Abstract
Although rifamycins have excellent activity against Chlamydophila pneumoniae and Chlamydia trachomatis in vitro, concerns about the possible development of resistance during therapy have discouraged their use for treatment of chlamydial infections. Rifalazil, a new semisynthetic rifamycin with a long half-life, is the most active antimicrobial against C. pneumoniae and C. trachomatis in vitro, indicating its potential for treatment of acute and chronic C. pneumoniae and C. trachomatis infections. We investigated the effect of serial passage of two C. pneumoniae isolates and two serotypes of C. trachomatis in subinhibitory concentrations of rifalazil and rifampin on the development of phenotypic and genotypic resistance. C. trachomatis developed resistance to both antimicrobials within six passages, with higher level resistance to rifampin (128 to 256 μg/ml) and lower level resistance to rifalazil (0.5 to 1 μg/ml). C. pneumoniae TW-183 developed only low-level resistance to rifampin (0.25 μg/ml) and rifalazil (0.016 μg/ml) after 12 passages. C. pneumoniae CWL-029 failed to develop resistance to either drug. Two unique mutations emerged in the rpoB gene of rifampin (L456I) and rifalazil (D461E)-resistant C. pneumoniae TW-183. A single mutation (H471Y) was detected in both rifampin- and rifalazil-resistant C. trachomatis UW-3/Cx/D, and a unique mutation (V136F) was found in rifalazil-resistant BU-434/L2. No mutations were detected in the entire rpoB gene of rifampin-resistant BU-434/L2. This is the first description of antibiotic resistance-associated mutations in C. pneumoniae and of rifampin resistance in C. trachomatis not associated with mutations in the rpoB gene.
Chlamydophila pneumoniae is a common cause of respiratory infections, and Chlamydia trachomatis is the most common sexually transmitted bacterial disease. In addition, persistent C. trachomatis and C. pneumoniae infections have been associated with the development of infertility, asthma, chronic obstructive pulmonary diseases, atherosclerosis, and Alzheimer's disease (3, 12, 16, 19, 20), and therapeutic trials with antimicrobial agents are in progress for some of these conditions. Yet the optimal treatment regimens for C. pneumoniae are unknown, and resistance to available antibiotics is beginning to emerge in C. trachomatis (21) and Chlamydia suis, a related veterinary pathogen (17). Alternative or improved therapies for acute and chronic chlamydial infections would be desirable.
Rifamycins have excellent activity against C. pneumoniae and C. trachomatis in vitro, but concerns about the emergence of resistance during therapy have discouraged their use for treatment of chlamydial infections in humans (25). Resistance to this class of antibiotics frequently occurs in other species, correlating with point mutations in their target gene, encoding RNA polymerase beta (rpoB) (2, 4, 14). The emergence of resistance to rifampin in vitro has been demonstrated in C. trachomatis (9, 15, 22, 25, 26). In contrast, no resistance has developed in C. trachomatis propagated in the presence of the rifamycin derivatives rifabutin and 3-azinomethyl-rifamycin under the same in vitro conditions (26, 29).
Rifalazil is a new semisynthetic rifamycin with a long half-life (60 h) and is the most potent antimicrobial in vitro against C. pneumoniae and C. trachomatis (23, 24). Therefore, it may have potential for short- and long-term therapy of acute and chronic C. pneumoniae and C. trachomatis infections.
In this study, we investigated the effect of serial passage of C. pneumoniae and C. trachomatis in subinhibitory concentrations of rifalazil or rifampin on the development of phenotypic and genotypic resistance in vitro.
MATERIALS AND METHODS
Selection of resistance.
HEp-2 cell monolayers (ATCC CCL-23) were seeded into six-well plates and inoculated with two C. pneumoniae isolates, TW-183 (ATCC VR-2282) and CDC/CWL-029 (ATCC VR-1310), and two C. trachomatis strains, BU-434/L2 (ATCC VR-902B) and UW-3/Cx/D (ATCC VR-885), at high multiplicities of infection. The medium was then replaced with one containing one-eighth to one-half the previously determined MIC of rifalazil or rifampin and incubated for 2 to 4 days at 35°C. Six passages for C. trachomatis and 12 passages for C. pneumoniae were performed in the presence of the same antimicrobials at the above or twofold increasing concentrations. After 6 and 12 passages, drug susceptibility testing was performed.
Susceptibility testing.
In vitro susceptibility testing of C. pneumoniae and C. trachomatis was performed in cell culture with HEp-2 cells grown in 96-well microtiter plates. Each well was inoculated with 0.1 ml of the microorganism diluted to yield 104 inclusion-forming units per ml. The plates were centrifuged at 2,000 × g for 1 h. The wells were then aspirated and overlaid with 0.1 ml of medium containing 1 μg of cycloheximide per ml and serial twofold dilutions of rifampin or rifalazil. After incubation at 35°C for 72 h, the cultures were fixed and stained for inclusions with Pathfinder Chlamydia Culture Confirmation monoclonal antibody (Bio-Rad Laboratories, Redmond, Wash.). The MIC was defined as the lowest antibiotic concentration at which no inclusions were seen. The minimum bactericidal concentration was determined by freezing the cultures at −70°C, thawing the cultures, passaging the disrupted cell monolayers onto new cells, incubating the cells for 48 h for C. trachomatis and 72 h for C. pneumoniae, and then fixing and staining the cells as described above. The minimum bactericidal concentration was defined as the lowest antibiotic concentration that resulted in no inclusions after passage. All tests were run in triplicate.
PCR and sequencing.
DNA from the original and resistant C. pneumoniae and C. trachomatis cultures was extracted with the DNeasy Tissue Kit (Qiagen, Valencia, Calif.). PCR primers were designed to amplify a 593-bp and a 610-bp PCR fragment covering the predicted rifamycin resistance region of the rpoB genes of C. pneumoniae and C. trachomatis, respectively, based on homology to the rpoB sequence of Mycobacterium tuberculosis (6, 28). The primers used were Cpn_rpoB_forward, 5′-CTAATCGACGTGTCCGCTC T-3′, and Cpn_rpoB_reverse, 5′-TTCGAAAGCTTCTCCAGCAT-3′, for C. pneumoniae and L2_rpoB_forward, 5′-ATGGGCGATGAGAAGACATC-3′, and L2_rpoB_reverse, 5′-CCCAGCATACGGGAGTTTTA-3′, for C. trachomatis.
PCR was performed with the ProofStart DNA polymerase kit (Qiagen, Valencia, Calif.) at 95°C for 5 min, then 40 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 60 sec. The PCR products were purified (QIAquick PCR purification kit; Qiagen) and sequenced in both directions (GeneWiz, North Brunswick, N.J.). The sequences were analyzed with BLAST 2 (http://www.ncbi.nlm.nih.gov/BLAST/bl2seq/bl2.html) and compared to the reference C. pneumoniae TW-183 (accession number AE017157), C. pneumoniae CDC/CWL-029 (accession number AE001593), and C. trachomatis serotype D (accession number NC_000117) sequences deposited in GenBank.
Additionally, the entire 3,759-bp rpoB gene of C. trachomatis 434/L2 from the original and resistant cultures was amplified and sequenced in both directions, with overlap, with the following primer pairs: D_whole_rpoB_F, 5′-TTCTTTTACCAGGGCTGCAT-3′; D_whole_rpoB_R, 5′-TCTAGCGTCTCTGCATTGGA-3′; D_rpoB_1_F, 5′-ACCATAGGGCGAACATCAA G-3′; D_rpoB_1_R, 5′-CAAGGGAGTGGTTTCCAAGA-3′; D_rpoB_2_F, 5′-ACCTGTCCAAGGTTCATTCG-3′; D_rpoB_2_R, 5′-ACGGTTCCTCCTGGTACAGA-3′; D_rpoB_3_F, 5′-GACAAGCGATCCTTTCTGCT-3′; D_rpoB_3_R, 5′-TCAGGAAGATGGGGTAGTCG-3′; D_rpoB_4_F, 5′-CATGTTCCGGAGTTGGATCT-3′; D_rpoB_4_R, 5′-GCTGGGTTTGAAGTTCGAGA-3′; D_rpoB_5_F, 5′-GCACGACACGATAAGGAGTCT-3′; D_rpoB_5_R, 5′-TATGCGCTTATTCTTCGATGC-3′; D_rpoB_6_F, 5′-CGTCGTCTAATGGGAATCCT-3′; D_rpoB_6_R, 5′-TCTCAAGTCCACCGTTCTCC-3′; D_rpoB_7_F, 5′-TGTCGAAGACAGCTTCTAACCA-3′; and D_rpoB_7_R, 5′-TCTAGCGTCTCTGCATTGGA-3′.
Nucleotide sequence accession number.
The sequence of the 434/L2 rpoB gene was submitted to GenBank (accession number AY623623).
RESULTS
The MICs of both rifampin and rifalazil increased for C. pneumoniae and C. trachomatis within six passages in subinhibitory concentrations of both drugs (Table 1). The MICs for C. trachomatis UW-3/Cx/D and BU-434/L2 increased 1.6- to 3.2 × 104- and 5 × 102-fold for rifampin and rifalazil, respectively. In contrast, the MICs of rifampin and rifalazil for C. pneumoniae TW-183 increased only 7.5- and 4-fold after six passages and 31.25- and 16-fold after 12 passages. Cross-resistance to both rifamycins was demonstrated with both species. Regardless of whether rifampin or rifalazil was used during selection, the resistant C. pneumoniae TW-183 had the same MICs to rifampin (both 0.25 μg/ml) and similar MICs to rifalazil (0.008 and 0.016 μg/ml). Resistant C. trachomatis BU-434/L2 strains both had MICs of 256 μg/ml to rifampin and 1.0 μg/ml to rifalazil. C. pneumoniae CWL-029 did not develop resistance after passage in subinhibitory concentrations of either rifampin or rifalazil.
TABLE 1.
Activity of rifampin and rifalazil against C. pneumoniae and C. trachomatis after passage in subinibitory concentrations of drug and associated mutations
| Species | Strain | Drug | MICa (μg/ml)
|
Mutation | Corresponding codon in E. coli | ||
|---|---|---|---|---|---|---|---|
| Initial | 6 passages | 12 passages | |||||
| C. pneumoniae | TW-183 | Rifamycin | 0.008 | 0.060 | 0.25 | L456I | 511 |
| Rifalazil | 0.001 | 0.004 | 0.016 | D461E | 516 | ||
| CWL029 | Rifamycin | 0.016 | 0.016 | 0.016 | None | ||
| Rifalazil | 0.001 | 0.001 | 0.001 | None | |||
| C. trachomatis | UW-3/Cx/D | Rifamycin | 0.008 | 128 | ND | H471Y | 526 |
| Rifalazil | 0.001 | 0.5 | ND | H471Y | 526 | ||
| BU-434/L2 | Rifamycin | 0.008 | 256 | ND | None | ||
| Rifalazil | 0.002 | 1.0 | ND | V136F | 146 | ||
Values are geometric means of three parallel tests for each strain-drug combination. ND, not determined.
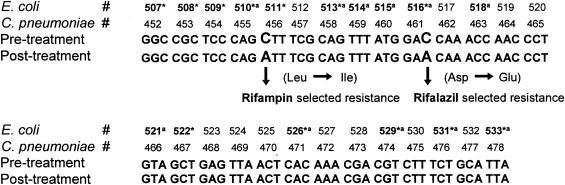
Two different mutations were found in the predicted rifamycin resistance region of the rpoB gene in rifampin- and rifalazil-resistant C. pneumoniae TW-183 (Fig. 1). Resistance to rifampin was associated with nucleotide changes at codon 456 resulting in an amino acid change from leucine to isoleucine. Resistance to rifalazil was associated with a nucleotide mutation at codon 461 causing replacement of aspartic acid with glutamic acid. No mutations were found in the predicted rifamycin resistance region of the rpoB genes in nonresistant C. pneumoniae CWL-029 after passaging in the presence of either rifamycin. The sequence of the rifamycin resistance region in C. pneumoniae CWL-029 exactly matched the sequence in the untreated C. pneumoniae TW-183.
FIG. 1.
Mutations in the predicted rifamycin resistance region of the rpoB gene of C. pneumoniae TW-183. Frequent genetic alteration sites reported in rifamycin-resistant E. coli (14) and M. tuberculosis (28) are marked with an asterisk and with the letter a, respectively.
The same mutations were detected at codon 471 in the rpoB genes of both rifampin- and rifalazil-resistant C. trachomatis UW-3/Cx/D, leading to a histidine-to-tyrosine change (Fig. 2). No mutations were found in the predicted rifamycin resistance regions I and II of the rpoB gene of resistant C. trachomatis BU-434/L2. However, sequencing of the entire rpoB gene showed a mutation upstream of that predicted resistance region at codon 136 (146, Escherichia coli numbering) in rifalazil-resistant C. trachomatis (Fig. 3). This mutation results in replacement of valine with phenylalanine. Interestingly, no mutations were found in the entire rpoB gene of the highly rifampin-resistant C. trachomatis BU-434/L2. Single-nucleotide polymorphisms of the rpoB gene of BU-434/L2 were found at 33 loci compared to the rpoB gene of serotype D.
FIG. 2.
Mutations in the predicted rifamycin resistance region of the rpoB gene of C. trachomatis D. Frequent genetic alteration sites reported in rifamycin-resistant E. coli (14) and M. tuberculosis (28) are marked with an asterisk and with the letter a, respectively.
FIG. 3.
Mutation at the beginning region of the rpoB gene of C. trachomatis 434/L2. *, mutation site associated with rifampin resistance in E. coli (18).
DISCUSSION
Rifampin and other rifamycins have been shown to be very active in vitro against Chlamydia spp. with MICs ranging from 0.0075 to 0.03 μg/ml (10, 11, 23). The high activity, favorable pharmacokinetics, and excellent cell penetration suggested that this class of antimicrobials could be very effective against chlamydial infections. In a limited number of studies, treatment of C. trachomatis infections with rifampin has been found to be successful and as effective as treatment with tetracycline (25). No signs of emerging resistance of C. trachomatis to rifamycins have been reported in vivo. However, the results of several in vitro studies demonstrated that C. trachomatis easily and rapidly develops resistance after serial passage in subinhibitory concentrations of rifamycins in both eggs and tissue culture (9, 10, 15).
The risk of development of resistance and data on the emergence of resistance in other bacteria (2, 14, 15, 26, 28) have discouraged further evaluation of rifamycins for the treatment of human chlamydial infections. The development of a new generation of rifamycins with increased activities and enhanced pharmacokinetic properties may prove valuable in treating acute and chronic chlamydial infections (24). In fact, clinical trials utilizing new rifamycins for the treatment of nongonococcal urethritis caused by C. trachomatis are under way. There are no data on the possible emergence of resistance to any rifamycin in C. pneumoniae in vitro or in vivo.
Rifalazil is the most potent rifamycin against Chlamydia spp., with MICs 10- to 1,000-fold lower than those of azithromycin and levofloxacin (23, 24). Limited experience with the use of rifalazil for treatment of pulmonary tuberculosis in animals and humans has not documented clinically significant emergence of resistance (8). However, emergence of low-level resistance in C. trachomatis was reported in one in vitro study (R. J. Suchland, W. E. Stamm, E. Denamur and D. Rothstein. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-2130, 2003).
In this study, serial passage in subinhibitory concentrations of rifalazil and rifampin selected for phenotypic resistance in both chlamydial species. However, rifalazil MICs for resistant C. trachomatis (0.5 to 1.0 μg/ml) were more than 250-fold lower than the rifampin MICs (128 to 256 μg/ml). Similarly, the rifalazil MIC for resistant C. pneumoniae TW-183 (0.016 μg/ml) was 15.5-fold lower than the rifampin MIC (0.25 μg/ml).
Remarkably, C. pneumoniae appeared to be less prone to the development of resistance than C. trachomatis. One of the two C. pneumoniae isolates tested, CWL-029, failed to develop resistance to either drug after 12 passages, while the other isolate, TW-183, developed a low level of resistance. Given the favorable pharmacokinetic profile of rifalazil and its derivatives (24), this drug may maintain physiological levels well above the increased MIC. Data are very limited on achievable levels of rifalazil in humans and animals. One study in humans found plasma levels of rifalazil of 0.013 to 0.026 μg/ml (8). A study in dogs and rats found plasma rifalazil concentrations of 0.21 to 0.77 μg/ml and 0.08 to 0.25 μg/ml, respectively (13). The results in rats demonstrated much higher levels of rifalazil in tissues: 0.51 to 5.3 μg/ml in lungs, 1.6 to 50.4 μg/ml in spleen, and 2.6 to 19.5 μg/ml in liver. These data suggest that rifalazil, like other rifamycins, may have good tissue penetration and intracellular levels in humans as well. If so, rifalazil might be effective even if relative resistance should occur during therapy.
Based on data from other microorganisms, rifamycin resistance is most frequently associated with genetic alterations in cluster I (codons 507 to 533, E. coli) or II (codons 563 to 572) of the rpoB gene (14, 28), a region of high amino acid homology across species, including chlamydiae.
As predicted, the resistance of C. pneumoniae TW-183 to rifampin and rifalazil was associated with mutations at codons equivalent to E. coli codons 511 and 516, respectively. Mutations at codon 526 (E. coli) in C. trachomatis were found in the rpoB gene of rifampin- and rifalazil-resistant UW-3/Cx/D. This mutation was also found by Dreses-Werringloer et al. (9) in rifampin-resistant C. trachomatis serovar K, in addition to one at codon 522. The codons involved in our mutants, especially at position 526, have been associated with rifamycin resistance in other species (2, 4, 14, 28). The mutations at 526 also led to the highest degrees of resistance. Perhaps as significant as the site of the mutation is the nature of the amino acid changes. It is quite possible that conservative changes in C. pneumoniae were responsible for the lower-level resistance observed, whereas more radical amino acid changes caused higher-level resistance in C. trachomatis.
Rifalazil-resistant C. trachomatis BU-434/L2 demonstrated a mutation at the beginning of the rpoB gene at codon 146. This rare mutation has been previously reported in the rpoB gene of rifampin-resistant E. coli by Lisitsyn et al. (18). Intriguingly, although BU-434/L2 developed high-level phenotypic resistance to rifampin, no genetic alterations were detected in the entire rpoB gene. The lack of mutations in the rpoB gene is not entirely unexpected. While in most cases high-level rifamycin resistance is associated with rpoB gene alteration (28), other mechanisms of rifampin resistance have been identified and reported for other bacteria, including a rifampin efflux pump, rifampin glucosylation, ribosylation, and phosphorylation enzymes (1, 5, 7, 27).
This is the first description of any antibiotic resistance-associated mutations in C. pneumoniae and of high-level rifampin resistance in C. trachomatis not associated with mutations in the rpoB gene. The clinical significance of these findings is not clear and needs to be investigated.
REFERENCES
- 1.Abadi, F. J. R., P. E. Carter, P. Cash, and T. H. Pennington. 1996. Rifampin resistance in Neisseria meningitidis due to alterations in membrane permeability. Antimicrob. Agents Chemother. 40:646-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry-Damon, H., C.-D. Soussy, and P. Courvalin. 1998. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 42:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belland, R. J., S. P. Ouellette, J. Gieffers, and G. I. Byrne. 2004. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol. 6:117-127. [DOI] [PubMed] [Google Scholar]
- 4.Carter, P. E., F. J. Abadi, D. E. Yakubu, and T. H. Pennington. 1994. Molecular characterization of rifampin resistant Neisseria meningitidis. Antimicrob. Agents Chemother. 38:1256-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekaran, S., and D. Lalithakumari. 1998. Plasmid mediated rifampicin resistance in Pseudomonas fluorescens. J. Med. Microbiol. 47:197-200. [DOI] [PubMed] [Google Scholar]
- 6.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry 3rd, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 7.Dabbs, E. R., K. Yazawa, Y. Mikami, M. Miyaji, N. Morisaki, S. Iwasaki, and K. Furihata. 1995. Ribosylation by mycobacterial strains as a new mechanism of rifampin inactivation. Antimicrob. Agents Chemother. 39:1007-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietze, R., L. Teixeira, L. M. Rocha, M. Palaci, J. L. Johnson, C. Wells, L. Rose, K. Eisenach, and J. J. Ellner. 2001. Safety and bactericidal activity of rifalazil in patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 45:1972-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreses-Werringloer, U., I. Padubrin, L. Kohler, and A. P. Hudson. 2003. Detection of nucleotide variability in rpoB in both rifampin-sensitive and rifampin-resistant strains of Chlamydia trachomatis. Antimicrob. Agents Chemother. 47:2316-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreses-Werringloer, U., I. Padubrin, H. Zeidler, and L. Kohler. 2001. Effect of azithromycin and rifampin on Chlamydia trachomatis infection in vitro. Antimicrob. Agents Chemother. 45:3001-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freidank, H. M., P. Losch, H. Vogele, and M. Wiedmann-Al-Ahmad. 1999. In vitro susceptibilities of Chlamydia pneumoniae isolates from German patients and synergistic activity of antibiotic combinations. Antimicrob. Agents Chemother. 43:1808-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales, G. F., G. Munoz, R. Sanchez, R. Henkel, G. Gallegos-Avila, O. Diaz-Gutierrez, P. Vigil, F. Vasquez, G. Kortebani, A. Mazzolli, and E. Bustos-Obregon. 2004. Update on the impact of Chlamydia trachomatis infection on male fertility. Andrologia 36:1-23. [DOI] [PubMed] [Google Scholar]
- 13.Hosoe, K., T. Mae, E. Konishi, K. Fujii, K. Yamashita, T. Yamane, T. Hidaka, and T. Ohasi. 1996. Pharmacokinetics of KRM-1648, a new benzoxazinorifamycin, in rats and dogs. Antimicrob. Agents Chemother. 40:2749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin, D. J., and C. A. Gross. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45-58. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. B., G. L. Ridgeway, S. Boulding, and K. L. Hanley. 1983. In vitro activities of rifamycine alone and in combination with other antibiotics against Chlamydia trachomatis. Rev. Infect. Dis. 5:S556-S561. [DOI] [PubMed] [Google Scholar]
- 16.Kraft, M., G. H. Cassell, J. Pak, and R. J. Martin. 2002. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 121:1782-1788. [DOI] [PubMed] [Google Scholar]
- 17.Lenart, J., A. A. Andersen, and D. D. Rockey. 2001. Growth and development of tetracycline-resistant Chlamydia suis. Antimicrob. Agents Chemother. 45:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisitsyn, N. A., E. D. Sverdlov, E. P. Moiseyeva, O. N. Danilevskaya, and V. G. Nikiforov. 1984. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol. Gen. Genet. 196:173-174. [DOI] [PubMed] [Google Scholar]
- 19.Loeb, M. B., D. Molloy, M. Smieja, T. Standish, C. H. Goldsmith, J. Mahony, S. Smith, M. Borrie, E. Decoteau, W. Davidson, A. McDougall, J. Gnarpe, M. O'donnell, and M. Chernesky. 2004. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J. Am. Geriatr. Soc. 52:381-387. [DOI] [PubMed] [Google Scholar]
- 20.Mardh, P. A. 2004. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr. Opin. Infect. Dis. 17:49-52. [DOI] [PubMed] [Google Scholar]
- 21.Misyurina, O. Y., E. V. Chipitsyna, Y. P. Finashutina, V. N. Lazarev T. A. Akopian, A. M. Savicheva, and V. M. Govorun. 2004. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides. Antimicrob. Agents Chemother. 48:1347-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morse, R., K. O'Hanlon, M. Virji, and M. D. Collins. 1999. Isolation of rifampin resistant mutants of Listeria monocytogenes and their characterization by rpoB gene sequencing, temperature sensitivity for growth, and interaction with epithelial cell line. J. Clin. Microbiol. 37:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roblin, P. M., T. Reznik, A. Kutlin, and M. R. Hammerschlag. 2003. In vitro activities of rifamycin derivatives ABI-1648 (rifalazil, KRM-1648), ABI-1657, and ABI-1131 against Chlamydia trachomatis and recent clinical isolates of Chlamydia pneumoniae. Antimicrob. Agents Chemother. 47:1135-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothstein, D. M., A. D. Hartman, M. H. Cynamon, and B. I. Eisenstein. 2003. Development potential of rifalazil. Expert Opin. Investig. Drugs 12:255-271. [DOI] [PubMed] [Google Scholar]
- 25.Schachter, J. 1983. Rifampin in chlamydial infections. Rev. Infect. Dis. 5:S562-S564. [DOI] [PubMed] [Google Scholar]
- 26.Treharne, J. D., P. J. Yearsley, and R. C. Ballard. 1989. In vitro studies of Chlamydia trachomatis susceptibility and resistance to rifampin and rifabutin. Antimicrob. Agents Chemother. 33:1393-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tribuddharat, C., and M. Fennewald. 1999. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, B., H. Koga, H. Ohno, K. Ogawa, M. Fukuda, Y. Hirakata, S. Maesaki, K. Tomono, T. Tashiro, and S. Kohno. 1998. Relationship between antimicrobial activities of rifampicin, rifabutin and KRM-1648 and rpoB mutations of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 42:612-628. [DOI] [PubMed] [Google Scholar]
- 29.Zanetti, S., D. Usai, A. Nonis, and G. Fadda. 1996. In-vitro activity of 3-azinomethyl-rifamycin (SPA-S-565) against Chlamydia trachomatis. J. Antimicrob. Chemother. 37:357-359. [DOI] [PubMed] [Google Scholar]