Abstract
Objectives
Cellular movement and relocalisation are important for many physiologic properties. Local mesenchymal stem cells (MSCs) from injured tissues and circulating MSCs aid in fracture healing. Cytokines and chemokines such as Stromal cell-derived factor 1(SDF-1) and its receptor chemokine receptor type 4 (CXCR4) play important roles in maintaining mobilisation, trafficking and homing of stem cells from bone marrow to the site of injury. We investigated the differences in migration of MSCs from the femurs of young, adult and ovariectomised (OVX) rats and the effect of CXCR4 over-expression on their migration.
Methods
MSCs from young, adult and OVX rats were put in a Boyden chamber to establish their migration towards SDF-1. This was compared with MSCs transfected with CXCR4, as well as MSCs differentiated to osteoblasts.
Results
MSCs from OVX rats migrate significantly (p < 0.05) less towards SDF-1 (9%, sd 5%) compared with MSCs from adult (15%, sd 3%) and young rats (25%, sd 4%). Cells transfected with CXCR4 migrated significantly more towards SDF-1 compared with non-transfected cells, irrespective of whether these cells were from OVX (26.5%, sd 4%), young (47%, sd 17%) or adult (21%, sd 4%) rats. Transfected MSCs differentiated to osteoblasts express CXCR4 but do not migrate towards SDF-1.
Conclusions
MSC migration is impaired by age and osteoporosis in rats, and this may be associated with a significant reduction in bone formation in osteoporotic patients. The migration of stem cells can be ameliorated by upregulating CXCR4 levels which could possibly enhance fracture healing in osteoporotic patients.
Cite this article: A. Sanghani-Kerai, M. Coathup, S. Samazideh, P. Kalia, L. Di Silvio, B. Idowu, G. Blunn. Osteoporosis and ageing affects the migration of stem cells and this is ameliorated by transfection with CXCR4. Bone Joint Res 2017;6:–365. DOI: 10.1302/2046-3758.66.BJR-2016-0259.R1.
Keywords: Osteoporosis, Bone, Mesenchymal
Article focus
We aimed to investigate the migration of MSCs from young, adult and ovariectomised (OVX) rats and to enhance this migration by increasing the chemokine receptor type 4 (CXCR4) expression of these cells.
Key messages
CXCR4 improves migration of young, adult and OVX Mesenchymal stem cells (MSCs).
The migration of adult/old and osteopaenic MSCs may be impaired due to reduced levels of CXCR4.
Strengths and limitations
Strengths: This study is the first to assess the CXCR4 expression of MSCs from three different groups of rats: young, adult and osteopaenic.
Limitation: This was an in vitro study and further work needs to be done in vivo to establish a clear role of CXCR4 in cell migration.
Introduction
Bone fractures in the elderly caused by osteoporosis lead to loss of mobility, chronic pain and high cost to the individual and society.1 The common treatment for osteoporosis is the use of bisphosphonates, which increase bone mineral density and reduces the risk of osteoporotic fractures. However, its long-term use has raised concerns in regard to atypical subtrochanteric fractures of the femur.2
Mesenchymal stem cells (MSCs) are multipotent stem cells with the ability to self-renew and differentiate into various cell lineages. Cellular movement and relocalisation are crucial for many important physiologic properties such as embryonic development, neovascularisation and angiogenesis, immunologic responses, wound healing and organ repair. Both local MSCs from the injured tissue and circulating MSCs collaborate in the healing of organs during tissue and/or organ regeneration. Cytokines and chemokines play important roles in maintaining the mobilisation, trafficking and homing of stem cells from the bone marrow to the site of injury.3-5 During tissue regeneration, it has been suggested that local MSCs derived from the injured tissue and circulating MSCs work together in the healing of damaged organs. MSCs sense the tissue injury, migrate to the site of the damage and undergo differentiation.4,6 This may explain the increase of stem cells found in damaged tissues compared with normal healthy tissues such as impaired sites in the brain after hypoglossal nerve injury7 and cerebral injury.8 As a result of injury, the surviving cells may produce chemo-attractants such as Stromal cell-derived factor 1(SDF-1). With its receptor chemokine receptor type 4 (CXCR4), SDF-1 directs the migration of MSCs to the injury site.8
Wynn et al9 showed that when a neutralising anti-CXCR4 antibody was used to block CXCR4 expression, it inhibited MSC migration to the bone marrow by approximately 46%. This study also demonstrated a dose-dependent migration of MSCs towards SDF-1 in vitro. Blocking CXCR4 expression was observed to reduce cell migration significantly towards SDF-1.9 Lien et al10 transfected murine MSCs with CXCR4 and core-binding factor subunit alpha-1 (Cbfa-1) and injected these modified cells into osteopaenic mice. A complete recovery of bone stiffness and strength in these animals was seen after four weeks. A similar study by Cho et al11 also showed that over-expression of MSCs with CXCR4 and receptor activator of NF-kappa B (RANK-Fc) improved bone mineral density in ovariectomised mice (OVX), with CXCR4 acting as an important migratory factor and enhancing the therapeutic effects of RANK-Fc for bone loss. All of these studies highlight the importance of cell migration in bone regeneration, however, little information is available on the differences in migration capability of MSCs from osteopaenic, adult and young rats, and the scope of CXCR4 in improving the migration of MSCs from these three different groups.
In patients with osteoporosis, there is often an increased incidence of delayed union associated with fragility fractures. This may be connected to recruitment of stem cells to the fracture site and their differentiation to bone. Considering the significance of the SDF-1/CXCR4 axis in homing and engraftment of bone marrow cells and the potential of MSCs in bone regeneration therapy, we investigated the novel concept of over-expressing CXCR4 in stem cells and the way in which this affected the migratory capacities of MSCs isolated from young, old and OVX rats. We also investigated the effects and expression of CXCR4 in osteoblasts differentiated from these cells.
Materials and Methods
Cell isolation, cultivation and expansion
MSCs were harvested from young two- to four-week-old Wistar rats by flushing the femurs with Dulbecco's modified Eagle medium (DMEM) (Sigma Aldrich, Irvine, United Kingdom), 20% foetal calf serum (FCS) (First Link Ltd, Birmingham, United Kingdom) and 1% penicillin streptomycin (Sigma Aldrich). Cells were cultured at 37°C at 5% CO2. Media were changed after four days to remove non-adherent cells. After ten to 14 days of primary culture, when the cells were 70% to 80% confluent, they were passaged using Trypsin-EDTA (Sigma-Aldrich, United Kingdom). To characterise the MSCs, passage 3 (P3) cells were differentiated into osteoblasts, adipocytes and chondrocytes by the methods described in the supplementary material and were stained using Alizarin Red (Sigma Aldrich), Oil Red O (Sigma Aldrich) and Alcian Blue (Sigma Aldrich), respectively. For osteogenic differentiation, 30 000 cells were seeded in 48 well plates and the media were supplemented with 100 nM dexamethasone (Sigma Aldrich), 0.05 mM ascorbic acid (Sigma Aldrich) and 10 mM beta-glycerol phosphate (Sigma Aldrich).
Osteopaenic rats
The ovaries from six- to eight-month-old female Wistar rats (n = 6) were removed and the rats were left for four months after surgery before measuring their bone mineral density using peripheral quantitative computed tomography (pQCT) (Stratec XCT 1000; Stratec Biomedical Systems, Birkenfeld, Germany) and comparing them with non-OVX control rats of the same age (n = 6). Cells were obtained from the bone marrow of the OVX and control adult rats and cultured as stated above.
Preparation of the recombinant adenoviruses
The human CXCR4 cDNA (pCMV-XL5-CXCR4 Origene Technologies, Inc, Rockville, Maryland) was digested by restriction endonucleases NotI and XhoI (Fisher Scientific, Loughborough, United Kingdom) and then inserted into pShuttle-CMV (AdEasy XL Adenoviral Vector System; Stratagene, San Diego, California) to form pShuttle-CMV-CXCR4. The human CXCR4 gene and the pShuttle-CMV vector were both cut by restriction endonucleases NotI and XhoI (Fisher Scientific) and were then repaired to form blunt ends. The cuts were confirmed by gel electrophoresis. Human CXCR4 cDNA was then cloned into the pShuttle-CMV vector using DNA ligase. The presence of the insert was confirmed by restriction digest as well as by Sanger sequencing. The incorporated shuttle vector was then linearised with PmeI restriction endonuclease (Fisher Scientific) and transformed into BJ5183-AD-1 competent bacterial cells using electroporation (Fig. 1). A scrambled plasmid was created by inserting no gene into the pShuttle-CMV vector.
Fig. 1.
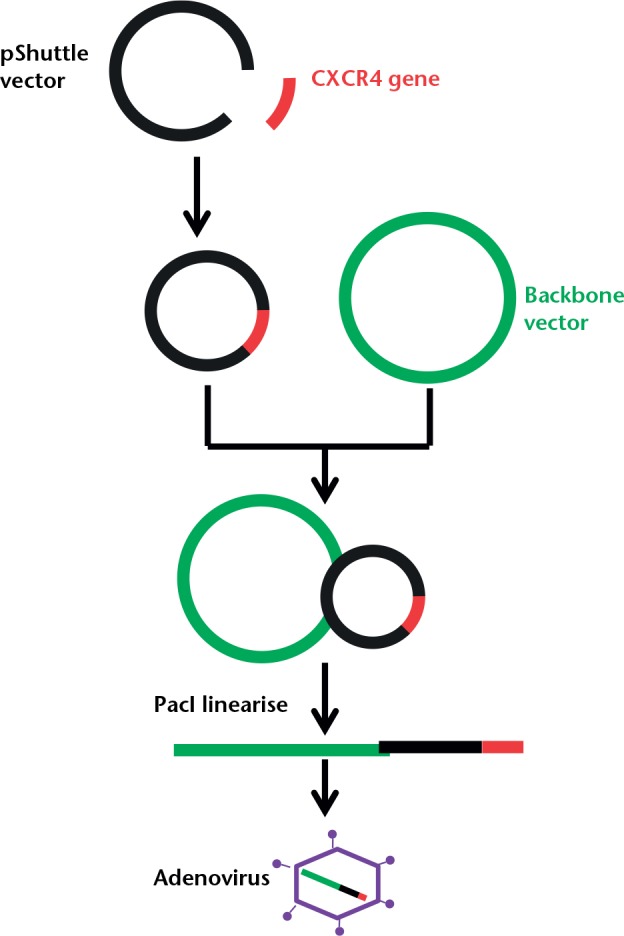
A schematic diagram showing the production stages of the recombinant adenovirus using the AdEasy XL Adenoviral Vector System (Stratagene, San Diego, California).
Following selection of the smallest colonies, the recombinant plasmid was collected using a miniprep kit (Thermofisher Scientific, Paisley, United Kingdom) and cut using the PacI restriction enzyme (ThermoFisher Scientific). The correctly identified copies were then largely expanded in bulk using the recombinant-deficient XL10-Gold strain (AdEasy XL Adenoviral Vector System; Stratagene, San Diego, California). Purified recombinant plasmid DNA, cut with PacI, was then used to transfect AD293 cells (AdEasy XL Adenoviral Vector System; Stratagene, San Diego, California). The viral particles were generated by alternate freezing and thawing of the infected AD293 cells and collecting the supernatant. An anti-hexon antibody stain, which statins infected cells dark brown, was used to determine the plaque-forming units/ml (pfu/ml) of the infected cells by counting the number of dark infected cells.
Sequencing method
A Polymerase chain reaction (PCR) was carried out on 3.5 μl (500 ng) of the purified plasmid DNA (pShuttle-CMV-CXCR4) using the Pshuttle CMV forward (5’GGTCTATATATAAGCAGAGCTG) and CXCR4 reverse primer (3’ATGAATGTCCACCTCGCTTT), sequencing buffer (ABI 4336699) and BigDye v3.1 (ABI 4337455; Thermo Fisher Scientific, Waltham, Massachusetts). PCR products were then purified using ethanol precipitation and centrifuged at 3000 rpm for 30 minutes. The products were then dried and washed with 70% ethanol, spun down and resuspended in Hi-Di Formamide (ABI 4440753), and analysed in a Sanger sequencer. A negative control with no DNA was also sequenced to validate the results.
Analysis of CXCR4 over-expression on infected cells
Young, adult and OVX MSCs from the third passage were infected with the CXCR4 adenovirus at a Multiplicity of Infection (MOI) of 800, and then trypsinised and centrifuged at 2000 rpm for ten minutes before being resuspended at 100 000 cells in phosphate-buffered saline (PBS). Cell aliquots were permeabilised at -20°C in methanol for ten minutes and incubated with primary CXCR4 antibody (Abcam, Cambridge, United Kingdom) for one hour at room temperature. The cells were washed in PBS and then incubated in secondary goat anti-rabbit antibody (Abcam, Cambridge, United Kingdom) for 30 minutes at room temperature. The negative control consisted of cells incubated in the secondary antibody only. Ten thousand cells were then analysed using a flow cytometry machine (CytoFLEX; Beckman Coulter, High Wycombe, United Kingdom). The results were compared with uninfected MSCs from the same rats.
Chemoinvasion assay
A chemoinvasion assay was used to evaluate the ability of MSCs, CXCR4-infected MSCs, scrambled infected MSCs and osteogenic differentiated MSCs to migrate towards SDF-1. To confirm osteogenic differentiation of MSCs cells were stained with Alizarin Red (Sigma Aldrich) and their osteocalcin expression analysed using immunocytochemistry after 21 days. A total of 25 000 osteogenic differentiated rBMCs and undifferentiated rBMCs were loaded separately in serum-free medium into upper compartments of the Boyden chamber, 5 µm pore size (Corning Inc., Corning, New York). The lower compartment of the Boyden chamber was filled with 100 ng/ml SDF-1 (Peprotech EC Ltd, London, United Kingdom) in DMEM, FCS and penicillin/streptomycin. The chambers were incubated at 37°C, 5% CO2, for 16 hours.
After 16 hours, the cells that migrated to the opposite side of the membrane were fixed in 10% formaldehyde (Sigma Aldrich) and stained with Crystal Violet (Sigma Aldrich). The migrated cells were counted by selecting six random fields at x20 magnification and calculating the percentage average number of cells. For each cell type (young, adult and OVX) and differentiation state (undifferentiated and osteogenic), the experiment was repeated three times. For the control, both the top and bottom of the chamber were filled with normal media with no SDF-1. In a similar but separate study, 10 000 cells transfected with CXCR4 and scrambled plasmid at MOI 800, and uninfected cells, were introduced into the chamber. The migrated cells were stained and counted after 16 hours and the data were normalised against uninfected cells.
Statistical analysis
Data were analysed using a Student t-test via SPSS Statistics (IBM Corp., Armonk, New York) and results were considered significant at the 0.05 level. All data has been represented as mean standard deviation.
Results
Establishment of the rat osteoporotic model
At four months post surgery, the average bone mineral density of the OVX rats was 538.2 g/cm3 (sd 23.3) in comparison with 666.9 g/cm3 (sd 46) for the sham rats, which was a reduction of 24% (sd 9%). Qualitative histomorphometrical results revealed significantly lower bone mass volume and thinner trabecular thickness in the OVX group, with a larger number of adipocytes occupying the stromal fraction of the bone marrow.
CXCR4 expression and migration of infected and uninfected cells
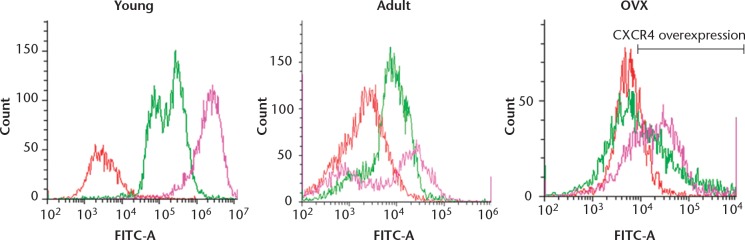
Non-transfected MSCs from younger rats expressed significantly greater amounts of CXCR4 (87.5%, sd 5.1%) in comparison with those isolated from adult (28.1%, sd 1.5%) and OVX rats (20.2%, sd 9.8%)(Fig. 2). Osteopaenia did not appear to be a factor in CXCR4 expression as the OVX rats expressed similar amounts to those of the control adult rats. However, we observed that a significantly lower number of MSCs from the OVX rats migrated towards SDF-1 compared with the adult and young rats. The migration of MSCs obtained from young rats was twice that seen in cells from adult rats (p = 0.023) and four times that of cells isolated from OVX rats (p = 0.013) (Fig. 3). Moreover, the migration of MSCs from young rats was significantly higher in plain media (without SDF-1) than those from adult and OVX rats.
Fig. 2.
Flow cytometry showing the chemokine receptor type 4 (CXCR4) expression of mesenchymal stem cells (MSCs) from young, adult control and ovariectomised (OVX) rats. The histograms show a comparison between the isotype control (red), CXCR4 expression in uninfected MSCs (green) and the over-expression of CXCR4 after transfection with the adenovirus (pink).
a) Graph showing the mean percentage migration of infected and uninfected cells from young, adult and ovariectomised (OVX) rats. b) The mean percentage migration of infected cells normalised against uninfected cells. *, ** and *** represent p < 0.05.
To enhance migration of MSCs, a human CXCR4 gene was introduced into rat MSCs through adenovirus infection. We tested the effect of CXCR4 transfected cells, scrambled plasmid-infected cells and uninfected cells on cell migration towards SDF-1 using the transwell migration assay. The data were normalised to the migration of uninfected cells to measure the effect of CXCR4 transfection on cells isolated from young, adult and OVX animals. After transfection with the CXCR4 virus, 92% (sd 2.6%), 44.2% (sd 8.2%) and 40% (sd 16.1%) of young, adult control and OVX MSCs, respectively, over-expressed CXCR4. Over-expression of CXCR4 significantly increased the migration of young MSCs towards SDF-1 by 80% in comparison with uninfected cells (p = 0.006). Although slightly fewer scrambled infected cells migrated towards SDF-1 in comparison with the uninfected cells (p = 0.03), there was a significant difference in the migration between the infected and scrambled infected cells (p = 0.003) towards SDF-1, demonstrating that over-expression of CXCR4 increased cell migration towards this cytokine. It was important to note that although the migration of young MSCs towards SDF-1 was greatest after CXCR4 transfection, the effect of CXCR4 was more profound in rat MSCs from OVX rats. A five-fold increase in migration of MSCs from OVX rats towards SDF-1was seen after CXCR4 transfection compared with uninfected OVX cells (p = 0.025) (Fig. 3). CXCR4 expression in OVX MSCs was doubled, while in young MSCs it only increased by 5% after CXCR4 transfection (Table I).
Table I.
The percentage chemokine receptor type 4 (CXCR4) expression before and after CXCR4 transfection in young, adult control and ovariectomised (OVX) mesenchymal stem cells
| Type of cells | CXCR4 expression before transfection (%) | CXCR4 expression after transfection (%) | Percentage increase (%) |
|---|---|---|---|
| Young MSCs | 87.5, sd5.1* | 92, sd 2.6* | 5.1 |
| Adult MSCs | 28.1, sd 1.5* | 44.2, sd 8.2* | 57.2 |
| OVX MSCs | 20.2, sd 9.8* | 40 sd, 16.1* | 97.9 |
represents significance p < 0.05.
MSC, mesenchymal stem cell; CXCR4, chemokine receptor type 4.
The migration capacity of MSCs decreases after osteogenic differentiation
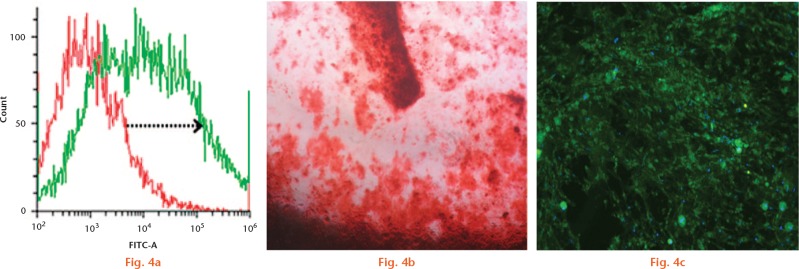
To determine whether osteogenic differentiation of rat MSCs affects migration towards SDF-1 and whether transfection with CXCR4 of these cells increases their migratory capacity, we repeated the migration assay on stem cells grown for 21 days in osteogenic medium. Positive differentiation of MSCs to osteoblasts was confirmed by osteocalcin staining and Alizarin Red (Sigma Aldrich) staining at day 21 (Figs 4b and 4c). CXCR4-infected cells were also differentiated to osteoblasts and differentiation significantly reduced their migration towards SDF-1 (6.7%, sd 2.3%) compared with undifferentiated MSCs (23.2 sd 4%) (p = 0.0006). Interestingly, we also observed that, when CXCR4-infected MSCs were differentiated to osteoblasts, their migration capability towards SDF-1 did not significantly improve (11.25%, sd 8.6%) whereas transfection significantly improved the migration of undifferentiated MSCs (47%, sd 17%) even though both sets of cells positively expressed CXCR4 (Fig. 5).
Flow cytometry to analyse the chemokine receptor type 4 (CXCR4) expression of rBMCs post osteogenesis. The arrow shows the positive shift of the CXCR4-expressing osteoblasts (a) Positive Alizarin Red (b) and osteocalcin staining (c) expressed at day 21 of osteogenic-differentiated rBMCs.
Fig. 5.
Graph showing the mean percentage and uninfected rBMCs, osteogenic-differentiated rBMCs and CXCR4-infected rBMCs migration of infected differentiated to osteoblasts towards SDF-1 in a Boyden chamber. *, ** and *** represents p < 0.05.
Discussion
These in vitro studies have highlighted the pivotal role that the SDF-1/CXCR4 axis plays in the homing of stem cells and the potential significance this may have in improving bone formation in osteoporosis. This study showed that cells transfected with CXCR4 increased migration towards SDF-1 in transwell chambers in comparison with uninfected cells. CXCR4 was therefore seen to play an essential role in stem cell migration in transwell assays. Due to the ageing process, bone composition, structure and function deteriorates which results in osteoporosis.12 It has also been demonstrated that MSCs from osteoporotic humans have impaired migration towards chemo-attractants such as foetal calf serum (FCS), bone morphogenetic protein (BMP)-2 and BMP-7 in Boyden chambers, as well as in micro-slide chemotaxis chambers.13 SDF-1 has previously been shown to recruit more host stem cells to a fracture defect site and to encourage osteogenic differentiation and production of bone.14
When considering the effect of age on cells, this study has also demonstrated that stem cells from OVX and adult rats express lower levels of CXCR4 on their surface compared with stem cells from young rats, which might explain their impaired migration towards SDF-1. These results are consistent with those reported by other studies.15,16 Liang et al17 intravenously injected lethally irradiated young or old Ly-5.1 mice with old or young Ly-5.2 bone marrow cells. The marrow from the recipients for Ly-5.2+ stem cells that had homed there was analysed after 24 hours. It was found that the older cells migrated two to three times less to the bone marrow niche compared with the young cells.17 This was most likely due to defective CXCR4 expression during ageing, reducing the number of CXCR4-positive cells in the bone marrow and in the circulation.16 This significance has been highlighted in the present study where the mobility of stem cells from OVX rats towards SDF-1 was increased when their CXCR4 expression was upregulated.
SDF-1 causes cell migration by binding with CXCR4, and therefore increased secretion of SDF-1 at the site of injury creates an environment that mediates the homing of circulating CXCR4-positive stem cells. The cells will subsequently differentiate into the surrounding tissue type and affect repair at the injury site.18 Poor homing ability of the stem cells to bone could result in a significant reduction in bone formation which ultimately contributes to osteoporosis.19
Oestrogen deficiency is the main cause that leads to postmenopausal osteoporosis. Oestrogen improves osteoblastic differentiation of hBMSCs through ER-α20 or activating Wnt/β-catenin signalling.21 Deficiency in oestrogen also affects the circulating levels of cytokines such as interleukin (IL)-1, tumour necrosis factor (TNF)-α and granulocyte macrophage colony-stimulating factor (GM-CSF). Increased levels of these cytokines due to reduction in oestrogen enhance bone resorption.22,23 The notch signalling pathway has been shown to induce osteogenic differentiation and inhibit adipogenic differentiation of BMSCs.24
A study performed by Fan et al25 has shown that oestrogen-upregulated Notch signalling enhanced the proliferation and differentiation of hBMCs. This could further explain the migration difference between the MSCs from the OVX and those from adult and younger rats, where the OVX rats were seen to perform much more poorly in the Boyden chamber. However, the current study did not measure the effect of oestrogen on the migration of stem cells from young, OVX or senile rats.
The differentiation of MSCs to osteoblasts is a complex process that involves the interaction of numerous hormones, autocrine and paracrine processes, and systemic growth factors.26 We found that stem cell migration towards SDF-1 is reduced after osteogenic induction even though the cells were over-expressed with CXCR4 prior to differentiation. The diminished migration of the differentiated MSCs may be a result of increased extra-cellular matrix deposition, and subsequently greater surface adherence. Matrix metalloproteinases (MMP) play a significant role in several differentiation events. MMPs are also involved in regulating osteoblast proliferation and apoptosis.27,28 However, it has been shown that MMPs inactivate SDF-1 and this may implicate the migration of osteoblasts.29 This could further imply that the SDF-1/CXCR4 axis is no longer required once the osteogenic differentiation pathway has been set in motion, as the migration of osteoblasts is no longer relevant once the stem cells have homed to the site of injury and started differentiating into bone cells.30 Additionally, the role of an osteoblast is to secrete osteoid in an orientated manner and migration of cells may impair this localised production. Liu et al30 found that when the cells were pre-treated with anti-SDF-1 and anti-CXCR4 antibody for six hours, expression of BMP-9-induced Runt-related transcription factor 2 (RUNX2) and Osterix (OSX), which are markers of osteogenesis, was reduced significantly, highlighting the importance of SDF-1/CXCR4 in initiating osteogenesis.30 However, although the CXCR4/SDF-1 axis may be involved in the induction of early stages of osteogenic differentiation, their levels were seen to diminish during osteogenesis.30,31
The importance of CXCR4 in improving bone loss in osteopaenic animals has been highlighted in previous studies. Lien et al’s10 study looked at the migration of MSCs transfected with CXCR4 and Cbfa-1 in osteopaenic mice, and Cho et al’s11 study similarly looked at the migration of stem cells transfected with CXCR4 and RANK-Fc in osteopaenic mice. However, this study emphasises the differences in CXCR4 expression between MSCs from ovariectomised rats, adult and young rats, as well as their differences in migration in vitro, which were improved after CXCR4 transfection. MSCs from OVX rats have poor migration ability, and CXCR4 was observed to be the most effective in improving their migration compared with MSCs from young rats. Moreover, we also looked at the migration of osteoblasts and showed that over-expressing differentiated rat MSCs with CXCR4 does not improve their migration.
The main limitation of this study is that it has been carried out in vitro and this is associated with cells in monolayer culture, with little surrounding extracellular matrix and other cell types. Therefore, this may influence the migration of MSCs. There may be other factors apart from CXCR4 that affect migration in vivo and these include integrins, cadherins, and other adhesion factors. For cells to migrate they must disrupt the cell-to-cell contact and cell-to-matrix contact and, for this, the cadherins and integrins contact must be disrupted through locally released enzyme. This has also not been investigated in this study. In a fracture situation, MSCs might migrate from the blood stream, bone marrow, muscle or periosteum. The periosteum is an important source of mesenchymal stem cells, but in this study we have only investigated stem cells derived from a bone marrow aspirate and it would be very interesting to measure the migration potential of MSCs derived from different sources.
This is a novel study because it shows that MSCs from osteopaenic rats have a poor migration capacity. This may be associated with the inability of osteoporotic patients to heal fractures. The reduced migration to SDF-1-producing cells may possibly indicate that there is a reduced stem cell number at the fracture site compared with that found in non-osteoporotic individuals, but further research in vivo needs to be done to validate the effect of CXCR4 on stem cell migration. Nevertheless, the maintained migration response upon upregulation of CXCR4 in rats illustrates the therapeutic potential of CXCR4-expressing MSCs in the treatment of osteoporotic fractures.
Footnotes
Author Contribution: A. Sanghani-Kerai, Acquisition of data, analysis of data, drafting the manuscript.
M. Coathup, Supervised the study, Reviewed and corrected paper, Study conception and design, Critical revision of the paper.
S. Samazideh, Acquisition of data, Reviewed and corrected paper.
P. Kalia, Revised the paper, Analysis of data.
L. Di Silvio, Reviewed and corrected paper, Analysis of data.
B. Idowu, Reviewed and corrected paper, Analysis of data.
G. Blunn, Supervised the study, Reviewed and corrected paper, Study conception and design, Critical revision.
ICMJE Conflicts of Interest: None declared.
Supplementary material
Details of differentiation and characterisation of stem cells can be found alongside the online version of this article at www.bjj.boneandjoint.org.uk
Funding Statement
G. Blunn reports funding received for board membership, consultancy and lectures, as well as royalties paid to their institution, none of which is related to this article.
References
- 1. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-656. [PMC free article] [PubMed] [Google Scholar]
- 2. Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res 2010;468:3384-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol 2006;34:967-975. [DOI] [PubMed] [Google Scholar]
- 4. Ito H. Chemokines in mesenchymal stem cell therapy for bone repair: a novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod Rheumatol 2011;21:113-121. [DOI] [PubMed] [Google Scholar]
- 5. Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem 2009;106:984-991. [DOI] [PubMed] [Google Scholar]
- 6. Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Homing genes, cell therapy and stroke. Front Biosci 2006;11:899-907. [DOI] [PubMed] [Google Scholar]
- 7. Ji JF, He BP, Dheen ST, Tay SSW. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004;22:415-427. [DOI] [PubMed] [Google Scholar]
- 8. Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 2004;101:18117-18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643-2645. [DOI] [PubMed] [Google Scholar]
- 10. Lien CY, Chih-Yuan Ho K, Lee OK, Blunn GW, Su Y. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res 2009;24:837-848. [DOI] [PubMed] [Google Scholar]
- 11. Cho SW, Sun HJ, Yang JY, et al. Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol Ther 2009;17:1979-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005;115:3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haasters F, Docheva D, Gassner C, et al. Mesenchymal stem cells from osteoporotic patients reveal reduced migration and invasion upon stimulation with BMP-2 or BMP-7. Biochem Biophys Res Commun 2014;452:118-123. [DOI] [PubMed] [Google Scholar]
- 14. Ho C-Y, Sanghani A, Hua J, et al. Mesenchymal Stem Cells with Increased Stromal Cell-Derived Factor 1 Expression Enhanced Fracture Healing. Tissue Eng Part A 2015;21:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang X, Su YP, Kong PY, et al. Human bone marrow mesenchymal stem cells expressing SDF-1 promote hematopoietic stem cell function of human mobilised peripheral blood CD34+ cells in vivo and in vitro. Int J Radiat Biol 2010;86:230-237. [DOI] [PubMed] [Google Scholar]
- 16. Shao H, Xu Q, Wu Q, et al. Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med 2011;15:2046-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 2005;106:1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stich S, Haag M, Häupl T, et al. Gene expression profiling of human mesenchymal stem cells chemotactically induced with CXCL12. Cell Tissue Res 2009;336:225-236. [DOI] [PubMed] [Google Scholar]
- 19. Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep 2014;12:41-47. [DOI] [PubMed] [Google Scholar]
- 20. Hong L, Colpan A, Peptan IA. Modulations of 17-β estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng 2006;12:2747-2753. [DOI] [PubMed] [Google Scholar]
- 21. Bhukhai K, Suksen K, Bhummaphan N, et al. A phytoestrogen diarylheptanoid mediates estrogen receptor/Akt/glycogen synthase kinase 3β protein-dependent activation of the Wnt/β-catenin signaling pathway. J Biol Chem 2012;287:36168-36178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem 2012;45:863-873. [DOI] [PubMed] [Google Scholar]
- 23. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995;332:305-311. [DOI] [PubMed] [Google Scholar]
- 24. Ugarte F, Ryser M, Thieme S, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol 2009;37:867-875. [DOI] [PubMed] [Google Scholar]
- 25. Fan J-Z, Yang L, Meng G-L, et al. Estrogen improves the proliferation and differentiation of hBMSCs derived from postmenopausal osteoporosis through notch signaling pathway. Mol Cell Biochem 2014;392:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells 2006;24:475-481. [DOI] [PubMed] [Google Scholar]
- 27. Karsdal MA, Larsen L, Engsig MT, et al. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 2002;277:44061-44067. [DOI] [PubMed] [Google Scholar]
- 28. Batouli S, Miura M, Brahim J, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 2003;82:976-981. [DOI] [PubMed] [Google Scholar]
- 29. Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 2004;110:3300-3305. [DOI] [PubMed] [Google Scholar]
- 30. Liu C, Weng Y, Yuan T, et al. CXCL12/CXCR4 signal axis plays an important role in mediating bone morphogenetic protein 9-induced osteogenic differentiation of mesenchymal stem cells. Int J Med Sci 2013;10:1181-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kortesidis A, Zannettino A, Isenmann S, et al. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 2005;105:3793-3801. [DOI] [PubMed] [Google Scholar]