate, with a Ki value of 1.52 μM.
FIG. 3.
Inhibitory effects of T-705RTP, rivabirin TP, T-705, and T-705RMP on influenza virus RNA polymerase activity. Results are means ± standard deviations (n = 3). *, results significantly different from those for the controls by the Tukey test (P < 0.01).
Effects on cellular DNA and RNA synthesis.
T-705 at concentrations up to 637 μM had little effect on either [3H]thymidine or [3H]uridine incorporation into the macromolecule fraction of MDCK cells. In contrast, ribavirin inhibited both cellular DNA and RNA synthesis in a dose-dependent manner, with IC50s of 2.17 and 16.0 μM, respectively (Table 3). The positive control compounds aphidicolin (a DNA polymerase inhibitor) and actinomycin D (an RNA synthesis inhibitor) completely inhibited the synthesis of DNA (95.9% inhibition) and RNA (99.6% inhibition) in host cells at 5 μM.
TABLE 3.
Inhibitory effects of T-705 and ribavirin on cellular DNA and RNA synthesis
| Compound | IC50 (μM)
|
|
|---|---|---|
| [3H]thymidine | [3H]uridine | |
| T-705 | >637 | >637 |
| Ribavirin | 2.17 | 16.0 |
| Aphidicolin | <5 | NTa |
| Actinomycin D | NT | <5 |
NT, not tested.
Inhibition of IMPDH activity.
T-705RMP exhibited a very weak inhibitory effect on the IMPDH activities of the host cells, with an IC50 of 601 μM. The IC50s of ribavirin MP and mycophenolic acid for the enzyme were 3.9 and 0.055 μM, respectively (Fig. 4).
DISCUSSION
The main object of this study was to clarify the mode of action of the anti-influenza virus activity of T-705. A time-of-addition experiment suggested that T-705 inhibits an early to middle stage of viral replication but not the adsorption or release stage. Addition of purines (i.e., adenine, guanine, and hypoxantine) and purine nucleosides (i.e., adenosine, guanosine, inosine, 2′-deoxyadenosine, and 2′-deoxyguanosine) but not pyrimidines resulted in a reduction of T-705 activity. This phenomenon suggests that T-705 acts like purines or purine nucleosides in viral RNA replication. In contrast, adenosine, inosine, and hypoxanthine did not inhibit the antiviral activity of ribavirin. Reversal of the ribavirin activity by guanosine but not by adenosine and inosine has also been demonstrated in other reports (3, 10, 13). Influenza virus RNA polymerase does not use the 2′-deoxynucleosides, but the activities of T-705 were found to be reversible by the addition of 2′-deoxyadenosine and 2′-deoxyguanosine. As we determined in a cell-based assay, various reversal sites like uptake and intracellular conversion, as well as polymerase activity, might be involved. Interestingly, despite these reversible activities of natural nucleic acids and nucleosides in vitro, T-705 has been shown to exhibit significant therapeutic efficacy in animal infection models (6, 16).
Apparently, it is hard to associate T-705 with a purine nucleoside base, but our HPLC radioactivity elution profiles also indicated that cellular enzymes could identify T-705 as nucleoside base and converted it to the phosphorylated metabolites. Analogues of a nucleic acid have been known to exhibit antiviral activity through intracellular metabolism to the phosphates. 1,3,4-Thiadiazol-2-ylcyanamide (LY217896), which had anti-influenza virus activity in vitro and in a mouse model of infection (8), was metabolized in BS-C-1 cells to the monophosphate form and inhibited IMPDH (1). It has been reported that ribavirin as a pseudobase could equivalently pair with both cytosine and uracil due to rotation of the carboxamide moiety and that RNA viruses, such as poliovirus and hepatitis C virus, use ribavirin triphosphate as a substrate for the viral RNA polymerase, and ribavirin incorporation was also found to be mutagenic (4, 12). T-705 also has a carboxamide moiety in its structure, and mutagenic activity might be involved in its antiviral activity. T-705RTP inhibited influenza virus RNA polymerase activity in a dose-dependent and a GTP-competitive manner. These results suggest that T-705 and the phosphorylated metabolites act as the pseudopurine, pseudo-purine nucleosides, and pseudo-purine nucleotides in the purine metabolic pathway.
It is noteworthy that T-705 inhibited viral replication without influencing the synthesis of cellular DNA or RNA. The high degree of selectivity of T-705 against influenza virus shown in this work corresponds with the findings that we presented in a previous report (6). The high error rate of RNA polymerase is considered to be one of the strategies exploited by RNA viruses to evade host immune pressure (14, 17), and the recognition of nucleotides by virus polymerase might be insufficient compared to that by host RNA polymerase.
IMPDH inhibitors, such as ribavirin and mycophenolic acid, inhibit the replication of both DNA and RNA virus in vitro due to a reduction of the GTP pool size in infected cells (15, 18, 20). The level of inhibition of IMPDH by T-705RMP was about 150-fold weaker than that by ribavirin MP, and it became apparent that the antiviral effect of T-705 is not a result of the inhibition of the cellular enzyme. This means that the mechanism of action of T-705 is not entirely the same as that of ribavirin.
The data presented here suggest that (i) T-705 can be converted to T-705RMP and T-705RTP by cellular kinases; (ii) T-705RTP is misidentified as a natural purine nucleotide by influenza virus polymerase; and (iii) host cell enzymes can differentiate T-705, T-705RMP, and T-705RTP from the natural nucleotides (Fig. 5).
In this report, we have demonstrated that T-705 has a mechanism of action different from those of ribavirin and the neuraminidase inhibitors because it selectively inhibits the virus RNA polymerase and not cellular RNA or DNA synthesis. This might be a cause of the lower level of toxicity of T-705 than that of ribavirin and its efficacy against amantadine and neuraminidase inhibitor-resistant viruses.
Although further studies on the mechanism of antiviral activity, toxicity, and pharmacokinetic profiles are needed, T-705 could be a useful orally active agent for the treatment of influenza virus infection.
Acknowledgments
We thank Yoshinobu Okuno (Osaka Prefectural Institute of Public Health) for providing the influenza viruses. We also thank Masahiko Kurokawa, Seiji Kageyama, and members of Department of Virology of the Toyama Medical and Pharmaceutical University for all the discussion and encouragement during the course of this work.
REFERENCES
- 1.Birch, G. M. 1995. The intracellular formation of a mononucleotide of the anti-influenza agent 1,3,4-thiadiazol-2-ylcyanamide (LY217896). Antivir. Chem. Chemother. 6:127-137. [Google Scholar]
- 2.Cheer, S. M., and A. J. Wagstaff. 2002. Spotlight on zanamivir in influenza. Am. J. Respir. Med. 1:147-152. [DOI] [PubMed] [Google Scholar]
- 3.Clercq, E. D. 1991. Antiviral activities of 5-ethynyl-1-β-ribofuranosylimidazole-4-carboxamide and related compounds. Antimicrob. Agents Chemother. 35:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty, S., D. Maag, J. J. Arnold. W. Zhong, J. Y. N. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez, H. 1986. Ribavirin: a clinical overview. Eur. J. Epidemiol. 2:1-14. [DOI] [PubMed] [Google Scholar]
- 6.Furuta, Y., K. Takahashi, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, Y. Watanabe, H. Narita, and K. Shiraki. 2002. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 46:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glezen, W. P. 1982. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 4:25-44. [DOI] [PubMed] [Google Scholar]
- 8.Hayden, F. G. 1990. Anti-influenza virus activity of the compound LY253963. Antivir. Res. 14:25-38. [DOI] [PubMed] [Google Scholar]
- 9.Hayden, F. G. 1992. Clinical and epidemiological importance of influenza A viruses resistant to amantadine and rimantadine. Med. Virol. 2:89-96. [Google Scholar]
- 10.Jordan, I. 1999. Inhibition of Borna disease virus replication by ribavirin. J. Virol. 73:7903-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser, L., C. Wat, T. Mills, P. Mahoney, P. Ward, and F. Hayden. 2003. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 163:1667-1672. [DOI] [PubMed] [Google Scholar]
- 12.Maag, D., C. Castro, Z. Hong, and C. E. Cameron. 2001. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of antiviral activity of ribavirin. J. Biol. Chem. 276:46094-46098. [DOI] [PubMed] [Google Scholar]
- 13.Oxford, J. S. 1975. Inhibition of replication of influenza A and B viruses by a nucleoside analogue (ribavirin). J. Gen. Virol. 28:409-414. [DOI] [PubMed] [Google Scholar]
- 14.Price, G. E. 2000. Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia. J. Exp. Med. 191:1853-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streeter, D. G. 1973. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA 70:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi, K., Y. Furuta, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, and K. Shiraki. 2003. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 14:235-241. [DOI] [PubMed] [Google Scholar]
- 17.Whitton, J. L., and M. B. Oldstone. 2001. The immune response to viruses, p. 285-320. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 18.Williams, R. H., D. H. Lively, D. C. DeLong, J. C. Cline, and M. J. Sweeny. 1968. Mycophenolic acid: antiviral and antitumor properties. J. Antibiot. 21:463-464. [DOI] [PubMed] [Google Scholar]
- 19.Witkowski, J. T. 1972. Design, synthesis, and broad spectrum antiviral activity of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J. Med. Chem. 15:1150-1154. [DOI] [PubMed] [Google Scholar]
- 20.Wray, S. K. 1985. Mode of action of ribavirin: effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antivir. Res. 5:29-37. [DOI] [PubMed] [Google Scholar]